Abstract
Chronic Mg2+ deficiency is the underlying cause of a broad range of health dysfunctions. As 25% of body Mg2+ is located in the skeletal muscle, Mg2+ transport and homeostasis systems (MgTHs) in the muscle are critical for whole-body Mg2+ homeostasis. In the present study, we assessed whether Mg2+ deficiency alters muscle fiber characteristics and major pathways regulating muscle physiology. C57BL/6J mice received either a control, mildly, or severely Mg2+-deficient diet (0.1%; 0.01%; and 0.003% Mg2+ wt/wt, respectively) for 14 days. Mg2+ deficiency slightly decreased body weight gain and muscle Mg2+ concentrations but was not associated with detectable variations in gastrocnemius muscle weight, fiber morphometry, and capillarization. Nonetheless, muscles exhibited decreased expression of several MgTHs (MagT1, CNNM2, CNNM4, and TRPM6). Moreover, TaqMan low-density array (TLDA) analyses further revealed that, before the emergence of major muscle dysfunctions, even a mild Mg2+ deficiency was sufficient to alter the expression of genes critical for muscle physiology, including energy metabolism, muscle regeneration, proteostasis, mitochondrial dynamics, and excitation–contraction coupling.
1. Introduction
Magnesium (Mg2+) intake is suboptimal in the population of Western countries, which results in an increased risk of latent Mg2+ deficiency with Western diet behavior [1]. In addition, there is an increased risk of low Mg2+ status in the elderly and after several current pharmacological treatments, such as proton pump inhibitors, thiazides, cetuximab, cisplatin, and some antibiotics [2,3]. In hospitalized patients, hypomagnesemia is a frequent finding often associated with other electrolyte disorders [4]. Clinical manifestations, depending on the severity and chronicity of deficiency, include a large variety of symptoms, e.g., neuromuscular symptoms (hyperexcitability, tetany, cramps, fasciculation, tremor, spasms, weakness), fatigue, tachycardia, anorexia, apathy, and behavioral alterations. In comparison to severe acute Mg2+ deficiency, the diagnosis of chronic latent Mg2+ deficiency is difficult, because magnesemia is often within reference intervals and results in nonspecific clinical symptoms [5,6]. However, it is well recognized that chronic Mg2+ deficiency contributes to a broad range of metabolic, cardiovascular, immune, and neurological disorders [7].
Mg2+ is the second (after K+) most abundant intracellular cation and Mg2+ is critical for a number of biological processes. Mg2+ is a natural Ca2+ antagonist, the activator of more than 200 enzymes, and the direct cofactor of over 600 enzymes [7]. In addition, intracellular Mg2+ is buffered by many biological molecules, including proteins, RNAs, DNA, and ATP. ATP is mainly bound to Mg2+, and MgATP2- is the active species in enzyme binding and energy production. Therefore, the intracellular concentration of Mg2+ must be tightly regulated. This is achieved through the activity of Mg2+ permeable channels and transporters. The last few years have seen rapid progress in the identification and characterization of Mg2+ transport and homeostasis systems (MgTHs), including transient receptor potential cation channel subfamily M member 6 (TRPM6) and 7 (TRPM7), magnesium transporter 1 (MagT1), magnesium transporter MRS2, solute carrier family 41 member 1 (Slc41a1) and 3 (Slc41a3), cyclin, CBS domain divalent metal cation transport mediator 1 (CNNM1) and 4 (CNNM4) [7,8,9]. Although the precise function of MgTHs is still under investigation, current knowledge suggests that cellular Mg2+ homeostasis is regulated by the combined action of several ubiquitous Mg2+ transporters.
Besides its role in energy production, in the skeletal muscle Mg2+ controls contraction by acting as a Ca2+ antagonist on Ca2+-permeable channels and Ca2+-binding proteins. Accordingly, Mg2+ deficiency decreases muscle strength [10]. Moreover, aging, frequently associated with low Mg2+ status, is characterized by the gradual decline of muscle mass and performance. About 25% of body Mg2+ is located in the skeletal muscle, which indicates that the expression of MgTHs is relevant to whole-body Mg2+ homeostasis. Gene and/or protein expression of ubiquitous MgTHs have been demonstrated in the skeletal muscle (http://www.proteinatlas.org/ accessed on 10 April 2021), but their specific functions in this tissue have not been elucidated. Moreover, to our knowledge, few studies on the regulation of MgTHs in skeletal muscle under pathophysiological conditions, including Mg2+ status, have been published [11].
We were interested in unveiling the early events occurring in the skeletal muscle in response to a low Mg2+-containing diet. The principal aim of this study was to individuate whether and how a short-term Mg2+-deficient diet modulates muscle fiber characteristics and cellular pathways critical for muscle physiology.
