Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/db Mice by Regulating Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Designs
2.3. Fasting Blood Glucose and Oral Glucose Tolerance Test (OGTT)
2.4. Insulin Tolerance Test (ITT)
2.5. Acute Intervention
2.6. Biochemical Analysis
2.7. Histology Staining
2.8. Real-Time Quantitative PCR
2.9. Western Blot Analysis
2.10. Gut Microbiota Analysis
2.11. Statistical Analysis
3. Results
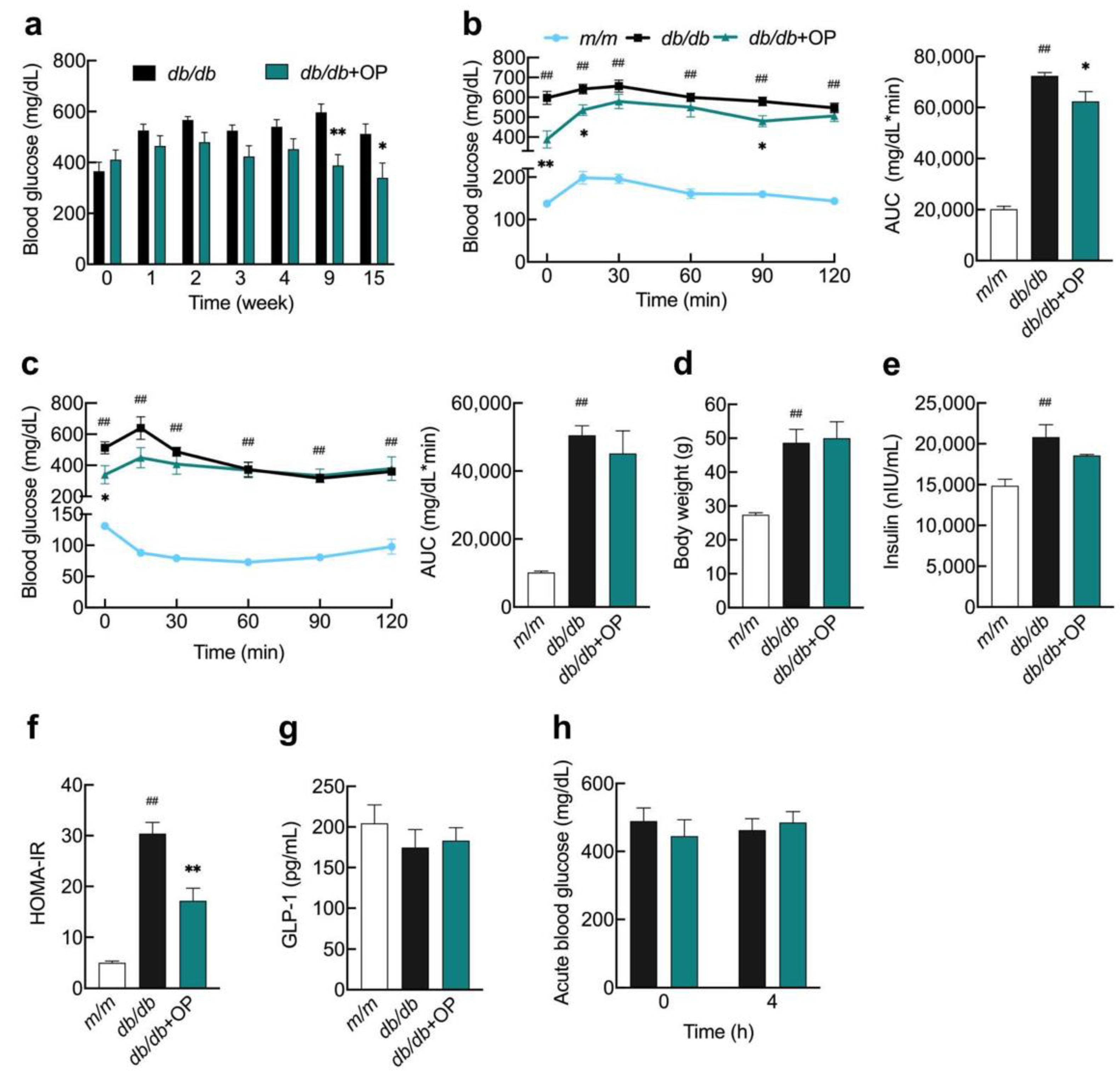
3.1. OP Alleviated Advanced Stage of Type 2 Diabetes in db/db Mice
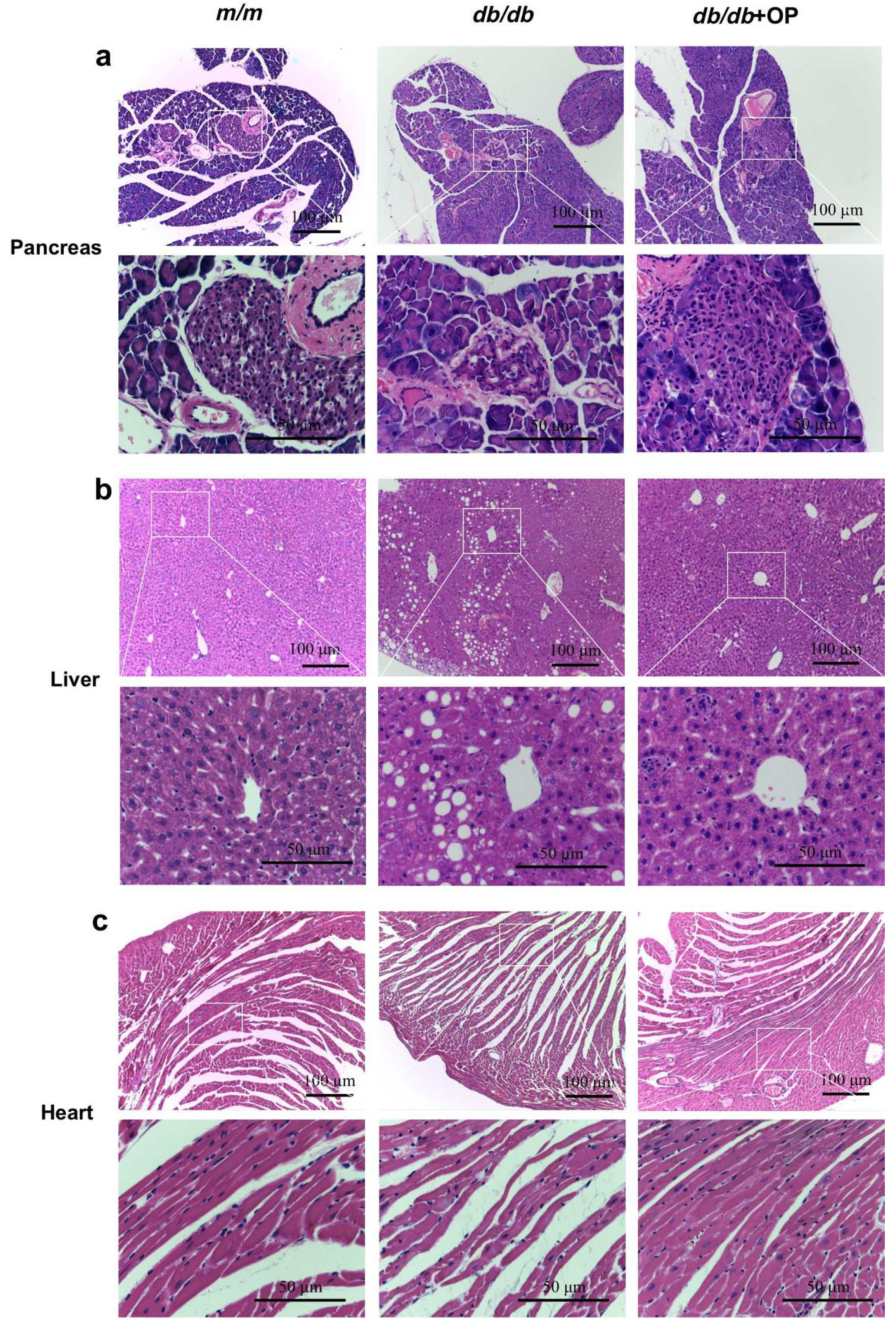
3.2. OP Treatment Restored Histopathological Features of Tissues in db/db Mice
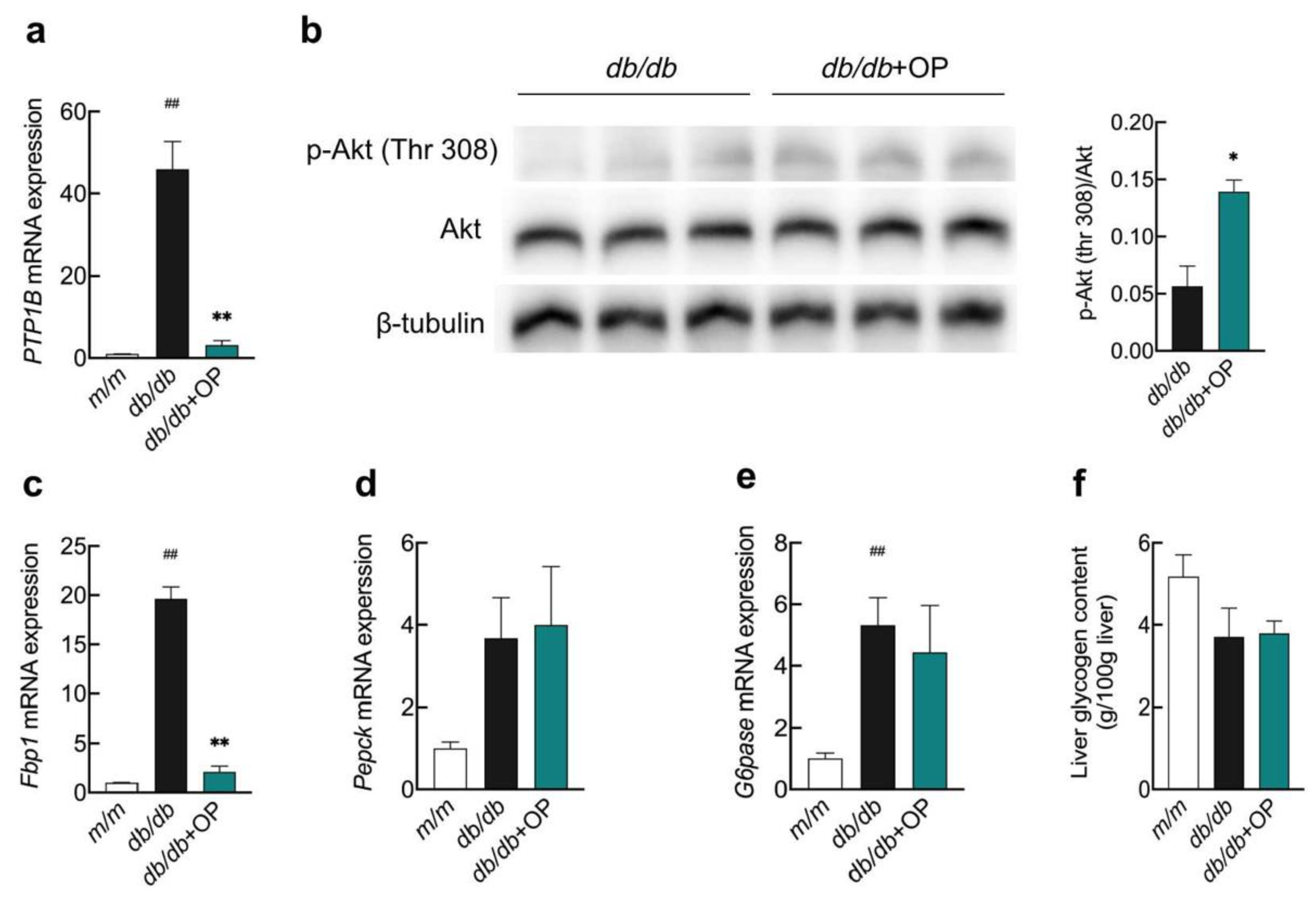
3.3. The Effects of OP on Hepatic Insulin Signaling and Key Enzymes Related to Hepatic Gluconeogenesis in db/db Mice
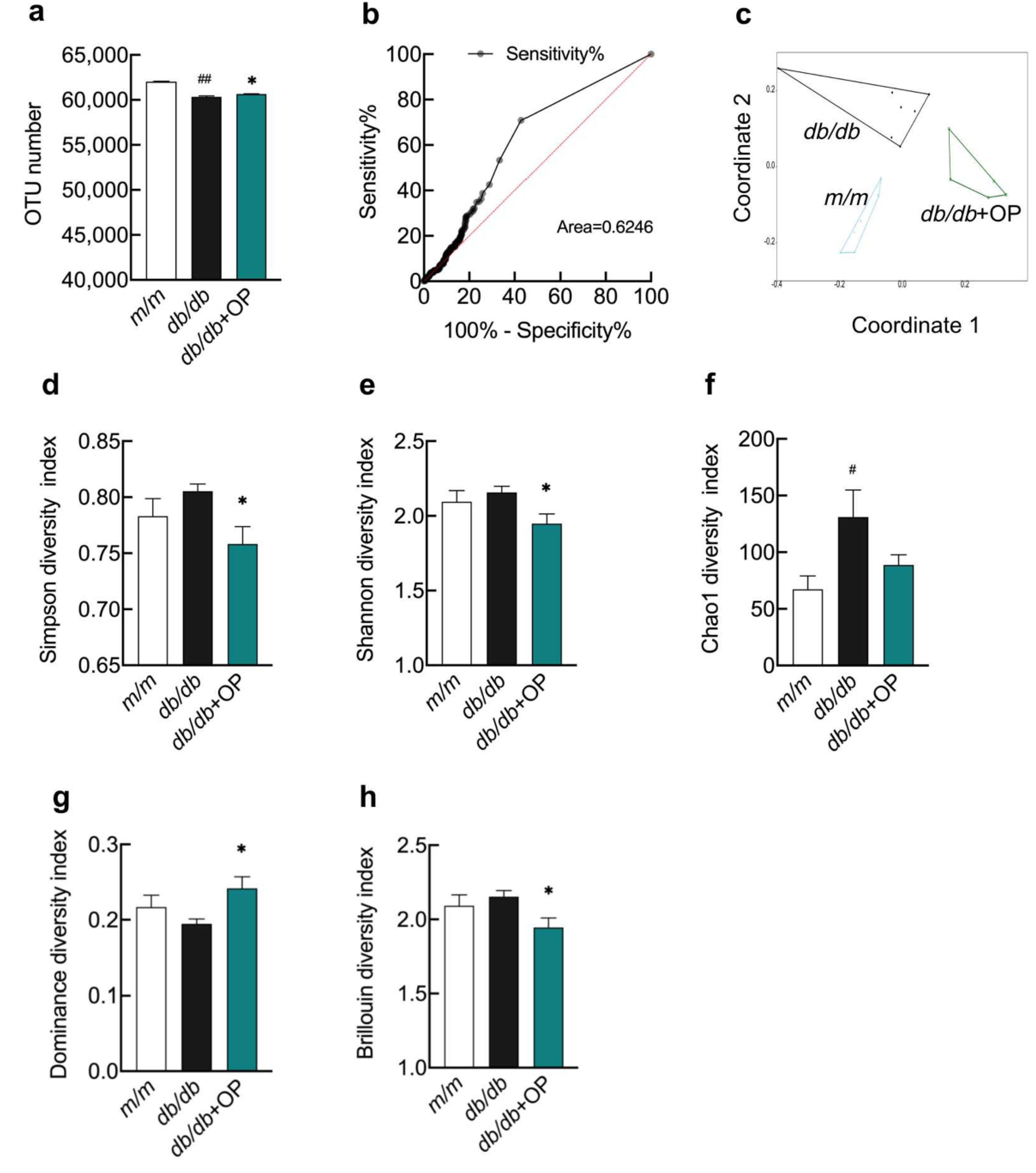
3.4. The Effects of OP Treatment on α-Diversity and β-Diversity of Gut Microbiota in db/db Mice
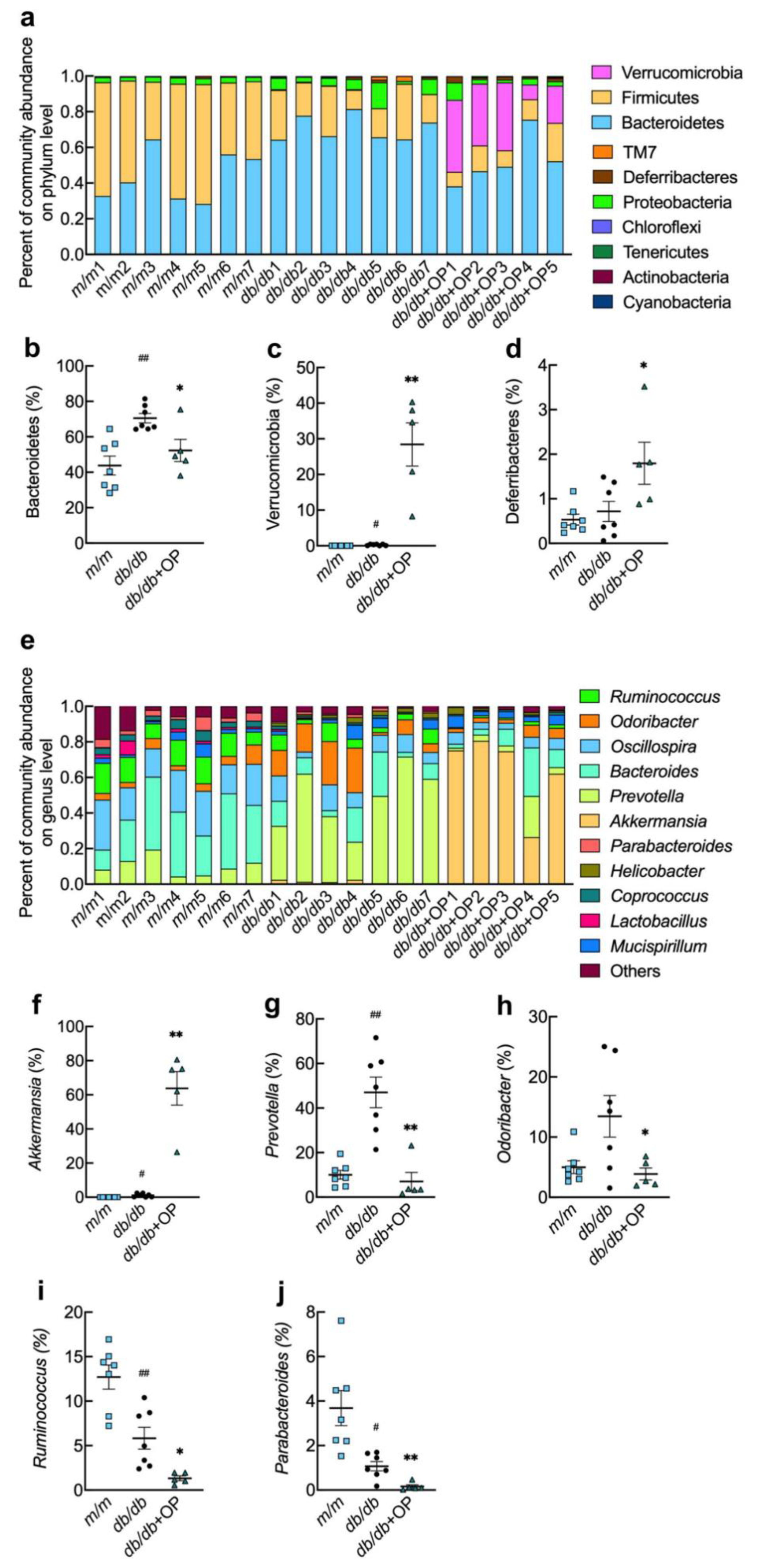
3.5. OP Treatment Changed the Gut Microbiota Composition in db/db Mice
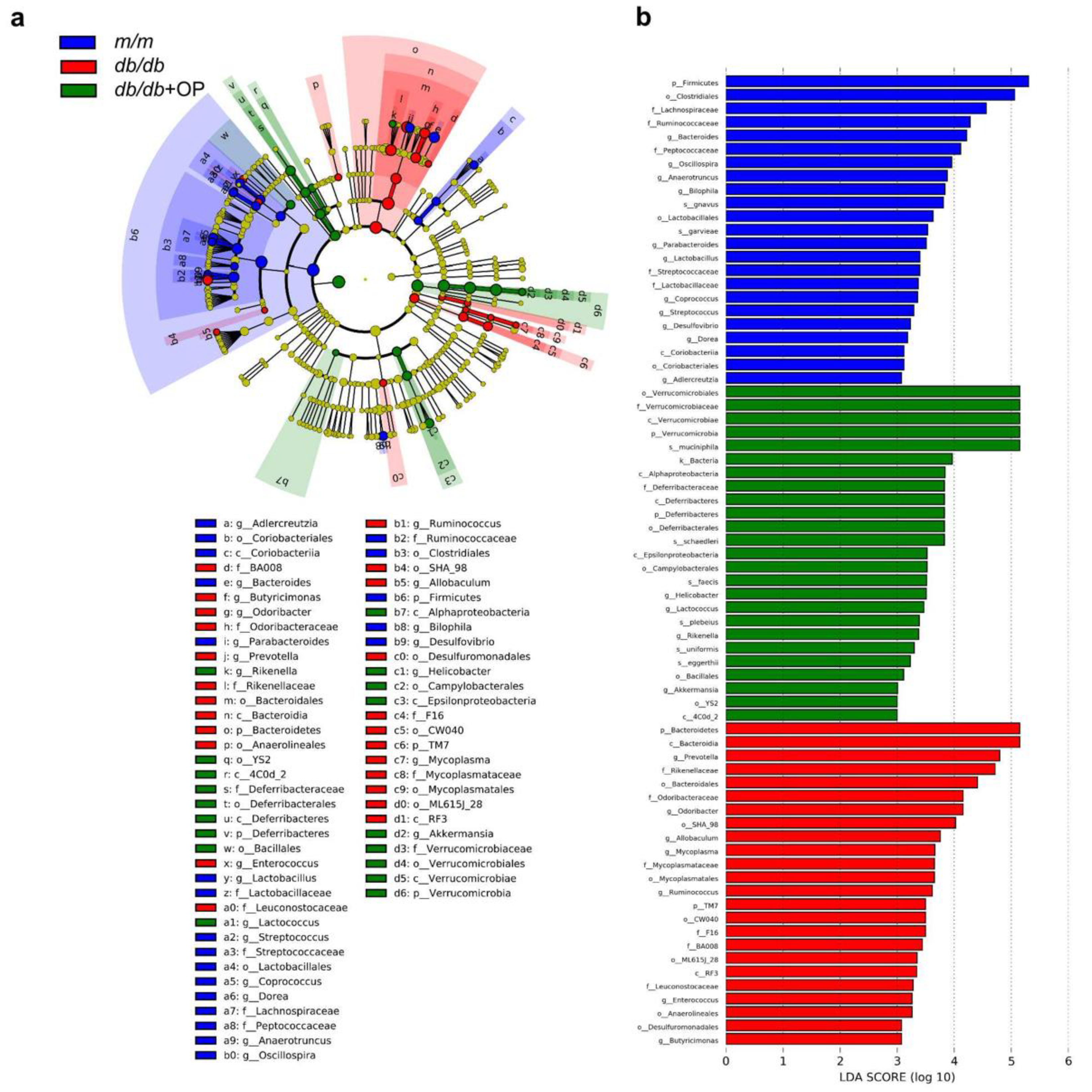
3.6. Key Phylotypes of Gut Microbiota Responding to OP Treatment
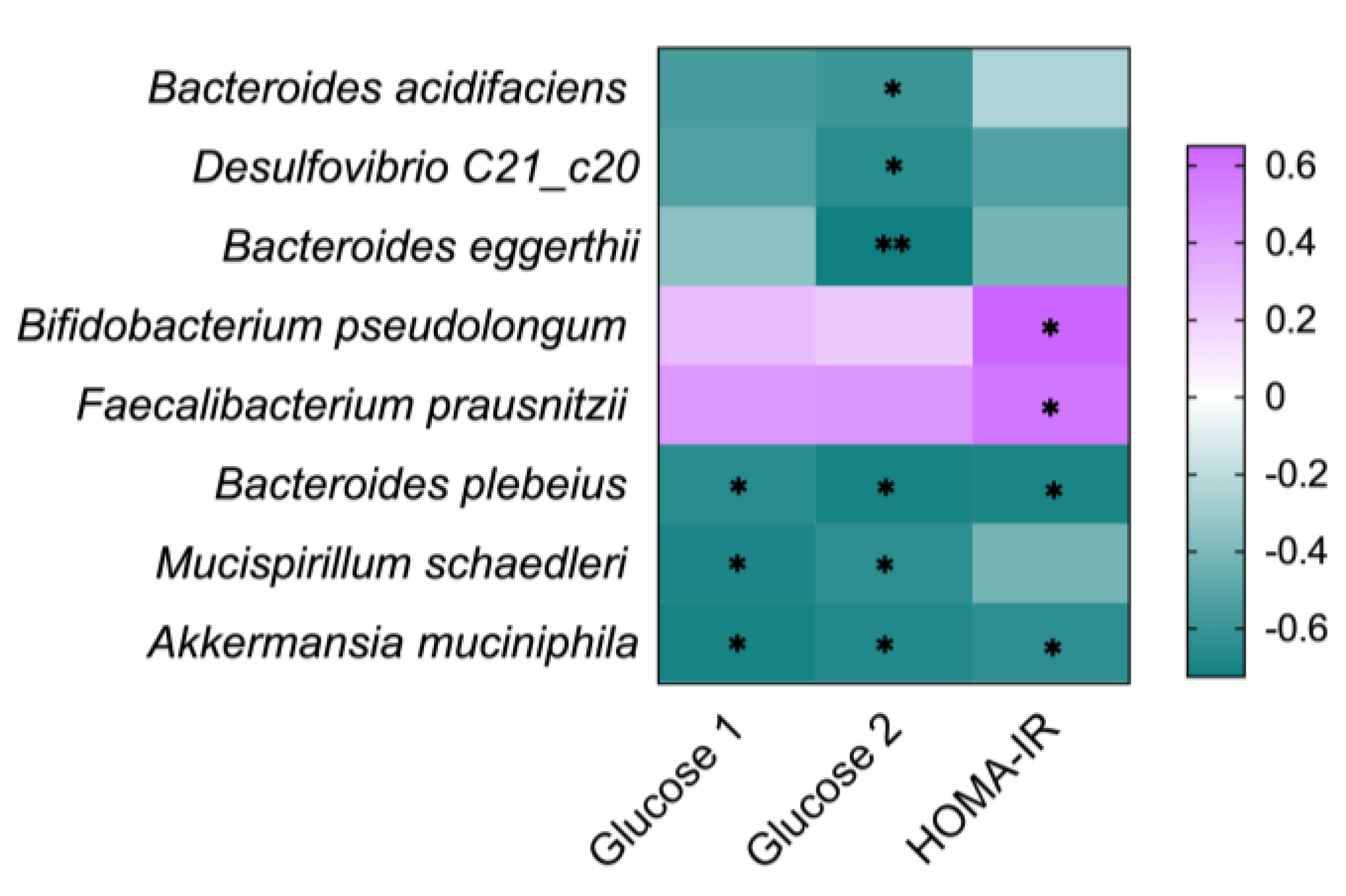
3.7. Correlation between the Gut Microbia and Diabetes-Related Indicators
