Impact of Glucosamine Supplementation on Gut Health
Abstract
:1. Introduction
2. Materials and Methods
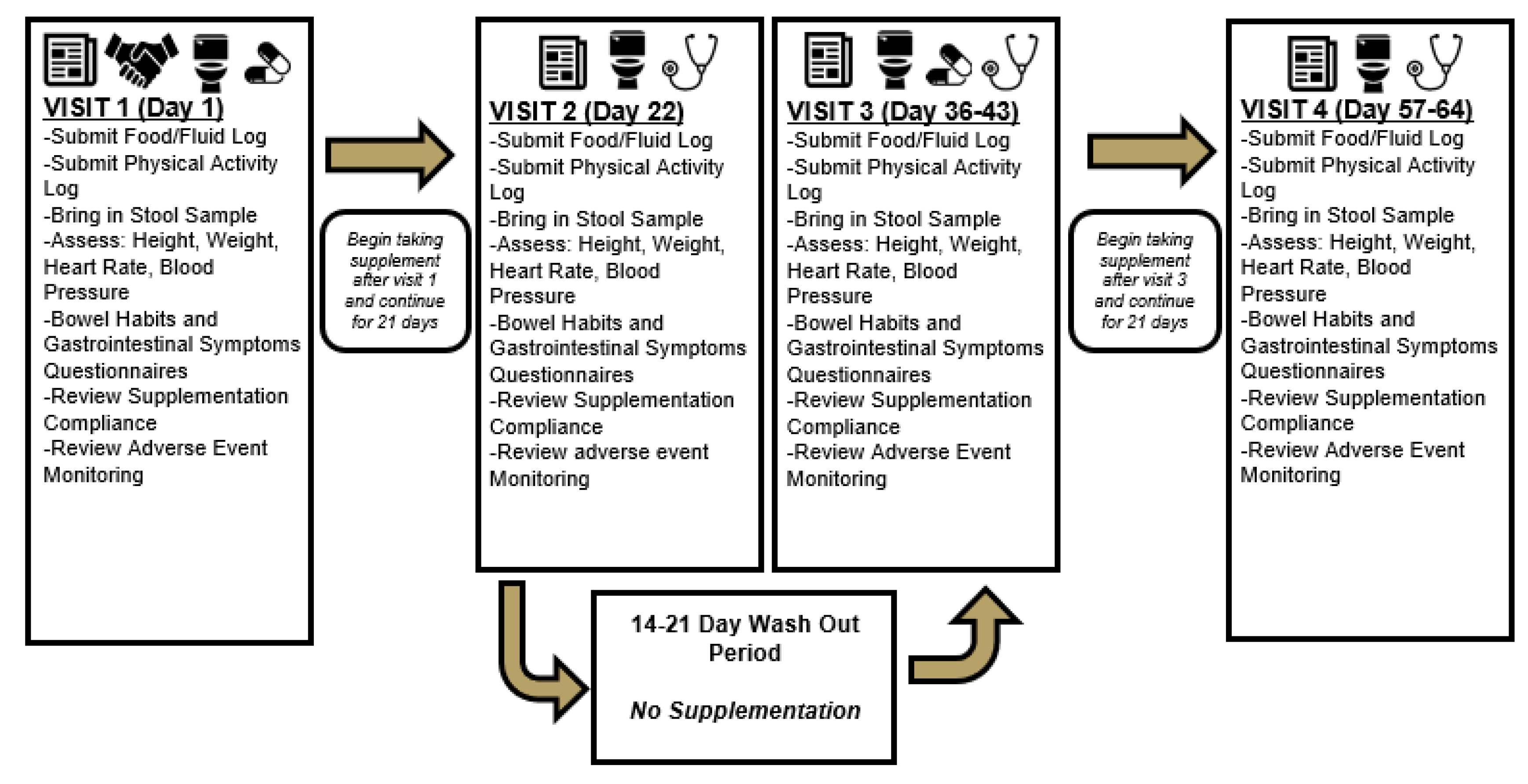
2.1. Experimental Design
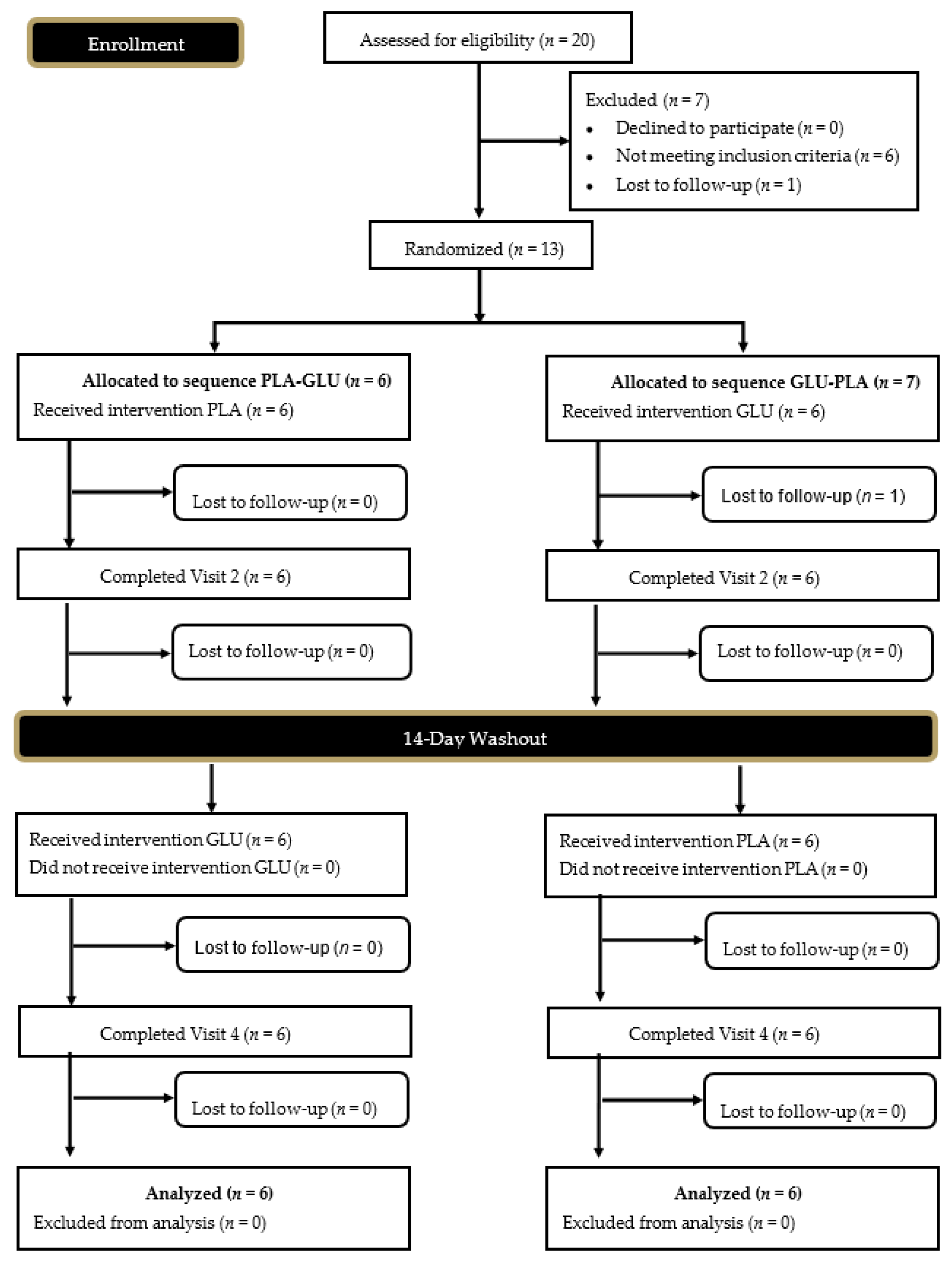
2.2. Study Participants
2.3. Anthropometrics
2.4. Dietary and Physical Activity Logs
2.5. Supplementation Protocol
2.6. Stool Quality and Gastrointestinal Questionnaires
2.7. Stool Collection
2.8. Fecal Microbiota Analysis
2.9. Fecal Metabolome Analysis
2.10. Statistical Analysis
3. Results
3.1. Adverse Events
3.2. Supplement Compliance
3.3. Baseline Differences
3.4. Participant Demographics and Hemodynamics
3.5. Bristol Stool Chart
3.6. Gastrointestinal Symptom Rating Scale
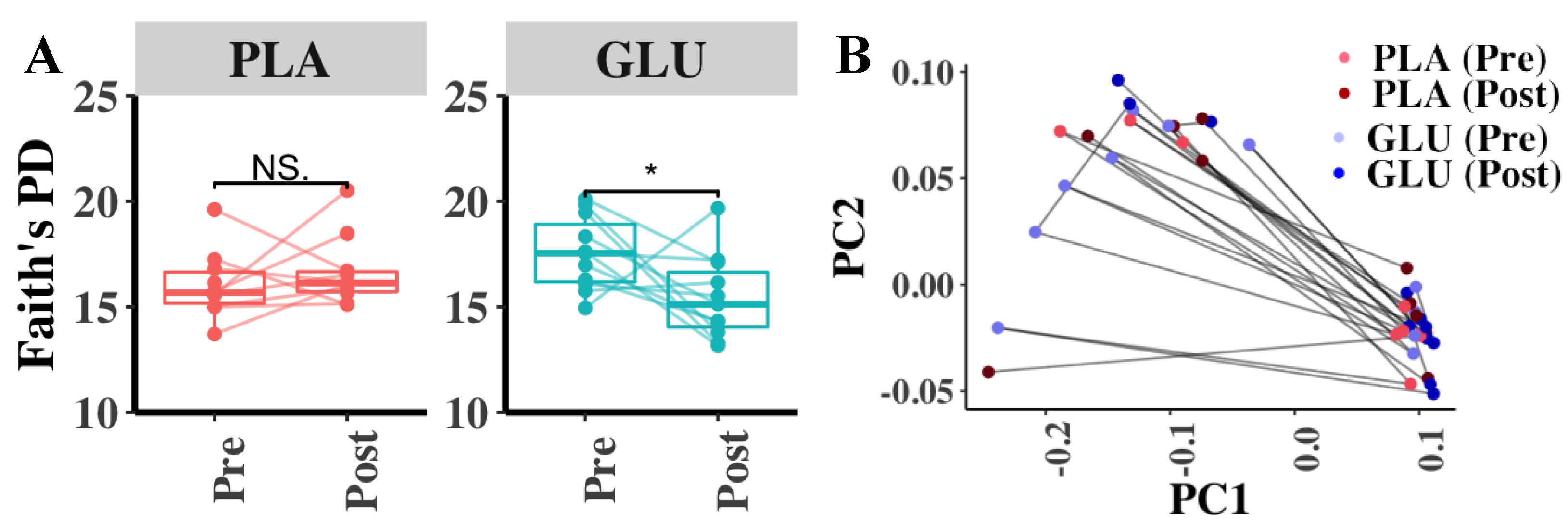
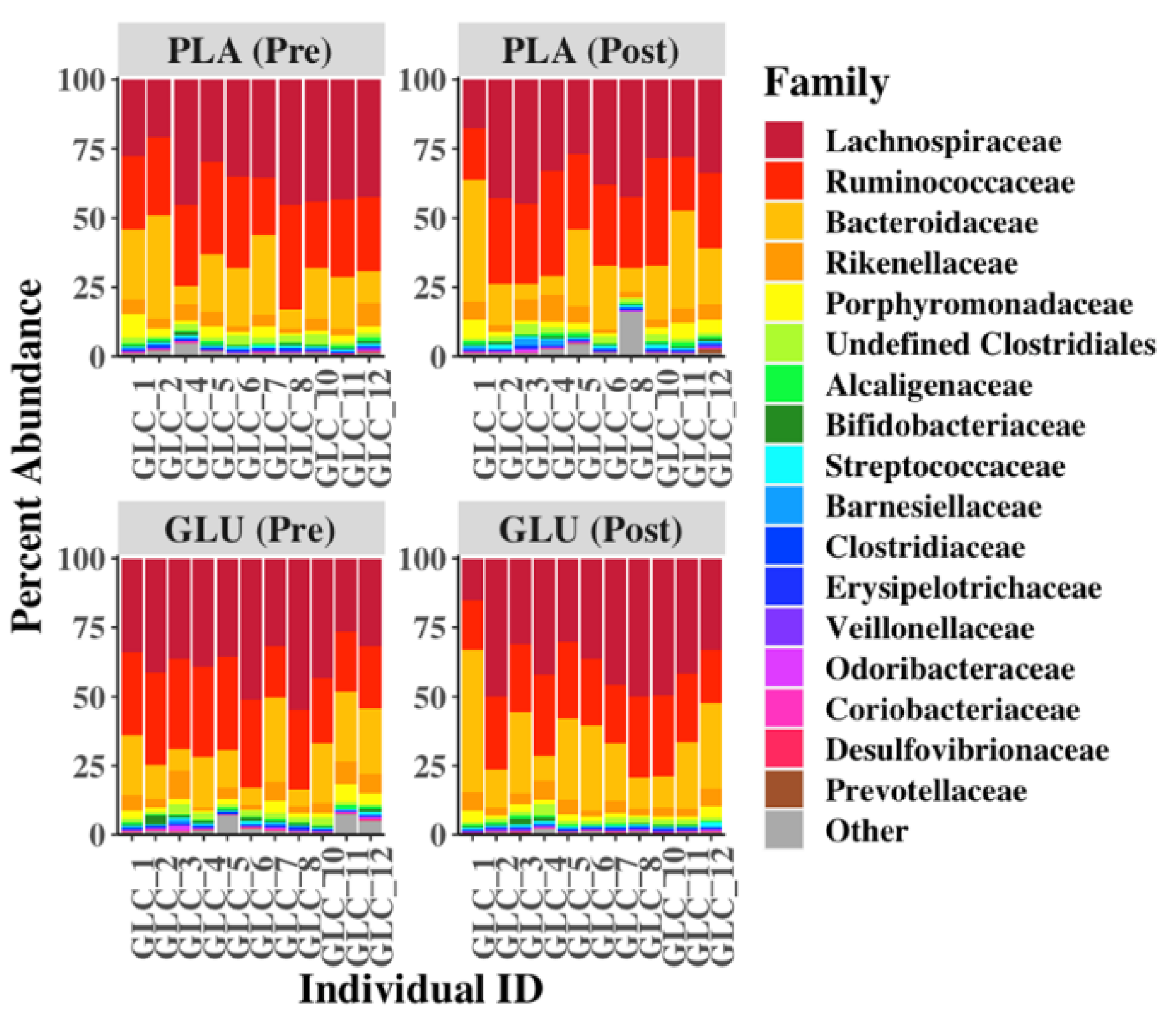
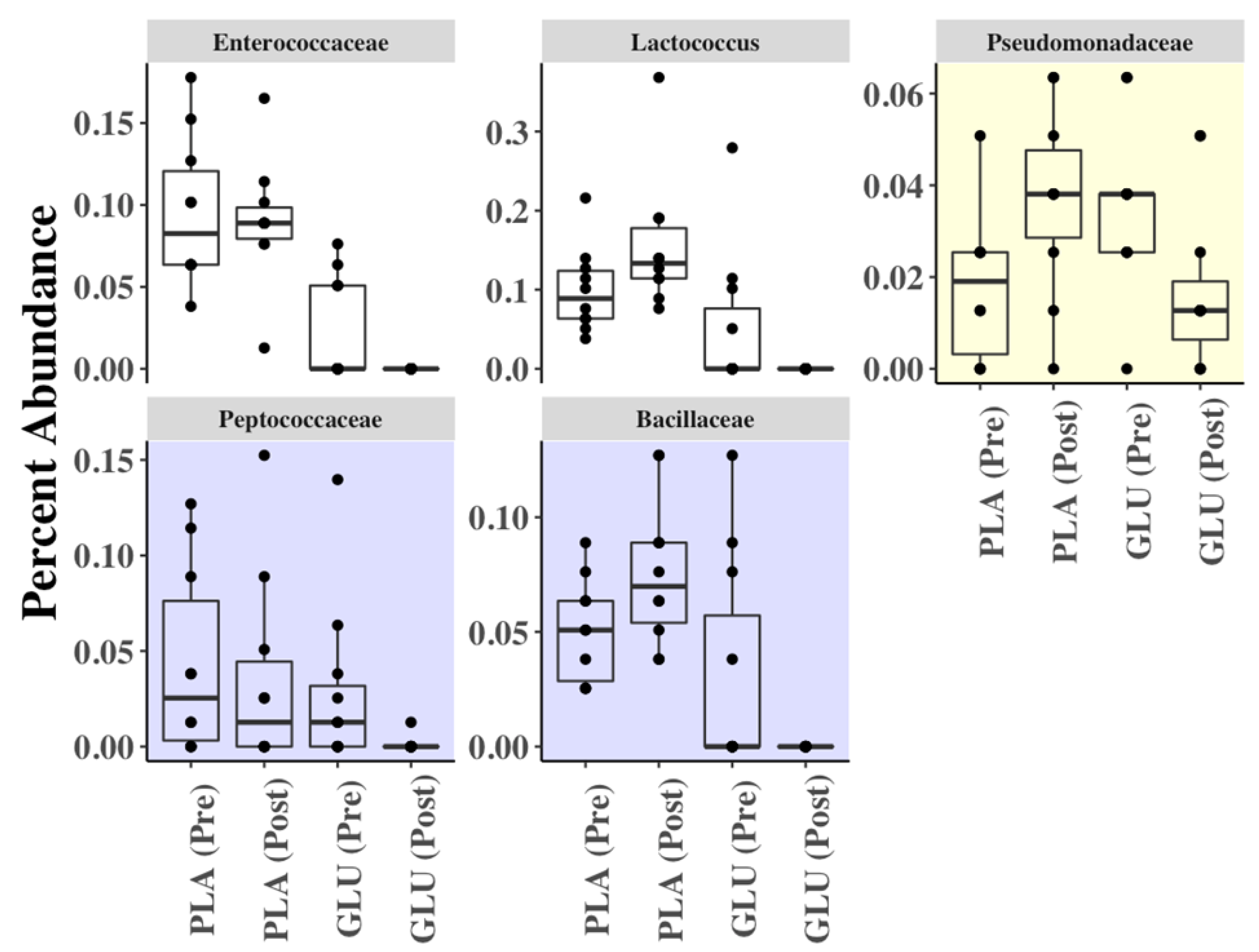
3.7. Fecal Microbiota Diversity
3.8. Fecal Metabolome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qato, D.M.; Alexander, G.C.; Conti, R.M.; Johnson, M.; Schumm, P.; Lindau, S.T. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008, 300, 2867–2878. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Mobasheri, A.; Marty, M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res. 2012, 14, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyere, O.; Pavelka, K.; Rovati, L.C.; Gatterova, J.; Giacovelli, G.; Olejarova, M.; Deroisy, R.; Reginster, J.Y. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: Results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthr. Cartil. 2008, 16, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Beaumont, G.; Ivorra, J.A.; Del Carmen Trabado, M.; Blanco, F.J.; Benito, P.; Martin-Mola, E.; Paulino, J.; Marenco, J.L.; Porto, A.; Laffon, A.; et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: A randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007, 56, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Ideno, Y.; Akai, M.; Seichi, A.; Hagino, H.; Iwaya, T.; Doi, T.; Yamada, K.; Chen, A.Z.; Li, Y.; et al. Effects of glucosamine in patients with osteoarthritis of the knee: A systematic review and meta-analysis. Clin. Rheumatol. 2018, 37, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, D.; Sang, L.; Wang, Y.; Shen, Y.; Zhuang, X.; Chu, M.; Jiang, L. Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: A network meta-analysis. Clin. Exp. Rheumatol. 2018, 36, 595–602. [Google Scholar]
- Bell, G.A.; Kantor, E.D.; Lampe, J.W.; Shen, D.D.; White, E. Use of glucosamine and chondroitin in relation to mortality. Eur. J. Epidemiol. 2012, 27, 593–603. [Google Scholar] [CrossRef]
