Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988–1994 to NHANES 2015–2016
Abstract
:1. Introduction
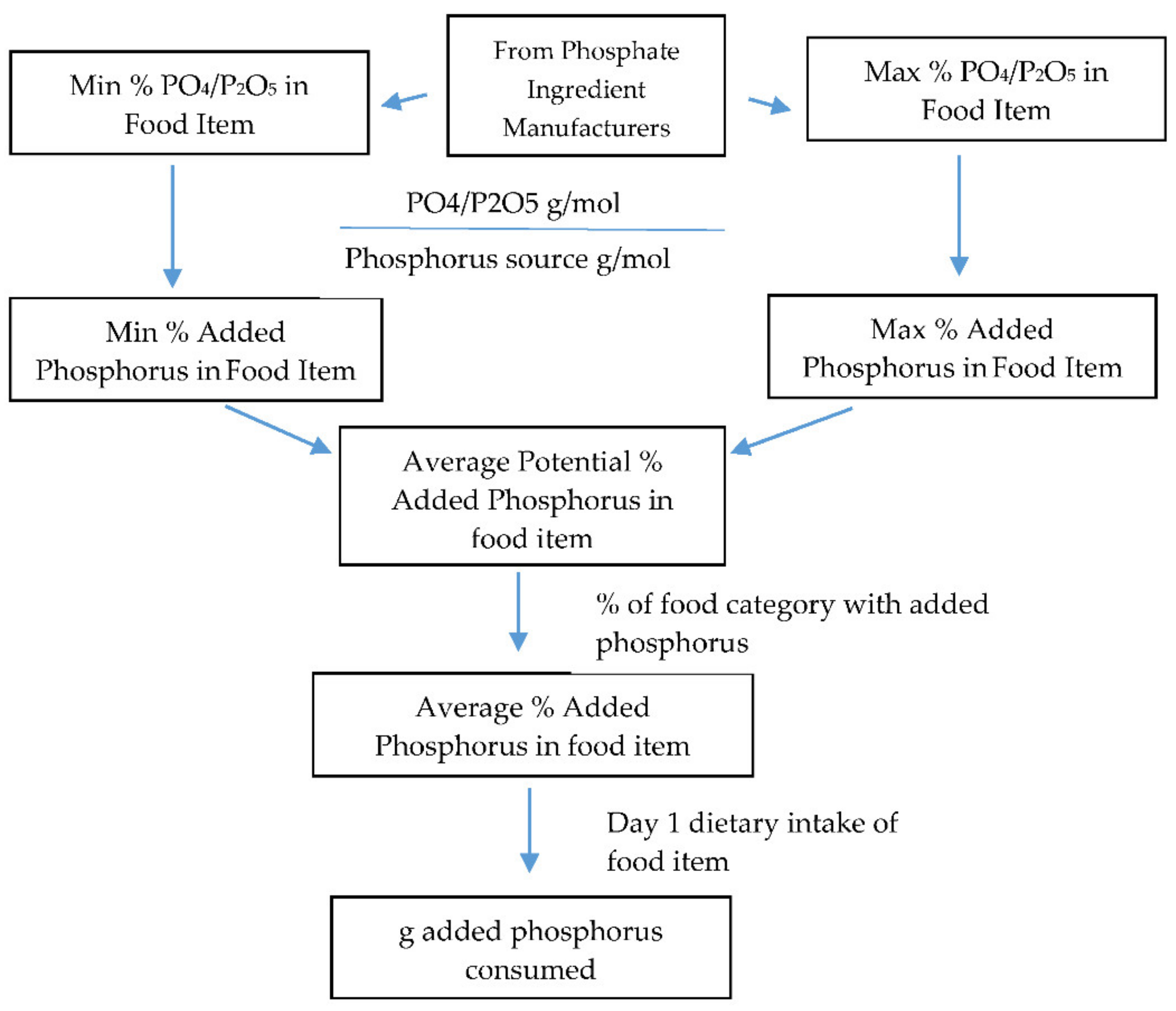
2. Materials and Methods
3. Results
3.1. Intake and Trends over Time
3.2. Intake and Trends over Time by Body Weight Status
3.3. Sources of Phosphorus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Phosphorus; National Academies Press: Washington, DC, USA, 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK109813/ (accessed on 23 February 2021).