2. Materials and Methods
2.1. Animals
The present study (APAFIS#14025-201803121538803) was approved by the Ethics Committee C2EA-02 and was conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. All animals were maintained in a temperature-controlled room (22 ± 1 °C) with a 12:12 h light:dark cycle and handled according to the recommendations of the Institutional Ethics Committee. Two-month-old male C57BL/6J mice were housed for one week in a standard environment with a control diet (0.1% Mg2+ wt/wt). The mice were then randomly divided into three groups, and over the following two weeks, each group (n = 12) was fed one of the three following diets: control diet (0.1% Mg2+ wt/wt), mildly Mg2+-deficient diet (0.01% Mg2+ wt/wt), or severely Mg2+-deficient diet (0.003% Mg2+ wt/wt). The Ca2+ content of the diets was 0.4% (wt/wt). All diets were prepared in our laboratory. Distilled water and food were available ad libitum. Quantitative magnetic resonance of live mice was carried out to estimate body fat and lean mass using an EcoMRI-100 analyzer (Echo Medical Systems LLC, Houston, TX, USA). At the end of the experiment, the animals were sacrificed, blood was collected from the heart in heparin-containing tubes, and gastrocnemius muscles were excised. Plasma was obtained by centrifugation (10 min, 3500 rpm, 4 °C) and frozen for later analysis. Muscles were weighed and (i) maintained in RNAlater (Qiagen, Courtaboeuf, France) overnight at 4 °C before extracting RNA, (ii) frozen in isopentane cooled on liquid N2 and stored at −80 °C for histology, or (iii) snap-frozen in liquid N2 for muscle Mg2+ measurements.
2.2. Mg2+ Analysis
Plasma magnesium was quantified using a Magnesium Calgamite kit according to the manufacturer’s instructions (Biolabo, Maizy, France). Erythrocytes were washed 3 times with a saline solution, hemolyzed in water, and centrifuged. Muscle samples were mineralized in 65% HNO3 for 48 h, and then 1.5 mL of deionized H2O and 0.8 mL of 18 mol/L NaOH were added. Magnesium analyses were performed on a chemistry analyzer (Indiko Plus, Thermo Fisher Scientific, Vantaa, Finland).
2.3. Fiber Morphometry and Capillary Network
Serial cross sections (10 µm thick) were obtained using a cryostat (Microm, Francheville, France) at −25 °C. Myofiber morphometry and capillarization were assessed on cross sections after labeling with anti-laminin-α1 (Sigma, Saint-Quentin-Fallavier, France) and anti-CD31 (M0823 from Dako, Glostrup, Denmark), respectively, and capturing images by a BX-51 microscope (Olympus, Rungis, France) according to [12,13]. On average, 710 ± 48 fibers were analyzed per subject. Fiber cross-sectional area (CSA) and perimeter were determined for each fiber, using the image processing software Visilog-6.9 (Noesis, Gif-sur-Yvette, France) as previously described [12]. A shape factor (perimeter2/4π CSA) was calculated, with a value of 1.0 indicating a circle and >1.0 an increasingly elongated ellipse. A mean of 229 ± 13 capillaries was analyzed per subject. Capillary density (CD) was expressed as the number of capillaries counted per square mm. Capillary-to-fiber ratio (C/F) was calculated as the ratio between the number of capillaries and the number of fibers present in the same area [13].
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
Independent RNA isolations were carried out for each gastrocnemius muscle sample. Total RNAs were extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen) following the manufacturer’s conditions. RNA concentrations were measured using a NanoDrop ND-1000 (LabTech, Ringmer, UK), and RNA quality was verified by 1% agarose gel electrophoresis. One μg total RNA was used as a template for single-strand cDNA synthesis using High-Capacity cDNA RT Kit (Applied Biosystems, Foster City, CA, USA) in a total volume of 20 µL containing 1 X RT buffer, 4 mM dNTP mix, 1 X random primers, 50 U reverse transcriptase and 20 U RNase inhibitor. The primer sequence is reported in Table 1. The reverse transcription reactions were run as follows: 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 s. PCR was carried out in a final volume of 20 µL containing 10 µL Power SYBR Green PCR Master Mix (Applied Biosystems), 0.4 µL of each primer at 10 pmol/µL, and 2 µL of the cDNA solution. qRT-PCR amplification was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad, Marnes-la-Coquette, France) with the following thermal cycler conditions: 15 min at 95 °C, followed by 45 cycles of 15 s at 95 °C, and 1 min at 60 °C. Raw data were analyzed using CFX Maestro (Bio-Rad) and compared by the ΔΔCt method. Results are expressed relative to the housekeeping gene (Actb) transcript quantity.

Table 1.
Sequence of primers used for qRT-PCR.
2.5. TaqMan Low-Density Array (TLDA)
A total of 500 ng (10 µL) cDNA of each sample was combined with 95 µL of nuclease-free water and 105 µL 2X TaqMan™ Fast Advanced Master Mix (Applied Biosystems) for the quantitative real-time PCR (qPCR) measurements. This mixture was divided equally over two sample-loading ports of the TLDA. The arrays were centrifuged once (1 min, 1300 rpm at room temperature) to equally distribute the sample over the wells. Subsequently, the card was sealed to prevent exchange between wells. qPCR amplification was performed using an Applied Biosystems 7900HT system with the following thermal cycler conditions: 2 min at 50 °C and 10 min at 94.5 °C, followed by 40 cycles of 30 s at 97 °C and 30 s at 59.7 °C. Raw data were analyzed using Sequence Detection System (SDS) Software v2.4 (Applied Biosystems). The expression of β-actin (Actb), β-glucuronidase (Gusb), and hypoxanthine phosphoribosyltransferase (Hprt) were used as controls. The genes analyzed are reported in Table 2.

Table 2.
Gene analyzed.