- Pocobelli, G.; Kristal, A.R.; Patterson, R.E.; Potter, J.D.; Lampe, J.W.; Kolar, A.; Evans, I.; White, E. Total mortality risk in relation to use of less-common dietary supplements. Am. J. Clin. Nutr. 2010, 91, 1791–1800. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Sun, D.; Zhou, T.; Ley, S.H.; Gustat, J.; Heianza, Y.; Qi, L. Association of habitual glucosamine use with risk of cardiovascular disease: Prospective study in UK Biobank. BMJ 2019, 365, l1628. [Google Scholar] [CrossRef] [Green Version]
- Kantor, E.D.; Newton, C.C.; Giovannucci, E.L.; McCullough, M.L.; Campbell, P.T.; Jacobs, E.J. Glucosamine use and risk of colorectal cancer: Results from the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control 2018, 29, 389–397. [Google Scholar] [CrossRef]
- Kantor, E.D.; Zhang, X.; Wu, K.; Signorello, L.B.; Chan, A.T.; Fuchs, C.S.; Giovannucci, E.L. Use of glucosamine and chondroitin supplements in relation to risk of colorectal cancer: Results from the Nurses’ Health Study and Health Professionals follow-up study. Int. J. Cancer 2016, 139, 1949–1957. [Google Scholar] [CrossRef]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol. Prev. Biomark. 2009, 18, 1419–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantor, E.D.; Lampe, J.W.; Peters, U.; Shen, D.D.; Vaughan, T.L.; White, E. Use of glucosamine and chondroitin supplements and risk of colorectal cancer. Cancer Causes Control 2013, 24, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Bak, Y.K.; Lampe, J.W.; Sung, M.K. Effects of dietary supplementation of glucosamine sulfate on intestinal inflammation in a mouse model of experimental colitis. J. Gastroenterol. Hepatol. 2014, 29, 957–963. [Google Scholar] [CrossRef]
- Salvatore, S.; Heuschkel, R.; Tomlin, S.; Davies, S.E.; Edwards, S.; Walker-Smith, J.A.; French, I.; Murch, S.H. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharm. 2000, 14, 1567–1579. [Google Scholar] [CrossRef]
- Adebowale, A.; Du, J.; Liang, Z.; Leslie, J.L.; Eddington, N.D. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharm. Drug Dispos. 2002, 23, 217–225. [Google Scholar] [CrossRef]
- Aghazadeh-Habashi, A.; Sattari, S.; Pasutto, F.; Jamali, F. Single dose pharmacokinetics and bioavailability of glucosamine in the rat. J. Pharm. Sci. 2002, 5, 181–184. [Google Scholar]
- Ibrahim, A.; Gilzad-kohan, M.H.; Aghazadeh-Habashi, A.; Jamali, F. Absorption and bioavailability of glucosamine in the rat. J. Pharm. Sci. 2012, 101, 2574–2583. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef] [Green Version]
- Shmagel, A.; Demmer, R.; Knights, D.; Butler, M.; Langsetmo, L.; Lane, N.E.; Ensrud, K. The Effects of Glucosamine and Chondroitin Sulfate on Gut Microbial Composition: A Systematic Review of Evidence from Animal and Human Studies. Nutrients 2019, 11, 294. [Google Scholar] [CrossRef] [Green Version]