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [Green Version]
- McClure, S.T.; Chang, A.R.; Selvin, E.; Rebholz, C.M.; Appel, L.J. Dietary Sources of Phosphorus among Adults in the United States: Results from NHANES 2001–2014. Nutrients 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the Health Impact of Phosphorus in the Food Supply: Issues and Considerations123. Adv. Nutr. 2014, 5, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Fiorito, L.M.; Mitchell, D.C.; Smiciklas-Wright, H.; Birch, L.L. Dairy and Dairy-Related Nutrient Intake During Middle Childhood. J. Am. Diet. Assoc. 2006, 106, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA, Agricultural Research Service. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat in America, NHANES 2015–2018. 2021. Available online: http://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 30 March 2021).
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Furst, P.; Gürtler, R.; Husøy, T.; et al. Scientific Opinion on the re-evaluation of phosphoric acid–phosphates–di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17, e05674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, R.; Jialal, I. Hyperphosphatemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551586/ (accessed on 31 January 2021).
- Schwarz, S.; Trivedi, B.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of Disorders in Mineral Metabolism with Progression of Chronic Kidney Disease. CJASN 2006, 1, 825–831. [Google Scholar] [CrossRef]
- Adeney, K.L.; Siscovick, D.S.; Ix, J.H.; Seliger, S.L.; Shlipak, M.G.; Jenny, N.S.; Kestenbaum, B.R. Association of Serum Phosphate with Vascular and Valvular Calcification in Moderate CKD. JASN 2009, 20, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum Phosphate Levels and Mortality Risk among People with Chronic Kidney Disease. JASN 2005, 16, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Voormolen, N.; Noordzij, M.; Grootendorst, D.C.; Beetz, I.; Sijpkens, Y.W.; van Manen, J.G.; Boeschoten, E.W.; Huisman, R.M.; Krediet, R.T.; Dekker, F.W.; et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol. Dial. Transplant. 2007, 22, 2909–2916. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. JASN 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of Elevated Serum PO4, Ca × PO4 Product, and Parathyroid Hormone with Cardiac Mortality Risk in Chronic Hemodialysis Patients. JASN 2001, 12, 2131–2138. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J.; Herzog, C.A.; Ishani, A.; Kalra, P.A. Serum Phosphorus Levels Associate with Coronary Atherosclerosis in Young Adults. JASN 2009, 20, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, R. Relations of Serum Phosphorus and Calcium Levels to the Incidence of Cardiovascular Disease in the Community. Arch. Intern. Med. 2007, 167, 879. [Google Scholar] [CrossRef] [Green Version]
- Sim, J.J.; Bhandari, S.K.; Smith, N.; Chung, J.; Liu, I.L.A.; Jacobsen, S.J.; Kalantar-Zadeh, K. Phosphorus and Risk of Renal Failure in Subjects with Normal Renal Function. Am. J. Med. 2013, 126, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampila, L.E. Applications and functions of food-grade phosphates. Ann. N. Y. Acad. Sci. 2013, 1301, 37–44. [Google Scholar] [CrossRef]
- Moore, L.W.; Nolte, J.V.; Gaber, A.O.; Suki, W.N. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 2015, 102, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Parpia, A.; L’abbe, M.; Goldstein, M.; Arcand, J.; Magnuson, B.; Darling, P. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients with Chronic Kidney Disease. J. Ren. Nutr. 2017, 28, 83–90. [Google Scholar] [CrossRef]
- León, J.B.; Sullivan, C.M.; Sehgal, A.R. The Prevalence of Phosphorus Containing Food Additives in Top Selling Foods in Grocery Stores. J. Ren. Nutr. 2013, 23, 265–270.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cupisti, A.; Benini, O.; Ferretti, V.; Gianfaldoni, D.; Kalantar-Zadeh, K. Novel Differential Measurement of Natural and Added Phosphorus in Preserved versus Non-Enhanced Ham. J. Ren. Nutr. 2012, 22, 533–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrigan, A.; Klinger, A.; Choquette, S.S.; Luzuriaga-McPherson, A.; Bell, E.K.; Darnell, B.; Gutiérrez, O.M. Contribution of Food Additives to Sodium and Phosphorus Content of Diets Rich in Processed Foods. J. Ren. Nutr. 2014, 24, 13–19, 19e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eicher-Miller, H.A.; Fulgoni, V.L.; Keast, D.R. Contributions of Processed Foods to Dietary Intake in the US from 2003–2008: A Report of the Food and Nutrition Science Solutions Joint Task Force of the Academy of Nutrition and Dietetics, American Society for Nutrition, Institute of Food Technologists, and International Food Information Council1234. J. Nutr. 2012, 142, 2065S–2072S. [Google Scholar] [CrossRef]
- NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (accessed on 24 January 2021).