2.6. Western Blot
Gastrocnemius muscles were mechanically shredded in a Potter homogenizer with lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate) containing protease inhibitors. Total proteins were quantified using the Bradford reagent (Sigma-Aldrich, St. Louis, MO, USA). Equal amounts of proteins were separated by SDS–PAGE on 4–20% Mini-PROTEAN TGX Stain-free Gels (Bio-Rad, Hercules, CA, USA) and transferred to nitrocellulose membranes by using Trans-Blot® TurboTM Transfer Pack (Bio-Rad). After blocking with bovine serum albumin (BSA), Western blot analysis was performed using primary antibodies against Myog, Opa1 (BD Biosciences, St. Diego, CA, USA), Gapdh, and Mfn2 (Santa-Cruz Biotechnology, Dallas, TX, USA). The filters were washed and incubated with secondary antibodies conjugated to horseradish peroxidase (Amersham Pharmacia Biotech Italia, Cologno Monzese, Italy) were used. The immunoreactive proteins were detected with ClarityTM Western ECL substrate (Bio-Rad) and images were captured with a ChemiDoc MP Imaging System (Bio-Rad). The nitrocellulose sheets were used as control loading. Densitometry of the bands was performed with the software ImageLab (Bio-Rad). The Western blots shown are representative and the densitometric analysis was performed on three independent experiments.
2.7. Statistical Analysis
Data are presented as means ±SE. To determine whether or not data sets were normally distributed, the Shapiro–Wilk and Kolmogorov–Smirnov normality tests were performed. When data were normally distributed, statistical comparisons between groups were performed applying either Student’s t test or one-way ANOVA, followed by a Tukey’s post hoc test, as appropriate. Mann–Whitney U tests were performed when data in at least one group were not normally distributed. Correction for multiple testing was performed with R according to [14], and TLDA significance was set at q-value < 0.05. Univariate linear Pearson’s regression was carried out to investigate relationships between MgTHs mRNA levels and body weight gain. Statistical analyses were performed using XLSTAT (Addinsoft, Paris, France), and significance was set at P < 0.05.
3. Results
3.1. Mg2+-Deficient Diet Reduces Muscle Mg2+ Concentrations but Does Not Affect Fiber Characteristics
To investigate the modulation of the expression of Mg2+ transport and homeostasis systems (MgTHs) in response to Mg2+ status, we used a model of C57BL/6J mice receiving a mildly or severely Mg2+-deficient diet and compared it to mice under an Mg2+-sufficient diet [15,16]. After 14 days of diet, mice fed a mildly or severely Mg2+-deficient diet exhibited a 26% and 75% reduction in plasma Mg2+ concentration, respectively (Figure 1a). However, no significant change in erythrocyte Mg2+ concentration was detected (Figure 1b). In parallel, moderate and severe Mg2+ deficiencies resulted in a 4.8% and 5.4% decrease in intramuscular Mg2+ concentrations, respectively, compared to the control Mg2+-sufficient diet (Figure 1c).
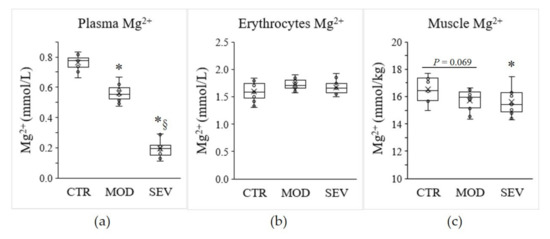
Figure 1.
Mg2+ status in mice. Mg2+ concentrations were measured in (a) plasma, (b) erythrocytes, and (c) gastrocnemius muscle of mice fed either a control (CTR), a mildly (MOD), or a severely (SEV) Mg2+-deficient diet. Data (N = 12 per group) are presented as box-and-whisker plots (centerline, median; box limits, first and third quartiles; whiskers, 1.5 x interquartile range; points, outliers; x in the box, mean). * Significant difference (P < 0.05) from the CTR group. § Significant difference (P < 0.05) from the MOD group.
Moderate and severe Mg2+ deficiencies were associated with a significant decline in body weight gain (Figure 2a), and magnetic resonance imaging (EchoMRI) indicated modest trends for whole-body fat and lean mass reductions (Figure 2b,c). Nonetheless, gastrocnemius muscle weight did not differ between Mg2+-deficient and Mg2+-sufficient diets (Figure 3a). Semiquantitative histology further indicated that Mg2+ deficiency was not associated with a detectable variation in muscle fiber cross-sectional area, shape, capillary density (CD), or capillary-to-fiber ratio (C/F) (Figure 3b–d).
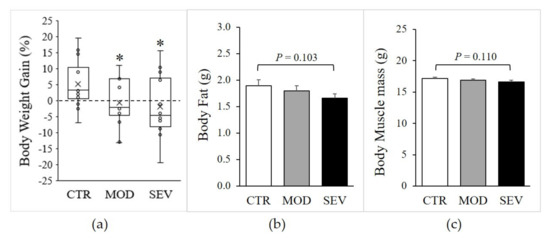
Figure 2.
Body composition of mice fed Mg2+-deficient diet. Mice were fed either a control (CTR), a mildly (MOD), or a severely (SEV) Mg2+-deficient diet: (a) body weight, data (N = 12) presented as box-and-whisker plots, (b) body fat, and (c) body muscle mass were determined by EcoMRI; results are means + SE (N = 12). * Significant difference (P < 0.05) from the CTR group.
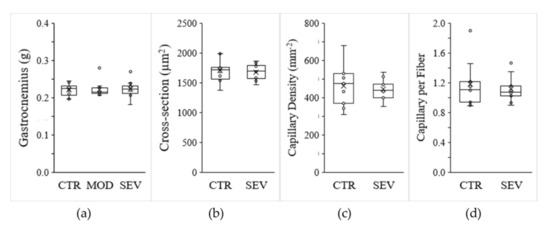
Figure 3.