- Benjdia, A.; Berteau, O. Sulfatases and radical SAM enzymes: Emerging themes in glycosaminoglycan metabolism and the human microbiota. Biochem. Soc. Trans. 2016, 44, 109–115. [Google Scholar] [CrossRef]
- Jin, M.; Iwamoto, T.; Yamada, K.; Satsu, H.; Totsuka, M.; Shimizu, M. Disaccharide derived from chondroitin sulfate A suppressed CpG-induced IL-6 secretion in macrophage-like J774.1 cells. Cytokine 2010, 51, 53–59. [Google Scholar] [CrossRef]
- Segarra, S.; Martinez-Subiela, S.; Cerda-Cuellar, M.; Martinez-Puig, D.; Munoz-Prieto, A.; Rodriguez-Franco, F.; Rodriguez-Bertos, A.; Allenspach, K.; Velasco, A.; Ceron, J. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: A randomized, controlled clinical trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Coulson, S.; Butt, H.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: Therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 2013, 21, 79–90. [Google Scholar] [CrossRef]
- Navarro, S.L.; Levy, L.; Curtis, K.R.; Lampe, J.W.; Hullar, M.A.J. Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans. Microorganisms 2019, 7, 610. [Google Scholar] [CrossRef] [Green Version]
- Burns, A.M.; Solch, R.J.; Dennis-Wall, J.C.; Ukhanova, M.; Nieves, C., Jr.; Mai, V.; Christman, M.C.; Gordon, D.T.; Langkamp-Henken, B. In healthy adults, resistant maltodextrin produces a greater change in fecal bifidobacteria counts and increases stool wet weight: A double-blind, randomized, controlled crossover study. Nutr. Res. 2018, 60, 33–42. [Google Scholar] [CrossRef]
- Fastinger, N.D.; Karr-Lilienthal, L.K.; Spears, J.K.; Swanson, K.S.; Zinn, K.E.; Nava, G.M.; Ohkuma, K.; Kanahori, S.; Gordon, D.T.; Fahey, G.C., Jr. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J. Am. Coll. Nutr. 2008, 27, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, G. Bristol Stool Chart: Prospective and monocentric study of "stools introspection" in healthy subjects. Prog. Urol. 2014, 24, 708–713. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjodin, I.; Dotevall, G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Martinic, A.; Barouei, J.; Bendiks, Z.; Mishchuk, D.; Heeney, D.D.; Martin, R.; Marco, M.L.; Slupsky, C.M. Supplementation of Lactobacillus plantarum Improves Markers of Metabolic Dysfunction Induced by a High Fat Diet. J. Proteome. Res. 2018, 17, 2790–2802. [Google Scholar] [CrossRef]