3.8. Predicted Metabolic Profile of the Gut Microbiota after OP Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Kang, J.; Liu, Q.; Tong, T.; Quan, H. Fighting Diabetes Mellitus: Pharmacological and Non-pharmacological Approaches. Curr. Pharm. Des. 2020, 26, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.; Weir, G.C.; Susan, B.W. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53, S16–S21. [Google Scholar]
- Kimura, T.; Kaneto, H.; Shimoda, M.; Hirukawa, H.; Okauchi, S.; Kohara, K.; Hamamoto, S.; Tawaramoto, K.; Hashiramoto, M.; Kaku, K. Protective effects of pioglitazone and/or liraglutide on pancreatic β-cells in db/db mice: Comparison of their effects between in an early and advanced stage of diabetes. Mol. Cell. Endocrinol. 2015, 400, 78–89. [Google Scholar] [CrossRef]
- Dalbøge, L.S.; Almholt, D.L.C.; Neerup, T.S.R.; Vassiliadis, E.; Vrang, N.; Pedersen, L.; Fosgerau, K.; Jelsing, J. Characterisation of Age-Dependent Beta Cell Dynamics in the Male db/db Mice. PLoS ONE 2013, 8, e82813. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Chen, H.-P.; He, Y.-Y.; Chen, W.-L.; Chen, J.-W.; Gao, L.; Hu, H.-Y.; Wang, J. Effects of Rich-Polyphenols Extract of Dendrobium loddigesii on Anti-Diabetic, Anti-Inflammatory, Anti-Oxidant, and Gut Microbiota Modulation in db/db Mice. Molecules 2018, 23, 3245. [Google Scholar] [CrossRef]