Mg2+-deficient diet and muscle characteristics. Mice were fed either a control (CTR), a mildly (MOD), or a severely (SEV) Mg2+-deficient diet: (a) gastrocnemius muscle weight; (b) muscle fiber cross-sectional area; (c) muscle capillary density; (d) number of capillaries per fiber. Data (N = 12 per group) are presented as box-and-whisker plots.
3.2. Mg2+-Deficient Diet Alters Expression of Muscle MgTHs
Although no evidence emerged in terms of atrophy or altered morphology or capillarization of muscle fibers, modest declines in muscular Mg2+ concentrations might nonetheless modulate specific mechanisms to maintain cellular Mg2+ homeostasis. We assessed whether Mg2+ deficiency could be associated with differential expression of MgTHs. qRT-PCR analyses performed on gastrocnemius muscle revealed significant declines in MagT1, CNNM2, CNNM4, and TRPM6 mRNAs in mice under Mg2+-deficient diets, compared to Mg2+-sufficient diets (Figure 4a). MagT1 is a controversial protein initially isolated as a critical mediator of Mg2+ homeostasis in eukaryotes [17] and then as an integral part of the N-linked glycosylation complex [18]. As MagT1 mutations associate with hypomagnesemia, we included MagT1 in the list of MgTHs. The mRNA levels of the other MgTHs, i.e., CNNM1, CNNM3, MRS2, Slc41a1, Slc41a2, and Slc41a3, were not altered by Mg2+ deficiency.
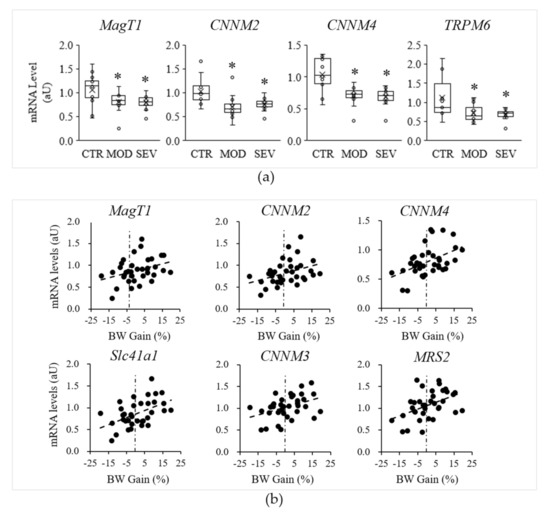
Figure 4.
Mg2+-deficient diet and muscle Mg2+ transport and homeostatic systems (MgTHs) mRNA levels. Mice were fed either a control (CTR), a mildly (MOD), or a severely (SEV) Mg2+-deficient diet: (a) gastrocnemius MgTHs mRNA levels; data (N = 12) are presented as box-and-whisker plots; * indicates significant difference (P < 0.05) from the CTR group; (b) examples of linear Pearson’s regressions between muscle MgTHs mRNA levels and body weight (BW) gain.
Interestingly, regression analyses performed with all mice indicated positive correlation between body weight gain and several MgTHs mRNAs, i.e., MagT1 (r = 0.35, P = 0.038), CNNM2 (r = 0.38, P = 0.022), CNNM3 (r = 0.40, P = 0.015), CNNM4 (r = 0.48, P = 0.003), MRS2 (r = 0.45, P = 0.006), Slc41a1 (r = 0.50, P = 0.002) (Figure 4b).
3.3. Mild Mg2+ Deficiency Alters the Expression of Genes Important for Muscle Energy Metabolism and Regeneration
As Mg2+ is the activator or cofactor of a number of enzymes, modest declines in intramuscular Mg2+ concentrations might modulate biological processes that are critical for muscle physiology. Initially, to assess muscle stress, we evaluated the expression of Trim72, Chac1, and Ddit3. Trim72 codes for a protein specifically located in the sarcolemma and involved in membrane repair [19]. Chac1 encodes a protein acting downstream of ATF4 implicated in muscle atrophy [20], and Ddit3 encodes a member of the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors and inhibits myogenesis [21]. We found that severely and mildly Mg2+-deficient diets did not modulate Trim72 (Figure 5). Moreover, severe Mg2+ deficiency, but not mild Mg2+ deficiency, upregulated the stress genes Chac1 and Ddit3 (Figure 5).
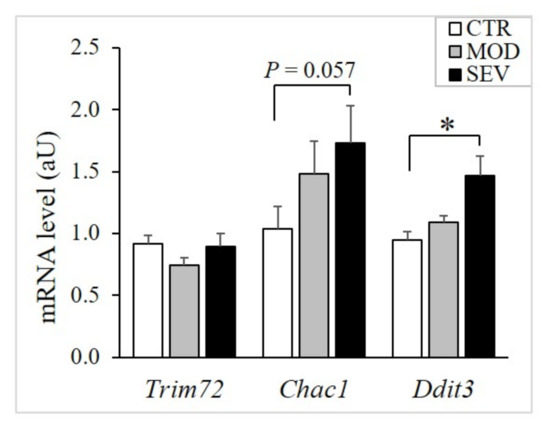
Figure 5.
Muscle mRNA levels of stress genes in mice fed Mg2+-deficient diet. TaqMan low-density array (TLDA) was used to measure mRNA levels in gastrocnemius muscle of mice fed either a control (CTR; white bars), a mildly (MOD; grey bars), or a severely Mg2+-deficient diet (SEV; black bars). * indicates significant difference (P < 0.05) from the CTR group.