- Xue, Z.; Kable, M.E.; Marco, M.L. Impact of DNA Sequencing and Analysis Methods on 16S rRNA Gene Bacterial Community Analysis of Dairy Products. mSphere 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Mills, D.A. Facility-specific "house" microbiome drives microbial landscapes of artisan cheesemaking plants. Appl. Environ. Microbiol. 2013, 79, 5214–5223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; Rankin, K.N.; Wagner, J.; Fu, H.; Chang, D.; Weljie, A.M.; Saude, E.J.; Lix, B.; Adamko, D.J.; Shah, S.; et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 2007, 79, 6995–7004. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lonnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Parenti, M.; Grip, T.; Lonnerdal, B.; Timby, N.; Domellof, M.; Hernell, O.; Slupsky, C.M. Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: A randomized controlled trial. Sci. Rep. 2019, 9, 11589. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. The R Project for Statistical Computing. Available online: https:www.r-project.org (accessed on 1 March 2021).
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-scale Meta-omics Studies [PrePrint]. Microbiology 2021. [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbo, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. Gut microbiota-derived metabolites in obesity: A systematic review. Biosci. Microbiota Food Health 2020, 39, 65–76. [Google Scholar] [CrossRef] [Green Version]
| PLA | GLU | PLA vs. GLU | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | p | |
| Body Mass (kg) | 77.0 ± 13.3 | 76.6 ± 12.6 | 0.15 | 76.5 ± 12.7 | 76.8 ± 12.5 | 0.60 | 0.43 |
| Heart Rate (beats/min) | 66.8 ± 10.4 | 65.8 ± 7.9 | 0.69 | 66.8 ± 7.7 | 63.1 ± 8.1 | 0.22 | 0.62 |
| Systolic BP (mmHg) | 123.6 ± 12.2 | 116.7 ± 13.3 | 0.04 | 121.7 ± 14.2 | 119.4 ± 10.8 | 0.60 | 0.09 |
| Diastolic BP (mmHg) | 74.3 ± 7.0 | 72.1 ± 8.2 | 0.02 | 73.9 ± 9.3 | 77.0 ± 9.8 | 0.33 | 0.11 |
| Physical Activity (min/day) | 51 ± 29 | 53 ± 29 | 0.90 | 45 ± 24 | 32 ± 20 | 0.33 | 0.56 |
| Energy (kcal/day) | 2037 ± 621 | 2091 ± 742 | 0.32 | 2146 ± 663 | 2085 ± 530 | 0.82 | 0.92 |
| Carbohydrate (g/day) | 213 ± 50 | 210 ± 91 | 0.76 | 209 ± 69 | 189 ± 36 | 0.80 | 0.82 |
| Protein (g/day) | 124 ± 68 | 118 ± 51 | 0.78 | 124 ± 52 | 123 ± 46 | 0.93 | 0.90 |
| Fat (g/day) | 75 ± 28 | 87 ± 35 | 0.08 | 89 ± 14 | 91 ± 36 | 0.35 | 0.86 |
| PLA | GLU | PLA vs. GLU | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p | d | Pre | Post | p | d | p * | % | d | 95% CI | |
| Bristol Stool | 3.1 ± 0.8 | 4.0 ± 1.4 | 0.10 | 0.79 | 3.3 ± 0.8 | 3.6 ± 0.8 | 0.10 | 0.38 | 0.20 | −19.9 | −0.61 | (−1.83, 0.74) |
| GSRS1 | 1.3 ± 0.6 | 1.5 ± 0.7 | 0.08 | 0.31 | 1.2 ± 0.4 | 1.5 ± 0.7 | 0.18 | 0.53 | 1.00 | 31.3 | 0.69 | (−0.42, 0.42) |
| GSRS2 | 1.0 ± 0.0 | 1.1 ± 0.3 | 0.32 | 0.47 | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.56 | 0.28 | 1.00 | −0.9 | 0.00 | (−0.30, 0.30) |
| GSRS3 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.00 | 0.00 | 1.0 ± 0.0 | 1.2 ± 0.4 | 0.16 | 0.71 | 0.16 | 20.0 | 1.00 | (−0.09, 0.45) |