- Li, B.-Y.; Xu, X.-Y.; Gan, R.-Y.; Sun, Q.-C.; Meng, J.-M.; Shang, A.; Mao, Q.-Q. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods 2019, 8, 440. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, K.; Tong, T. Efficacy and Mechanisms of Oleuropein in Mitigating Diabetes and Diabetes Complications. J. Agric. Food Chem. 2021, 69, 6145–6155. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antiox-idant molecule in the treatment of metabolic syndrome. Phyther. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Hou, D.; Sun, W.; Liu, T.H.; Wu, L.L.; Qin, L.L.; Guo, X.; Xu, G.Y.; Zhang, Z.; Hou, Y.; Zhang, L.; et al. The mechanism behind the effects of oleuropein on insulin resistance in the liver in db/db mice. World Sci. Technol. Tradit. Chin. Med. 2016, 18, 1133–1138. [Google Scholar]
- Qadir, N.M.; Ali, K.A.; Qader, S.W. Antidiabetic Effect of Oleuropein from Olea europaea Leaf against Alloxan Induced Type 1 Diabetic in Rats. Braz. Arch. Biol. Technol. 2016, 59, 16150116. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Yasunaga, M.; Shichiri, M.; Ishida, N.; Koike, T.; Matsuo, T.; Abe, H.; Yoshida, Y.; Nakajima, Y. Oleuro-pein-rich diet attenuates hyperglycemia and impaired glucose tolerance in