- Dwyer, J.; Picciano, M.F.; Raiten, D.J.; Members of the Steering Committee. Collection of Food and Dietary Supplement Intake Data: What We Eat in America–NHANES. J. Nutr. 2003, 133, 590S–600S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. 2020. What We Eat in America Food Categories 2017–2018. Available online: https://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 1 March 2021).
- Krebs-Smith, S.M.; Kott, P.S.; Guenther, P.M. Mean proportion and population proportion: Two answers to the same question? J. Am. Diet. Assoc. 1989, 89, 671–676. [Google Scholar] [PubMed]
- Yamamoto, K.T.; Robinson-Cohen, C.; de Oliveira, M.C.; Kostina, A.; Nettleton, J.A.; Ix, J.H.; Nguyen, H.; Eng, J.; Lima, J.A.; Siscovick, D.S.; et al. Dietary phosphorus is associated with a significant increase in left ventricular mass. Kidney Int. 2013, 83, 707–714. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.H.; Rue, T.C.; Kestenbaum, B. Serum Phosphorus Concentrations in the Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney Dis. 2009, 53, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, R.; Gona, P.; Benjamin, E.J.; Wang, T.J.; Aragam, J. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur. J. Heart Fail. 2010, 12, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itkonen, S.T.; Karp, H.J.; Kemi, V.E.; Kokkonen, E.M.; Saarnio, E.M.; Pekkinen, M.H.; Kärkkäinen, M.U.; Laitinen, E.K.A.; Turanlahti, M.I.; Lamberg-Allardt, C.J. Associations among total and food additive phosphorus intake and carotid intima-media thickness—A cross-sectional study in a middle-aged population in Southern Finland. Nutr. J. 2013, 12, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemi, V.E.; Kärkkäinen, M.U.M.; Lamberg-Allardt, C.J.E. High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females. Br. J. Nutr. 2006, 96, 545–552. [Google Scholar]
- Flegal, K.M. Prevalence and Trends in Obesity Among US Adults, 1999–2000. JAMA 2002, 288, 1723. [Google Scholar] [CrossRef]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, C.M.; Leon, J.B.; Sehgal, A.R. Phosphorus containing food additives and the accuracy of nutrient databases: Implications for renal patients. J. Ren. Nutr. 2007, 17, 350–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benini, O.; D’Alessandro, C.; Gianfaldoni, D.; Cupisti, A. Extra-Phosphate Load From Food Additives in Commonly Eaten Foods: A Real and Insidious Danger for Renal Patients. J. Ren. Nutr. 2011, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Mehta, O. Phosphorus and Potassium Content of Enhanced Meat and Poultry Products: Implications for Patients Who Receive Dialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Krekel, C.; McClure, S.T.; Chang, A.R. Improving Estimates of Phosphorus Additive Content: Manufacturers Needed. J. Ren. Nutr. 2016, 26, e27–e30. [Google Scholar] [CrossRef] [Green Version]