| GSRS4 | 1.6 ± 0.7 | 1.2 ± 0.4 | 0.05 | 0.70 | 1.8 ± 1.0 | 1.2 ± 0.4 | 0.08 | 0.79 | 0.45 | −11.3 | −0.41 | (−1.07, 0.53) |
| GSRS5 | 1.5 ± 1.2 | 1.3 ± 0.5 | 0.71 | 0.22 | 1.3 ± 0.9 | 1.6 ± 1.2 | 0.32 | 0.28 | 0.33 | 35.4 | 0.48 | (−0.46, 1.37) |
| GSRS6 | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.32 | 0.28 | 1.5 ± 0.7 | 2.2 ± 1.3 | 0.10 | 0.67 | 0.16 | 37.6 | 0.77 | (−0.32, 1.41) |
| GSRS7 | 1.7 ± 1.3 | 2.4 ± 1.3 | 0.03 | 0.54 | 1.9 ± 1.2 | 2.1 ± 1.2 | 0.32 | 0.17 | 0.03 | −30.7 | −0.40 | (−0.81, −0.10) |
| GSRS8 | 1.0 ± 0.0 | 1.1 ± 0.3 | 0.32 | 0.47 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.0 | 0.00 | 0.56 | −10 | −0.31 | (−0.45, 0.27) |
| GSRS9 | 2.1 ± 1.1 | 2.6 ± 1.5 | 0.25 | 0.38 | 1.8 ± 0.8 | 2.5 ± 1.2 | 0.08 | 0.69 | 0.87 | 8.8 | 0.08 | (−1.51, 1.69) |
| GSRS10 | 1.1 ± 0.3 | 1.4 ± 0.5 | 0.18 | 0.73 | 1.8 ± 1.4 | 1.1 ± 0.3 | 0.10 | 0.69 | 0.10 | −64.9 | −1.29 | (−2.20, 0.20) |
| GSRS11 | 1.0 ± 0.0 | 1.2 ± 0.4 | 0.16 | 0.71 | 1.0 ± 0.0 | 1.7 ± 1.6 | 0.11 | 0.62 | 0.29 | 55.0 | 0.68 | (−0.55, 1.64) |
| GSRS12 | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| GSRS13 | 1.3 ± 0.7 | 1.1 ± 0.3 | 0.41 | 0.37 | 1.6 ± 1.0 | 1.1 ± 0.3 | 0.10 | 0.68 | 0.08 | −15.5 | −0.43 | (−0.59, 0.04) |
| GSRS14 | 1.0 ± 0.0 | 1.1 ± 0.3 | 0.32 | 0.47 | 1.3 ± 0.9 | 1.6 ± 0.9 | 0.08 | 0.33 | 0.16 | 13.0 | 0.29 | (−0.09, 0.45) |
| GSRS15 | 1.4 ± 0.7 | 1.2 ± 0.4 | 0.48 | 0.35 | 1.7 ± 1.1 | 1.1 ± 0.3 | 0.07 | 0.74 | 0.20 | −23.8 | −0.67 | (−1.21, 0.30) |
| Diarrhea | 2.0 ± 0.0 | 2.3 ± 0.7 | 0.18 | 0.61 | 2.3 ± 0.9 | 3.3 ± 1.6 | 0.02 | 0.77 | 0.13 | 30.6 | 0.76 | (−0.36, 1.81) |
| Ingestion | 5.9 ± 2.1 | 7.3 ± 2.8 | 0.09 | 0.57 | 6.5 ± 2.5 | 7.9 ± 3.0 | 0.07 | 0.51 | 0.88 | −0.4 | 0.04 | (−2.52, 2.70) |
| Constipation | 3.7 ± 1.3 | 3.6 ± 0.8 | 0.93 | 0.09 | 5.1 ± 3.5 | 3.3 ± 0.9 | 0.07 | 0.70 | 0.06 | −33.3 | −0.89 | (−3.70, 0.24) |
| Abdom Pain | 4.3 ± 1.9 | 4.0 ± 1.2 | 0.55 | 0.19 | 4.3 ± 1.7 | 4.2 ± 1.7 | 0.67 | 0.06 | 0.86 | 4.2 | 0.11 | (−1.05, 1.41) |
| Reflux | 2.0 ± 0.0 | 2.1 ± 0.3 | 0.32 | 0.47 | 2.1 ± 0.3 | 2.4 ± 0.7 | 0.18 | 0.56 | 0.16 | 8.4 | 0.45 | (−0.09, 0.45) |
| PLA vs. GLU | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PLA | GLU | p‡ | % | d | 95% CI | ||||
| Formate | Pre | 244 ± 116 | d = 0.09 | 298 ± 166 | d = 0.43 | 0.25 | 30.7 | 0.49 | (−270, 81) |
| Post | 255 ± 135 | p * = 0.56 | 403 ± 299 | p * = 0.20 | |||||
| Acetate | Pre | 55,604 ± 17,431 | d = 0.19 | 40,675 ± 12,876 | d = 0.25 | 0.90 | 0.5 | −0.05 | (−14,026, 15,644) |
| Post | 59,417 ± 22,185 | p * = 0.70 | 43,680 ± 11,351 | p * = 0.46 | |||||
| Propionate | Pre | 15,419 ± 8193 | d = 0.18 | 12,596 ± 4145 | d = 0.01 | 0.48 | −10.0 | −0.22 | (−3303, 6411) |
| Post | 17,040 ± 9419 | p * = 0.86 | 12,663 ± 5766 | p * = 0.96 | |||||