type 2 diabetes model mouse. J. Agric. Food Chem. 2015, 63, 6715–6722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, H.; Wang, A. Oleuropein alleviates gestational diabetes mellitus by activating AMPK signaling. Endocr. Connect. 2021, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Obata, A.; Shimoda, M.; Okauchi, S.; Kanda-Kimura, Y.; Nogami, Y.; Moriuchi, S.; Hirukawa, H.; Kohara, K.; Nakanishi, S.; et al. Protective effects of the SGLT2 inhibitor luseogliflozin on pancreatic β-cells in db/db mice: The earlier and longer, the better. Diabetes Obes. Metab. 2018, 20, 2442–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; He, X.; Sheng, Y.; Yang, C.; Li, H.; Xu, J.; Xu, W.; Huang, K. Caulis Spatholobi Ameliorates Obesity through Activating Brown Adipose Tissue and Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5150. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Inviron. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Petkov, V.; Manolov, P. Pharmacological analysis of the iridoid oleuropein. Arzneimittelforschung 1972, 22, 1476–1486. [Google Scholar]
- Alonso, J. Tratado de Fitofármacos y Nutracéuticos (Treaty of Phytopharmaceuticals and Nutraceuticals); Corpus Libros: Rosario, Argentina, 2004. [Google Scholar]