- Moser, M.; White, K.; Henry, B.; Oh, S.; Miller, E.R.; Anderson, C.A.; Benjamin, J.; Charleston, J.; Appel, L.J.; Chang, A.R. Phosphorus Content of Popular Beverages. Am. J. Kidney Dis. 2015, 65, 969–971. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E. Prevalence and characteristics of misreporting of energy intake in US adults: NHANES 2003–2012. Br. J. Nutr. 2015, 114, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
| Subject Group | NHANES Cycle | Total Phosphorus | Added Phosphorus | Natural Phosphorus | % As Added |
|---|---|---|---|---|---|
| All (n = 55,744) | 1988–1994 | 1292 (10.7) | 178.1 (2.9) | 1113.4 (9.9) | 14.6 (0.2) |
| 2001–2002 | 1330 (16.1) | 192.6 (3.4) | 1137.8 (15.9) | 15.2 (0.3) | |
| 2003–2004 | 1324 (16.5) | 188.0 (3.2) | 1136.2 (15.6) | 15.1 (0.2) | |
| 2005–2006 | 1359 (16.4) | 183.6 (4.9) | 1175.4 (16.0) | 14.2 (0.3) | |
| 2007–2008 | 1331 (21.2) | 174.9 (3.7) | 1156.2 (21.1) | 13.8 (0.3) | |
| 2009–2010 | 1417 (11.4) | 181.6 (2.6) | 1235.4 (10.6) | 13.2 (0.2) | |
| 2011–2012 | 1430 (9.90) | 172.3 (3.9) | 1257.3 (11.5) | 12.5 (0.3) | |
| 2013–2014 | 1406 (12.4) | 175.5 (3.3) | 1230.4 (12.7) | 13.0 (0.3) | |
| 2015–2016 | 1398 (17.0) | 155.2 (4.1) | 1242.7 (15.6) | 11.6 (0.2) | |
| P 3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Cycle Trend | |||||
| Beta 4 | 15.06 (1.89) | −3.13 (0.46) | 18.19 (1.83) | −0.43 (0.03) | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Yearly Trend | |||||
| Beta 5 | 5.70 (0.65) | −0.92 (0.16) | 6.62 (0.62) | −0.14 (0.01) | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Males (n = 27,616) | 1988–1994 | 1538 (14.9) | 209.9 (4.0) | 1328.2 (13.9) | 14.4 (0.2) |
| 2001–2002 | 1551 (27.3) | 219.8 (6.3) | 1330.7 (26.3) | 14.7 (0.3) | |
| 2003–2004 | 1546 (23.7) | 216.2 (5.2) | 1329.8 (22.2) | 14.7 (0.3) | |
| 2005–2006 | 1594 (21.4) | 216.3 (6.9) | 1377.5 (20.4) | 14.3 (0.4) | |
| 2007–2008 | 1548 (24.9) | 198.7 (5.6) | 1349.8 (24.5) | 13.4 (0.4) | |
| 2009–2010 | 1656 (16.8) | 215.3 (4.7) | 1441.0 (15.9) | 13.3 (0.3) | |
| 2011–2012 | 1675 (16.5) | 199.4 (5.8) | 1475.8 (16.9) | 12.4 (0.3) | |
| 2013–2014 | 1635 (18.1) | 200.5 (5.6) | 1434.1 (16.8) | 12.7 (0.3) | |
| 2015–2016 | 1612 (23.2) | 177.0 (6.0) | 1435.3 (21.7) | 11.5 (0.3) | |
| P 3 | 0.0084 | <0.0001 | 0.0001 | <0.0001 | |
| Cycle Trend | |||||