To reduce the potential interferences due to the activation of the stress response and to mimic chronic latent deficiency conditions, further investigations were focused on mild Mg2+ deficiency. Moreover, TaqMan low-density array (TLDA) analyses revealed that mild Mg2+ deficiency was sufficient to rapidly alter the expression of genes important for lipid and carbohydrate metabolism (Figure 6a). These included Slc2a4 and Slc6a8, coding for Glut4 and creatine transporter (CT)-1, respectively, Gapdh, Cs (citrate synthase), Plin2, a marker of intramyocellular lipid droplets, and the transcription factors Creb1, Srebf1, and Srebf2. Mild Mg2+ deficiency also rapidly altered the expression of genes involved in muscle regeneration (Figure 6b), i.e., Myog (myogenin), Mef2c (myocyte enhancer factor 2C), Mstn (myostatin), and its receptors (Acvr2a and Acvr2b).
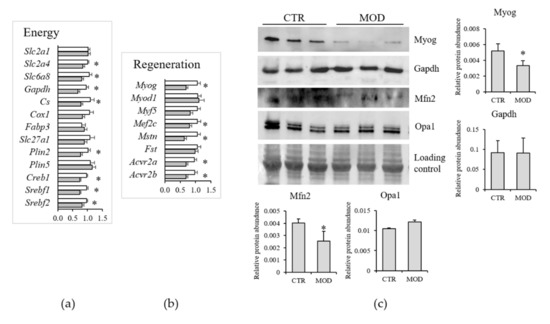
Figure 6.
Muscle mRNA levels of genes involved in energy metabolism (a) and muscle regeneration (b) in mice fed a mildly Mg2+-deficient diet. Results are means ± SE (N = 12). White columns CTR, grey columns mild Mg2+ deficiency. (c) Western blot was performed on 40 µg of lysates using specific antibodies against Myog, Gapdh, Mfn1, and Opa1. A representative western is shown. Densitometry was performed on three different blots. * indicates significant difference (P < 0.05) from the CTR group.
We tested the total amounts of some proteins by Western blot on lysates from gastrocnemius muscles of 12 animals under control or mildly Mg2+-deficient diet. As shown in Figure 6c, we found a significant reduction of Myog and no modulation of Gapdh.
3.4. Mild Mg2+ Deficiency Alters Expression of Genes Important for Muscle Proteostasis
Amongst the transduction pathways implicated in the regulation of muscle atrophy [22,23], mild Mg2+ deficiency was associated with a decreased expression of Fbxo32 (MAFbx), Zeb1, Fbxo31, Atf4, and Eif4ebp1, while the mRNA levels of other major regulators, e.g., Trim63 (MuRF1), Foxo3, Mdm2, Fbxo30 (Musa1), Fbxo21 (Smart), Gadd45a, and Cdkn1a (p21) did not significantly change (Figure 7a).
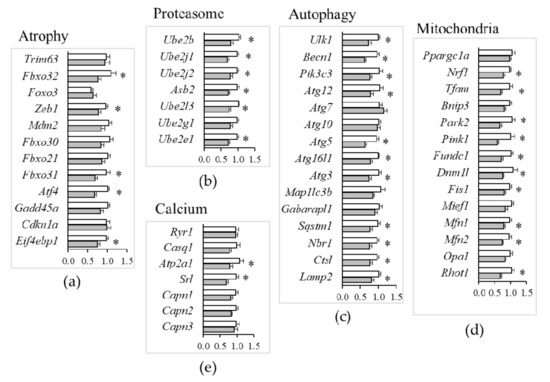
Figure 7.
Muscle gene expression in mice fed a mildly Mg2+-deficient diet. TaqMan low-density array (TLDA) was used to measure mRNA levels of genes critical for muscle physiology of mice fed either a control (white bars) or a mildly (grey bars) Mg2+-deficient diet. Genes involved in muscle atrophy (a), proteostasis (b), autophagy (c), mitochondrial dynamics (d) and calcium homeostasis (e) were analyzed. Results are means ± SE (N = 12). * indicates significant difference (P < 0.05) from the CTR group.
In agreement with the downregulation of major atrogenes by mild Mg2+ deficiency, e.g., Fbxo32 (MAFbx), there was also evidence for altered expression of many genes involved in protein catabolism. This encompassed the downregulation of (i) E2-activating genes (Ube2b, Ube2j1, Ube2j2, Asb2, Ube2l3, Ube2e1) of the ubiquitin–proteasome system (Figure 7b); (ii) genes regulating initiation (Ulk1, Becn1, Pik3c3), elongation (Atg12, Atg5, Atg16l1, Atg3), substrate/cargo recruitment (Sqstm1, Nbr1) and lysosomal proteolysis (Ctsl, Lamp2) in autophagy (Figure 7c). However, Mg2+ deficiency did not affect the expression of Ca2+-dependent proteases (Capn1, Capn2, Capn3 calpains) (Figure 7e).
3.5. Mild Mg2+ Deficiency, Mitochondria, and Ca2+ Homeostasis
Mitochondria play multifaceted roles in essential aspects of skeletal muscle cell physiology [24]. Our TLDA analyses indicated that a mild Mg2+ deficiency is sufficient to downregulate genes regulating mitophagy (Park2, Pink1, Fundc1) (Figure 7d), in addition to reducing the expression of genes implicated in mitogenesis (Nrf1, Tfam), mitochondrial fission (Dnm1l, Fis1), and fusion (Mfn1, Mfn2, Rhot1) (Figure 7d). By Western blot, we confirmed the downregulation of mitofusin (Mfn)2 (Figure 6c). Opa1, instead, was not modulated, both at the RNA and the protein levels (Figure 6c and Figure 7d).