| Butyrate | Pre | 15,244 ± 9517 | d = 0.08 | 11,370 ± 5781 | d = 0.35 | 0.51 | 14.3 | 0.19 | (−6234, 3366) |
| Post | 15,978 ± 7809 | p * = 0.87 | 13,538 ± 6558 | p * = 0.14 | |||||
| Iso-butyrate | Pre | 1787 ± 984 | d = 0.45 | 2097 ± 887 | d = 0.12 | 0.11 | −38.6 | −0.59 | (−193, 1612) |
| Post | 2356 ± 1474 | p * = 0.52 | 1956 ± 1334 | p * = 0.73 | |||||
| Isovalerate | Pre | 1542 ± 967 | d = 0.37 | 1885 ± 886 | d = 0.16 | 0.09 | −18.9 | −0.33 | (−365, 1377) |
| Post | 1956 ± 1226 | p * = 0.68 | 1712 ± 1304 | p * = 0.67 | |||||
| Valerate | Pre | 2674 ± 1362 | d = 0.24 | 2691 ± 1314 | d = 0.08 | 0.22 | −36.0 | −0.53 | (−110, 1283) |
| Post | 3063 ± 1833 | p * = 0.80 | 2574 ± 1519 | p * = 0.78 | |||||
| Total SCFA | Pre | 92,514 ± 35,299 | d = 0.20 | 71,612 ± 22,891 | d = 0.20 | 0.81 | −1.3 | −0.08 | (−21,884, 27,156) |
| Post | 100,064 ± 41,479 | p * = 0.80 | 76,527 ± 25,492 | p * = 0.49 | |||||
| PLA vs. GLU | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PLA | GLU | p‡ | % | d | 95% CI | ||||
| Alanine | Pre | 2503 ± 1098 | d = 0.31 | 2667 ± 1134 | d = 0.46 | 0.24 | −33.4 | −0.72 | (−688, 2415) |
| Post | 2928 ± 1636 | p * = 0.62 | 2228 ± 721 | p * = 0.16 | |||||
| Glutamate | Pre | 7857 ± 2856 | d = 0.20 | 8882 ± 3831 | d = 0.78 | 0.01 | −30.7 | −0.90 | (801, 4504) |
| Post | 8400 ± 2641 | p * = 0.68 | 6773 ± 2223 | p * = 0.04 | |||||
| Glycine | Pre | 1082 ± 438 | d = 0.27 | 1207 ± 514 | d = 0.53 | 0.22 | −34.5 | −0.76 | (−293, 1091) |
| Post | 1238 ± 684 | p * = 0.63 | 965 ± 399 | p * = 0.09 | |||||
| Isoleucine | Pre | 904 ± 402 | d = 0.28 | 1029 ± 462 | d = 0.59 | 0.17 | −40.5 | −0.84 | (−202, 995) |
| Post | 1049 ± 603 | p * = 0.50 | 777 ± 388 | p * = 0.05 | |||||
| Leucine | Pre | 1303 ± 611 | d = 0.10 | 1422 ± 622 | d = 0.58 | 0.23 | −28.4 | −0.64 | (−307, 1101) |
| Post | 1372 ± 737 | p * = 0.68 | 1094 ± 496 | p * = 0.05 | |||||
| Lysine | Pre | 1658 ± 748 | d = 0.22 | 1834 ± 815 | d = 0.55 | 0.07 | −32.1 | −0.73 | (−56, 1194) |
| Post | 1847 ± 943 | p * = 0.59 | 1454 ± 554 | p * = 0.11 | |||||
| Methionine | Pre | 374 ± 367 | d = 0.08 | 424 ± 290 | d = 0.28 | 0.55 | −23.7 | −0.18 | (−263, 458) |
| Post | 399 ± 292 | p * = 0.84 | 352 ± 210 | p * = 0.15 | |||||
| Phenylalanine | Pre | 625 ± 311 | d = 0.04 | 629 ± 280 | d = 0.34 | 0.52 | −15.6 | −0.34 | (−237, 434) |
| Post | 638 ± 331 | p * = 0.70 | 544 ± 208 | p * = 0.26 | |||||
| Proline | Pre | 709 ± 304 | d = 0.02 | 687 ± 280 | d = 0.11 | 0.85 | −5.7 | −0.12 | (−413, 493) |
| Post | 716 ± 383 | p * = 0.99 | 654 ± 326 | p * = 0.63 | |||||
| Threonine | Pre | 768 ± 311 | d = 0.22 | 831 ± 355 | d = 0.56 | 0.16 | −31.8 | −0.73 | (−127, 641) |
| Post | 853 ± 455 | p * = 0.53 | 659 ± 252 | p * = 0.14 | |||||