- Soliman, G.A.; Saeedan, A.S.; Abdel-Rahman, R.F.; Ogaly, H.A.; Abd-Elsalam, R.M.; Abdel-Kader, M.S. Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study. Saudi Pharm. J. 2019, 27, 326–340. [Google Scholar] [CrossRef]
- Clewell, A.E.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Murbach, T.S.; Szakonyiné, I.P. A Comprehensive Toxicological Safety Assessment of an Extract of Olea Europaea, L. Leaves (Bonolive™). Int. J. Toxicol. 2016, 35, 208–221. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Fradkin, J.E.; Roberts, B.T.; Rodgers, G.P. What’s preventing us from preventing type 2 diabetes? N. Engl. J. Med. 2012, 367, 1177–1179. [Google Scholar] [CrossRef]
- Somineni, H.K.; Boivin, G.P.; Elased, K.M. Daily exercise training protects against albuminuria and angiotensin converting enzyme 2 shedding in db/db diabetic mice. J. Endocrinol. 2014, 221, 235–251. [Google Scholar] [CrossRef]
- Al-Azzawie, H.F.; Alhamdani, M.-S.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006, 78, 1371–1377. [Google Scholar] [CrossRef]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Oteo, J.A. Gut microbiota: A key player in health and disease. A review focused on obesity. J. Physiol. Biochem. 