Mg2+ may also modulate Ca2+-permeable channels and Ca2+-binding proteins. Ca2+ handling by the sarcoplasmic reticulum is a key feature in muscle contraction. Action potentials elicit contraction by the release of Ca2+ from the sarcoplasmic reticulum through the ryanodine receptors (Ryr1) that are regulated by calsequestrin-1 (Casq1). For muscle relaxation, Ca2+ is transported back to the sarcoplasmic reticulum by sarco/endoplasmic reticulum Ca2+-ATPases (Serca), regulated by sarcalumenins (Srl) [25,26]. It is noteworthy that our TLDA study emphasized that a mild Mg2+ deficiency did not affect the expression of genes involved in contraction (Ryr1, Casq1), while it downregulated the expression of those implicated in relaxation, Atp2a1 (Serca), and Srl (Figure 7e).
4. Discussion
Appropriate nutrition is indispensable for normal muscle metabolism and function [7]. In humans, cross-sectional associations between low Mg2+ intake and loss of skeletal mass and function were reported in large population cohorts [27,28,29].
To get insight into the role of Mg2+ in muscle health, we developed an experimental model in which mice were fed a moderately or severely Mg2+-deficient diet. Both these dietetic regimens significantly reduced serum Mg2+ levels, a useful biomarker of Mg2+ status [6]. Unexpectedly, we did not detect a reduction of erythrocytes Mg2+ concentration, differently from a previous report demonstrating the concomitant reduction of serum and erythrocyte Mg2 in animals fed the severely deficient diet [15]. We have no explanation at the moment for this discrepancy. Interestingly, we found that some Mg2+ transporters were significantly downregulated in the muscle. This finding explains the reduced amounts of intracellular Mg2+ in the muscle and might also represent an initial, adaptive response aimed at maintaining circulating Mg2+ as close to physiological levels as possible. In this perspective, it is noteworthy that 12–13 week-old Trpm6-deficient adult mice are hypomagnesemic and sarcopenic, events reversible upon supplementation with Mg2+ [30]. Additionally, CNNM2+/− mice were hypomagnesemic, but no data are available on the skeletal muscle yet [31]. In our experimental model, Mg2+ deficiency was associated with a significant decline in body weight gain. Interestingly, regression analysis revealed a positive correlation between body weight gain and the downregulation of MagT1, CNNM2, CNNM3, CNNM4, MRS2, Slc41a1 in the muscle. Indeed, redundancy among members of Mg2+ transporters likely enables functional compensation to maintain sufficient Mg2+ homeostasis resulting in normal body weight.
We found no structural signs of muscle atrophy after 14 days in Mg2+ deficient regimen. This finding might be due to the downregulation of the genes coding for myostatin and its receptors, which activate the ubiquitin–proteasome and autophagy pathways, thus resulting in muscle wasting [32]. Accordingly, TLDA analysis revealed the reduced expression of genes involved in the regulation of the proteasome and autophagy in mice fed a moderately low Mg2+ diet.
By TLDA analysis, we found significant differences in the expression of genes coding for proteins involved in energy metabolisms. Glucose transport into cells is the first step in glucose metabolism. We here describe the downregulation of the gene encoding the glucose transporter Glut4. Interestingly, in the gastrocnemius of type 2 diabetic rats, Glut4 was reduced, and Mg2+ supplementation was sufficient to revert it [33]. The low expression of Glut4 in Mg2+-deficient mice indicates that less glucose might be available for energy production, further impaired by the downregulation of Citrate synthase, involved in the first reaction of the Krebs cycle. Reduced amounts of Plin2, Srebf1, and Srebf2 transcripts might be predictive of altered lipid metabolism. It is noteworthy that Srebf1 and Srebf2 are downregulated in the skeletal muscle of diabetic individuals [34] and that the overexpression of Plin2 ameliorates insulin sensitivity in skeletal muscle [35]. Additionally, Slc6a8, coding for creatine transporter (CT)-1, is reduced in moderately Mg2+-deficient mice. In the skeletal muscle, the creatine system is fundamental for optimal energy utilization, especially at the beginning of exercise and during intense physical activity, because it serves as a first-line energy buffer that maintains ATP levels constant. CT1 is the major route for creatine entry in skeletal muscle cells and has a central role in ensuring high intracellular creatine content [36]. Consistently, CT1-deficient mice feature muscle atrophy, reduced strength, and endurance [37].
TLDA also disclosed the reduced expression of genes involved in mitophagy, fusion, and fission, thus indicating alterations of mitochondrial dynamics that might be accompanied by impaired energy production in Mg2+-deficient mice. Moreover, we found a marked reduction of the total amounts of Mfn2, a GTPase located on the outer mitochondrial membrane which is critical for mitochondrial fusion [38]. It is noteworthy that altered mitochondria have been implicated in sarcopenia in the elderly, in muscle atrophy associated with disuse, in muscular dystrophies, and in insulin resistance [24]. TLDA also shows the downregulation of genes that coordinate the different steps of autophagy in Mg2+-deficient mice. This result is in agreement with data from cultured cells. Indeed, both TRPM7 or MagT1 silencing and Mg2+ deficiency activate autophagy in human mesenchymal stem cells induced osteogenic differentiation [39]. Consistently, high concentrations of extracellular Mg2+ inhibit autophagy in chondrocyte ATDC5 cells [40].