| Tyrosine | Pre | 789 ± 468 | d = 0.21 | 874 ± 394 | d = 0.49 | 0.16 | −30.5 | −0.66 | (−128, 640) |
| Post | 879 ± 387 | p * = 0.60 | 707 ± 273 | p * = 0.14 | |||||
| Valine | Pre | 1180 ± 507 | d = 0.30 | 1351 ± 610 | d = 0.65 | 0.15 | −42.4 | −0.90 | (−239, 1329) |
| Post | 1376 ± 791 | p * = 0.46 | 1003 ± 451 | p * = 0.03 | |||||
| Total BCAAs | Pre | 3386 ± 1502 | d = 0.22 | 3802 ± 1674 | d = 0.62 | 0.18 | −36.5 | −0.80 | (−3413, 735) |
| Post | 3796 ± 2123 | p * = 0.54 | 2873 ± 1312 | p * = 0.04 | |||||
| Total AA | Pre | 19,752 ± 7608 | d = 0.24 | 21,838 ± 8104 | d = 0.68 | 0.06 | −31.0 | −0.89 | (−246, 13,390) |
| Post | 21,694 ± 8345 | p * = 0.59 | 17,208 ± 5190 | p * = 0.02 | |||||
| PLA vs. GLU | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PLA | p * | GLU | p * | p‡ | % | d | 95% CI | ||
| Amino Acid Breakdown Products | |||||||||
| Cadaverine | Pre | 157 ± 76 | d = 0.58 | 170 ± 52 | d = 0.09 | 0.23 | −29.1 | −0.73 | (−35, 128) |
| Post | 198 ± 66 | p * = 0.30 | 165 ± 56 | p * = 0.80 | |||||
| Putrescine | Pre | 90 ± 84 | d = 0.17 | 92 ± 59 | d = 0.38 | 0.88 | 5.0 | 0.06 | (−79, 69) |
| Post | 108 ± 120 | p * = 0.69 | 115 ± 61 | p * = 0.20 | |||||
| p-Cresol | Pre | 464 ± 298 | d = 0.25 | 597 ± 330 | d = 0.10 | 0.29 | −27.7 | −0.34 | (−144, 421) |
| Post | 557 ± 421 | p * = 0.98 | 551 ± 537 | p * = 0.72 | |||||
| Urocanate | Pre | 103 ± 29 | d = 0.46 | 104 ± 57 | d = 0.36 | 0.17 | −38.7 | −0.81 | (−21, 100) |
| Post | 124 ± 58 | p * = 0.21 | 85 ± 48 | p * = 0.30 | |||||
| Nucleotide Breakdown Products | |||||||||
| Hypoxanthine | Pre | 392 ± 224 | d = 0.13 | 414 ± 190 | d = 0.53 | 0.22 | −27.1 | −0.59 | (−83, 317) |
| Post | 420 ± 222 | p * = 0.73 | 325 ± 143 | p * = 0.06 | |||||
| Ribose | Pre | 1110 ± 552 | d = 0.74 | 1428 ± 760 | d = 0.15 | 0.01 | −37.4 | −0.77 | (158, 714) |
| Post | 1450 ± 339 | p * = 0.07 | 1332 ± 533 | p * = 0.65 | |||||
| Uracil | Pre | 866 ± 425 | d = 0.17 | 922 ± 352 | d = 0.74 | 0.04 | −34.7 | −0.81 | (26, 604) |
| Post | 943 ± 469 | p * = 0.65 | 684 ± 287 | p * = 0.02 | |||||
| Microbially Produced Fermentation Products | |||||||||
| Ethanol | Pre | 1293 ± 2442 | d = 0.45 | 169 ± 98 | d = 0.69 | 0.33 | 123.7 | 0.71 | (−2940, 1108) |
| Post | 481 ± 784 | p * = 0.43 | 272 ± 187 | p * = 0.04 | |||||
| Fumarate | Pre | 175 ± 75 | d = 0.41 | 225 ± 151 | d = 0.05 | 0.40 | −29.4 | −0.43 | (−84, 190) |
| Post | 221 ± 139 | p * = 0.19 | 218 ± 115 | p * = 0.84 | |||||
| Lactate | Pre | 38 ± 20 | d = 0.50 | 60 ± 49 | d = 0.40 | 0.56 | 107.7 | 0.40 | (−394, 229) |
| Post | 68 ± 82 | p * = 0.47 | 172 ± 396 | p * = 0.43 | |||||
| Methionine-sulfoxide | Pre | 241 ± 166 | d = 0.19 | 258 ± 191 | d = 0.33 | 0.29 | 37.4 | −0.51 | (−95, 282) |