| Beta 4 | 14.36 (2.80) | −3.95 (0.72) | 18.31 (2.66) | −0.40 (0.04) | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Yearly Trend | |||||
| Beta 5 | 5.22 (0.93) | −1.23 (0.25) | 6.45 (0.88) | −0.13 (0.01) | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Females (n = 28,128) | 1988–1994 | 1052 (10.1) | 147.2 (2.9) | 905.1 (8.7) | 14.8 (0.2) |
| 2001–2002 | 1115 (16.8) | 165.8 (4.1) | 949.5 (15.5) | 15.7 (0.4) | |
| 2003–2004 | 1108 (15.5) | 160.5 (3.7) | 947.3 (15.7) | 15.4 (0.4) | |
| 2005–2006 | 1130 (17.3) | 151.5 (3.8) | 978.0 (17.0) | 14.2 (0.4) | |
| 2007–2008 | 1122 (22.0) | 151.6 (3.9) | 970.3 (21.6) | 14.1 (0.4) | |
| 2009–2010 | 1185 (12.4) | 148.9 (2.4) | 1036.0 (12.1) | 13.1 (0.2) | |
| 2011–2012 | 1191 (12.8) | 146.0 (4.1) | 1044.8 (12.0) | 12.7 (0.3) | |
| 2013–2014 | 1185 (13.4) | 151.4 (3.3) | 1033.4 (14.4) | 13.3 (0.3) | |
| 2015–2016 | 1189 (16.0) | 134.0 (3.4) | 1055.4 (14.3) | 11.6 (0.2) | |
| P 3 | <0.0001 | 0.0004 | <0.0001 | <0.0001 | |
| Cycle Trend | |||||
| Beta 4 | 15.91 (1.87) | −2.30 (0.45) | 18.21 (1.77) | −0.46 (0.04) | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Yearly Trend | |||||
| Beta 5 | 6.21 (0.63) | −0.61 (0.16) | 6.82 (0.59) | −0.15 (0.01) | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Subject Group | NHANES Cycle | Total Phosphorus | Added Phosphorus | Natural Phosphorus | % As Added |
|---|---|---|---|---|---|
| All (n = 55,202) | 1988–1994 | 17.75 (0.14) | 2.43 (0.04) | 15.32 (0.13) | 14.61 (0.21) |
| 2001–2002 | 17.31 (0.23) | 2.51 (0.05) | 14.80 (0.22) | 15.20 (0.27) | |
| 2003–2004 | 17.02 (0.18) | 2.42 (0.04) | 14.61 (0.18) | 15.09 (0.21) | |
| 2005–2006 | 17.42 (0.24) | 2.34 (0.06) | 15.08 (0.23) | 14.22 (0.33) | |
| 2007–2008 | 17.00 (0.25) | 2.21 (0.04) | 14.79 (0.24) | 13.64 (0.28) | |
| 2009–2010 | 17.97 (0.16) | 2.30 (0.03) | 15.67 (0.14) | 13.22 (0.22) | |
| 2011–2012 | 18.20 (0.20) | 2.19 (0.05) | 16.01 (0.19) | 12.51 (0.25) | |
| 2013–2014 | 17.83 (0.17) | 2.22 (0.04) | 15.61 (0.18) | 12.98 (0.25) | |
| 2015–2016 | 17.48 (0.21) | 1.93 (0.04) | 15.55 (0.21) | 11.53 (.22) | |
| P 3 | 0.2859 | <0.0001 | 0.3222 | <0.0001 | |
| Cycle Trend | |||||
| Beta 4 | 0.06 (0.02) | −0.06 (0.01) | 0.12 (0.02) | −0.43 (0.03) | |
| p-value | 0.0163 | <0.0001 | <0.0001 | <0.0001 | |
| Yearly Trend | |||||
| Beta 5 | 0.01 (0.01) | −0.02 (0.002) | 0.03 (0.01) | −0.14 (0.01) | |
| p-value | 0.0163 | <0.0001 | <0.0001 | <0.0001 | |
| Males (n = 27,356) | 1988–1994 | 19.44 (0.18) | 2.63 (0.05) | 16.80 (0.17) | 14.42 (0.25) |
| 2001–2002 | 18.57 (0.35) | 2.65 (0.08) | 15.92 (0.33) | 14.63 (0.32) | |
| 2003–2004 | 18.36 (0.25) | 2.57 (0.06) | 15.79 (0.24) | 14.75 (0.28) | |