Our study presents some limitations. First of all, the experiments were performed using young growing animals. It will be relevant to extend our studies using animals of different ages and to evaluate metabolic parameters related to glucose metabolism and lipid metabolism to obtain a complete overview of the fundamental function exerted by skeletal muscle. We also highlight that our study was performed on male mice. To identify potential gender-related differences, the same experiments should be performed on females. Another limitation is that only some proteins were evaluated. Further experiments are required to confirm the altered expressions at the protein level and/or their functional activity.
5. Conclusions
Our results emphasize that even a mild Mg2+ deficiency, as found in the Western population, is sufficient to modulate the gene expression of major pathways, mostly related to energy metabolism, proteostasis, autophagy, and mitochondrial dynamics in the skeletal muscle. Consequently, supplementing Mg2+ in Mg2+-deficient individuals might be a simple and costless countermeasure to maintain healthy muscles and metabolic balance.
Author Contributions
Conceptualization, A.M., D.B. (Daniel Béchet), and J.A.M.; data curation, D.B. (Dominique Bayle), A.M., and D.B. (Daniel Béchet); formal analysis, D.B. (Dominique Bayle), C.C.-G., M.Z., and M.M.-Z.; funding acquisition, S.C. and D.B. (Daniel Béchet); investigation, D.B. (Dominique Bayle) and C.C.-G.; Methodology, D.B. (Dominique Bayle), C.C.-G., M.G., S.C., M.Z., A.P.-S., and D.B. (Daniel Béchet); project administration, A.M., D.B. (Daniel Béchet), and J.A.M.; supervision, A.M., D.B. (Daniel Béchet), and J.A.M.; validation, S.C., A.M., D.B. (Daniel Béchet), and J.A.M.; writing—original draft preparation, D.B. (Daniel Béchet); writing—review and editing, A.M., D.B. (Daniel Béchet), and J.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the French government’s IDEX-ISITE initiative 16-IDEX-0001 (CAP 20-25) and, in part, by Università di Milano (Fondi del Piano di Sviluppo di Ricerca 2020).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee C2EA-02 of INRAE (Protocol Code 14025-201803121538803 of 25 April 2018).
Data Availability Statement
The data presented in this study are openly available in INRA Dataverse at https://data.inrae.fr/dataset.xhtml?persistentId=doi:10.15454/IVBODD (accessed on 24 June 2021).
Acknowledgments
Acknowledgements to the animal facility staff in charge of the experimental protocol and to Séverine Valero for the technical assistance and plasma magnesium analysis. The authors acknowledge support from the University of Milan through the APC initiative.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 2020, 6, e05390. [Google Scholar] [CrossRef] [PubMed]
- Lo Piano, F.; Corsonello, A.; Corica, F. Magnesium and elderly patient: The explored paths and the ones to be explored: A review. Magnes. Res. 2019, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kisters, K.; Gröber, U. Magnesium and thiazide diuretics. Magnes. Res. 2018, 31, 143–145. [Google Scholar] [CrossRef]
- Hansen, B.A.; Bruserud, Ø. Hypomagnesemia in critically ill patients. J. Intensive Care 2018, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, M.J. Update on the assessment of magnesium status. Br. J. Nutr. 2008, 99, S24–S36. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, M.; Hubert, J.; Mazur, A. Methods of assessment of magnesium status in humans: A systematic review. Magnes. Res. 2011, 24, 163–180. [Google Scholar] [CrossRef] [Green Version]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Mascarell, P.; González-Recio, I.; Fernández-Rodríguez, C.; Oyenarte, I.; Müller, D.; Martínez-Chantar, M.L.; Martínez-Cruz, L.A. Current Structural Knowledge on the CNNM Family of Magnesium Transport Mediators. Int. J. Mol. Sci. 2019, 20, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolisek, M.; Sponder, G.; Pilchova, I.; Cibulka, M.; Tatarkova, Z.; Werner, T.; Racay, P. Magnesium Extravaganza: A Critical Compendium of Current Research into Cellular Mg(2+) Transporters Other than TRPM6/7. Rev. Physiol. Biochem. Pharmacol. 2019, 176, 65–105. [Google Scholar] [CrossRef] [PubMed]
- Carvil, P.; Cronin, J. Magnesium and implications on muscle function. Strength Cond. J. 2010, 32, 48–54. [Google Scholar] [CrossRef]
- Coudy-Gandilhon, C.; Gueugneau, M.; Taillandier, D.; Combaret, L.; Polge, C.; Roche, F.; Barthélémy, J.-C.; Féasson, L.; Maier, J.A.; Mazur, A.; et al. Magnesium transport and homeostasis-related gene expression in skeletal muscle of young and old adults: Analysis of the transcriptomic data from the PROOF cohort Study. Magnes. Res. 2019, 32, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Gueugneau, M.; Coudy-Gandilhon, C.; Théron, L.; Meunier, B.; Barboiron, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Picard, B.; et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 566–576. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Meunier, B.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Roche, F.; Féasson, L.; Barthélémy, J.-C.; et al. Lower skeletal muscle capillarization in hypertensive elderly men. Exp. Gerontol. 