| Post | 278 ± 222 | p * = 0.42 | 202 ± 149 | p * = 0.39 | |||||
| Phenylacetate | Pre | 888 ± 577 | d = 0.28 | 1095 ± 511 | d = 0.27 | 0.22 | −30.6 | −0.4 | (−210, 791) |
| Post | 1082 ± 772 | p * = 0.78 | 999 ± 786 | p * = 0.70 | |||||
| Succinate | Pre | 924 ± 642 | d = 0.15 | 635 ± 289 | d = 0.34 | 0.94 | 8.0 | 0.03 | (−497, 462) |
| Post | 1029 ± 712 | p * = 0.99 | 758 ± 430 | p * = 0.48 | |||||
| Likely Host Derived | |||||||||
| 3-Hydroxybutyrate | Pre | 74 ± 37 | d = 0.35 | 89 ± 62 | d = 0.26 | 0.98 | 4.3 | 0.02 | (−60, 58) |
| Post | 60 ± 42 | p * = 0.53 | 76 ± 35 | p * = 0.41 | |||||
| N-Acetylglucosamine | Pre | 260 ± 216 | d = 0.12 | 249 ± 147 | d = 0.41 | 0.56 | −34.9 | −0.48 | (−248, 425) |
| Post | 286 ± 207 | p * = 0.81 | 187 ± 152 | p * = 0.16 | |||||
| Likely Diet Derived | |||||||||
| Glucosamine | Pre | n.d. | n.d. | ||||||
| Post | n.d. | n.d. | |||||||
| Glucose | Pre | 3650 ± 3069 | d = 0.16 | 2229 ± 2054 | d = 0.45 | 0.38 | 61.4 | 0.59 | (−5329, 2252) |
| Post | 3217 ± 2414 | p * = 0.90 | 3334 ± 2753 | p * = 0.23 | |||||
| Glycerol | Pre | 6013 ± 2267 | d = 0.22 | 7458 ± 7347 | d = 0.18 | 0.55 | −33.0 | −0.40 | (−5965, 10,342) |
| Post | 7141 ± 7055 | p * = 0.62 | 6397 ± 3575 | p * = 0.47 | |||||
| Nicotinate | Pre | 371 ± 153 | d = 0.31 | 327 ± 162 | d = 0.41 | 0.76 | −6.6 | −0.13 | (−105, 139) |
| Post | 332 ± 95 | p * = 0.27 | 271 ± 106 | p * = 0.23 | |||||
| Xylose | Pre | 594 ± 317 | d = 0.23 | 441 ± 420 | d = 0.54 | 0.23 | 70.8 | 0.82 | (−912, 250) |
| Post | 525 ± 271 | p * = 0.83 | 702 ± 545 | p * = 0.31 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.M.; Finnegan, P.; Stecker, R.A.; Lee, H.; Ratliff, K.M.; Jäger, R.; Purpura, M.; Slupsky, C.M.; Marco, M.L.; Wissent, C.J.; et al. Impact of Glucosamine Supplementation on Gut Health. Nutrients 2021, 13, 2180. https://doi.org/10.3390/nu13072180
Moon JM, Finnegan P, Stecker RA, Lee H, Ratliff KM, Jäger R, Purpura M, Slupsky CM, Marco ML, Wissent CJ, et al. Impact of Glucosamine Supplementation on Gut Health. Nutrients. 2021; 13(7):2180. https://doi.org/10.3390/nu13072180
Chicago/Turabian StyleMoon, Jessica M., Peter Finnegan, Richard A. Stecker, Hanna Lee, Kayla M. Ratliff, Ralf Jäger, Martin Purpura, Carolyn M. Slupsky, Maria L. Marco, Craig J. Wissent, and et al. 2021. "Impact of Glucosamine Supplementation on Gut Health" Nutrients 13, no. 7: 2180. https://doi.org/10.3390/nu13072180
APA StyleMoon, J. M., Finnegan, P., Stecker, R. A., Lee, H., Ratliff, K. M., Jäger, R., Purpura, M., Slupsky, C. M., Marco, M. L., Wissent, C. J., Theodosakis, J., & Kerksick, C. M. (2021). Impact of Glucosamine Supplementation on Gut Health. Nutrients, 13(7), 2180. https://doi.org/10.3390/nu13072180