| 2005–2006 | 18.78 (0.29) | 2.54 (0.08) | 16.23 (0.29) | 14.29 (0.35) | |
| 2007–2008 | 18.30 (0.27) | 2.33 (0.06) | 15.96 (0.28) | 13.39 (0.37) | |
| 2009–2010 | 19.33 (0.24) | 2.53 (0.06) | 16.80 (0.22) | 13.36 (0.33) | |
| 2011–2012 | 19.78 (0.24) | 2.36 (0.08) | 17.42 (0.21) | 12.36 (0.33) | |
| 2013–2014 | 19.26 (0.26) | 2.34 (0.07) | 16.91 (0.24) | 12.68 (0.29) | |
| 2015–2016 | 18.75 (0.29) | 2.03 (0.05) | 16.73 (0.28) | 11.42 (0.31) | |
| P 3 | 0.0447 | <0.0001 | 0.8234 | <0.0001 | |
| Cycle Trend | |||||
| Beta 4 | 0.05 (0.03) | −0.06 (0.01) | 0.12 (0.03) | −0.40 (0.04) | |
| p-value | 0.1227 | <0.0001 | 0.0005 | <0.0001 | |
| Yearly Trend | |||||
| Beta 5 | 0.01 (0.01) | −0.02 (0.003) | 0.03 (0.01) | −0.14 (0.01) | |
| p-value | 0.1227 | <0.00012 | 0.0006 | <0.0001 | |
| Females (n = 27,846) | 1988–1994 | 16.12 (0.18) | 2.24 (0.05) | 13.88 (0.15) | 14.80 (0.23) |
| 2001–2002 | 16.07 (0.25) | 2.37 (0.07) | 13.70 (0.23) | 15.75 (0.41) | |
| 2003–2004 | 15.72 (0.30) | 2.26 (0.05) | 13.46 (0.30) | 15.42 (0.38) | |
| 2005–2006 | 16.09 (0.35) | 2.14 (0.07) | 13.95 (0.33) | 14.15 (0.35) | |
| 2007–2008 | 15.76 (0.30) | 2.08 (0.04) | 13.68 (0.28) | 13.86 (0.29) | |
| 2009–2010 | 16.66 (0.20) | 2.08 (0.04) | 14.58 (0.19) | 13.07 (0.25) | |
| 2011–2012 | 16.67 (0.22) | 2.02 (0.05) | 14.65 (0.21) | 12.65 (0.26) | |
| 2013–2014 | 16.46 (0.21) | 2.10 (0.06) | 14.36 (0.21) | 13.27 (0.34) | |
| 2015–2016 | 16.24 (0.20) | 1.83 (0.05) | 14.41 (0.19) | 11.63 (0.23) | |
| P 3 | 0.6450 | <0.0001 | 0.0274 | <0.0001 | |
| Cycle Trend | |||||
| Beta 4 | 0.07 (0.03) | −0.05 (0.01) | 0.12 (0.03) | −0.45 (0.04) | |
| p-value | 0.0156 | <0.0001 | <0.0001 | <0.0001 | |
| Yearly Trend | |||||
| Beta 5 | 0.02 (0.01) | −0.02 (0.003) | 0.04 (0.01) | −0.15 (0.01) | |
| p-value | 0.0156 | <0.0001 | <0.0001 | <0.0001 |
| Food Category | Consumer N | Mean (SE), mg/day | Percent Daily (SE), % |
|---|---|---|---|
| Total Phosphorus | |||
| Cheese | 13,219 | 80.32 (1.65) | 5.84 (0.11) |
| Pizza | 3853 | 57.93 (1.64) | 4.21 (0.12) |
| Chicken, whole pieces | 8755 | 49.98 (1.30) | 3.63 (0.10) |
| Milk, reduced fat | 6495 | 46.09 (1.37) | 3.35 (0.10) |
| Eggs and omelets | 9057 | 42.70 (0.84) | 3.10 (0.06) |
| Yeast breads | 16,634 | 38.80 (0.63) | 2.82 (0.04) |
| Cold cuts and cured meats | 7455 | 37.60 (0.94) | 2.73 (0.07) |
| Nuts and seeds | 6480 | 37.10 (1.16) | 2.70 (0.08) |
| Meat mixed dishes | 4360 | 32.66 (0.93) | 2.37 (0.07) |
| Burritos and tacos | 2327 | 31.92 (1.61) | 2.32 (0.11) |
| Beef, excludes ground | 4218 | 29.68 (0.98) | 2.16 (0.07) |
| Fish | 3338 | 29.40 (1.26) | 2.14 (0.09) |