2016, 76, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondón, L.J.; Groenestege, W.M.T.; Rayssiguier, Y.; Mazur, A. Relationship between low magnesium status and TRPM6 expression in the kidney and large intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R2001–R2007. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Blommaert, E.; Péanne, R.; Cherepanova, N.A.; Rymen, D.; Staels, F.; Jaeken, J.; Race, V.; Keldermans, L.; Souche, E.; Corveleyn, A.; et al. Mutations in MAGT1 lead to a glycosylation disorder with a variable phenotype. Proc. Natl. Acad. Sci. USA 2019, 116, 9865–9870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.; Masumiya, H.; Weisleder, N.; Matsuda, N.; Nishi, M.; Hwang, M.; Ko, J.-K.; Lin, P.; Thornton, A.; Zhao, X.; et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009, 11, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, S.M.; Bullard, S.A.; Basisty, N.; Marcotte, G.R.; Skopec, Z.P.; Dierdorff, J.M.; Al-Zougbi, A.; Tomcheck, K.C.; DeLau, A.D.; Rathmacher, J.A.; et al. Activating transcription factor 4 (ATF4) promotes skeletal muscle atrophy by forming a heterodimer with the transcriptional regulator C/EBPβ. J. Biol. Chem. 2020, 295, 2787–2803. [Google Scholar] [CrossRef] [Green Version]
- AlSudais, H.; Lala-Tabbert, N.; Wiper-Bergeron, N. CCAAT/Enhancer Binding Protein β inhibits myogenic differentiation via ID3. Sci. Rep. 2018, 8, 16613. [Google Scholar] [CrossRef]
- Ebert, S.M.; Al-Zougbi, A.; Bodine, S.C.; Adams, C.M. Skeletal Muscle Atrophy: Discovery of Mechanisms and Potential Therapies. Physiology 2019, 34, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y., II; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Hepple, R.T. Editorial: Mitochondria in Skeletal Muscle Health, Aging and Diseases. Front. Physiol. 2016, 7, 446. [Google Scholar] [CrossRef] [Green Version]
- Leberer, E.; Timms, B.G.; Campbell, K.P.; MacLennan, D.H. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 10118–10124. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Gourbeyre, O.; Chambon, C.; Combaret, L.; Polge, C.; Taillandier, D.; Attaix, D.; Friguet, B.; Maier, A.B.; et al. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genom. 2014, 15, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; de Groot, L.C.P.G.M.; Mijnarends, D.M.; de Vries, J.H.M.; Verlaan, S.; Meijboom, S.; Luiking, Y.C.; Schols, J.M.G.A. Differences in Nutrient Intake and Biochemical Nutrient Status Between Sarcopenic and Nonsarcopenic Older Adults-Results From the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Orsso, C.E.; Tibaes, J.R.B.; Oliveira, C.L.P.; Rubin, D.A.; Field, C.J.; Heymsfield, S.B.; Prado, C.M.; Haqq, A.M. Low muscle mass and strength in pediatrics patients: Why should we care? Clin. Nutr. 2019, 38, 2002–2015. [Google Scholar] [CrossRef]
- Chubanov, V.; Ferioli, S.; Wisnowsky, A.; Simmons, D.G.; Leitzinger, C.; Einer, C.; Jonas, W.; Shymkiv, Y.; Bartsch, H.; Braun, A.; et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife 2016, 5. [Google Scholar] [CrossRef]
- Franken, G.A.C.; Seker, M.; Bos, C.; Siemons, L.A.H.; van der Eerden, B.C.J.; Christ, A.; Hoenderop, J.G.J.; Bindels, R.J.M.; Müller, D.; Breiderhoff, T.; et al. Cyclin M2 (CNNM2) knockout mice show mild hypomagnesaemia and developmental defects. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Morakinyo, A.O.; Samuel, T.A.; Adekunbi, D.A. Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr. Regul. 2018, 52, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewter, C.; Berger, D.; Considine, R.V.; Medina, G.; Rochford, J.; Ciaraldi, T.; Henry, R.; Dohm, L.; Flier, J.S.; O’Rahilly, S.; et al. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes 2002, 51, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Bosma, M.; Hesselink, M.K.C.; Sparks, L.M.; Timmers, S.; Ferraz, M.J.; Mattijssen, F.; van Beurden, D.; Schaart, G.; de Baets, M.H.; Verheyen, F.K.; et al. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 2012, 61, 2679–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brault, J.J.; Abraham, K.A.; Terjung, R.L. Muscle creatine uptake and creatine transporter expression in response to creatine supplementation and depletion. J. Appl. Physiol. 2003, 94, 2173–2180. [Google Scholar] [CrossRef] [Green Version]
- Stockebrand, M.; Sasani, A.; Das, D.; Hornig, S.; Hermans-Borgmeyer, I.; Lake, H.A.; Isbrandt, D.; Lygate, C.A.; Heerschap, A.; Neu, A.; et al. A Mouse Model of Creatine Transporter Deficiency Reveals Impaired Motor Function and Muscle Energy Metabolism. Front. Physiol. 2018, 9, 773. [Google Scholar] [CrossRef]
- Filadi, R.; Pendin, D.; Pizzo, P. Mitofusin 2: From functions to disease. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Romeo, V.; Locatelli, L.; Cazzaniga, A.; Maier, J.A.M. TRPM7 and MagT1 in the osteogenic differentiation of human mesenchymal stem cells in vitro. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Yue, J.; Jin, S.; Gu, S.; Sun, R.; Liang, Q. High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway. J. Cell. Physiol. 2019, 234, 23190–23201. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).