| Milk, nonfat | 2688 | 28.46 (1.46) | 2.07 (0.10) |
| Natural Phosphorus | |||
| Pizza | 3853 | 57.93 (1.64) | 4.84 (0.14) |
| Cheese | 13,219 | 53.81 (1.11) | 4.49 (0.09) |
| Milk, reduced fat | 6495 | 46.09 (1.37) | 3.85 (0.11) |
| Eggs and omelets | 9057 | 42.70 (0.84) | 3.56 (0.07) |
| Chicken, whole pieces | 8755 | 42.40 (1.10) | 3.54 (0.09) |
| Nuts and seeds | 6480 | 37.10 (1.16) | 3.10 (0.09) |
| Meat mixed dishes | 4,360 | 32.54 (0.93) | 2.72 (0.08) |
| Yeast breads | 16,634 | 32.44 (0.54) | 2.71 (0.04) |
| Burritos and tacos | 2327 | 31.92 (1.61) | 2.66 (0.13) |
| Beef, excludes ground | 4218 | 29.68 (0.98) | 2.48 (0.08) |
| Cold cuts and cured meats | 7455 | 29.60 (0.76) | 2.47 (0.06) |
| Milk, nonfat | 2688 | 28.46 (1.46) | 2.38 (0.12) |
| Fish | 3338 | 28.13 (1.20) | 2.35 (0.10) |
| Milk, whole | 4684 | 26.65 (1.03) | 2.22 (0.09) |
| Added Phosphorus | |||
| Cheese | 13,219 | 26.52 (0.56) | 14.95 (0.29) |
| Soft drinks | 14,356 | 16.60 (0.55) | 9.36 (0.31) |
| Cakes and pies | 5278 | 16.22 (0.44) | 9.15 (0.23) |
| Rolls and buns | 6998 | 10.79 (0.30) | 6.08 (0.16) |
| Cookies and brownies | 8830 | 10.60 (0.19) | 5.98 (0.12) |
| Doughnuts, sweet rolls, pastries | 4564 | 10.23 (0.28) | 5.77 (0.15) |
| Tortillas | 4770 | 10.06 (0.59) | 5.67 (0.33) |
| Diet soft drinks | 5299 | 9.53 (0.31) | 5.37 (0.16) |
| Cold cuts and cured meats | 7455 | 8.00 (0.19) | 4.51 (0.10) |
| Chicken, whole pieces | 8755 | 7.58 (0.20) | 4.27 (0.11) |
| Yeast breads | 16,634 | 6.36 (0.09) | 3.58 (0.06) |
| Biscuits, muffins, quick breads | 3611 | 5.84 (0.25) | 3.30 (0.14) |
| Nutritional beverages | 481 | 5.61 (0.51) | 3.16 (0.28) |
| Cream and cream substitutes | 8250 | 4.97 (0.17) | 2.81 (0.10) |
| Pancakes, waffles, French toast | 1925 | 4.60 (0.20) | 2.59 (0.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulgoni, K.; Fulgoni, V.L., III. Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988–1994 to NHANES 2015–2016. Nutrients 2021, 13, 2249. https://doi.org/10.3390/nu13072249
Fulgoni K, Fulgoni VL III. Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988–1994 to NHANES 2015–2016. Nutrients. 2021; 13(7):2249. https://doi.org/10.3390/nu13072249
Chicago/Turabian StyleFulgoni, Kristin, and Victor L. Fulgoni, III. 2021. "Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988–1994 to NHANES 2015–2016" Nutrients 13, no. 7: 2249. https://doi.org/10.3390/nu13072249
APA StyleFulgoni, K., & Fulgoni, V. L., III. (2021). Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988–1994 to NHANES 2015–2016. Nutrients, 13(7), 2249. https://doi.org/10.3390/nu13072249