2015, 71, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; De Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced lep-tin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.L.; Dong, X.Y.; Sha, L.P.; Lei, H.; Zhang, X.X.; Zhu, L.L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus: Via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.P.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes patho-physiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-X.; Ren, H.; Gao, Y.-F.; Lee, C.-Y.; Li, S.-F.; Zhang, F.; Li, L.; Chen, H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Göker, M.; Gronow, S.; Zeytun, A.; Nolan, M.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Cheng, J.F.; Pitluck, S.; et al. Com-plete genome sequence of Odoribacter splanchnicus type strain (1651/6 T). Stand. Genom. Sci. 2011, 4, 200–209. [Google Scholar] [CrossRef]
- Wang, Y.; Ames, N.P.; Tun, H.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High Molecular Weight Barley β-Glucan Alters Gut Microbiota Toward Reduced Cardiovascular Disease Risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Jing, N.; Zhao, Y.; Yang, X. EGCG regulates fatty acid metabolism of high-fat diet-fed mice in association with enrichment of gut Akkermansia muciniphila. J. Funct. Foods 2020, 75, 104261. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Zhang, Y.; Zheng, X. Dietary anthocyanin-rich extract of açai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice. Nutrients 2021, 86, 111176. [Google Scholar] [CrossRef]
- Régnier, M.; Rastelli, M.; Morissette, A.; Suriano, F.; Le Roy, T.; Pilon, G.; Delzenne, N.M.; Marette, A.; Van Hul, M.; Cani, P.D. Rhu-barb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansia muciniphila in mice. Nutrients 2020, 12, 2932. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Zhang, X.; Wang, Z.; Gao, Z.; Liu, G.; Hu, H.; Zou, L.; Li, X. Insulin Sensitivity-Enhancing Activity of Phlorizin Is Associated with Lipopolysaccharide Decrease and Gut Microbiota Changes in Obese and Type 2 Diabetes (db/db) Mice. J. Agric. Food Chem. 2016, 64, 7502–7511. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Wang, X.; Lei, L.; Liu, M.Z.; Li, R.C.; Sun, S.J.; Liu, S.N.; Huan, Y.; Zhou, T.; Liu, Q.; et al. Berberine combined with stachy-ose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phyther. Res. 2019, 34, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-F.; Su, S.-L.; Guo, J.-M.; Zhu, Y.; Zhao, M.; Duan, J.-A. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J. Funct. Foods 2017, 36, 362–374. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bac-teroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Zheng, J.L.; Li, H.; Zhang, X.J.; Jiang, M.; Luo, C.Q.; Lu, Z.M.; Xu, Z.H.; Shi, J.S. Prebiotic mannan-oligosaccharides augment the hy-poglycemic effects of metformin in correlation with modulating gut microbiota. J. Agric. Food Chem. 2018, 66, 5821–5831. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.; Elliott, B. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Montalibet, J.; Kennedy, B.P. Therapeutic strategies for targeting PTP1B in diabetes. Drug Discov. Today Ther. Strat. 2005, 2, 129–135. [Google Scholar] [CrossRef]
- Zinker, B.A.; Rondinone, C.M.; Trevillyan, J.M.; Gum, R.J.; Clampit, J.E.; Waring, J.F.; Xie, N.; Wilcox, D.; Jacobson, P.; Frost, L.; et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 11357–11362. [Google Scholar] [CrossRef] [PubMed]
- Porcu, C.; Sideri, S.; Martini, M.; Cocomazzi, A.; Galli, A.; Tarantino, G.; Balsano, C. Oleuropein Induces AMPK-Dependent Autophagy in NAFLD Mice, Regardless of the Gender. Int. J. Mol. Sci. 2018, 19, 3948. [Google Scholar] [CrossRef]
- Van Poelje, P.D.; Potter, S.C.; Chandramouli, V.C.; Landau, B.R.; Dang, Q.; Erion, M.D. Inhibition of fructose 1,6-bisphosphatase re-duces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes 2006, 55, 1747–1754. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Chi, T.Z.; Lin, T.S.; Das, S.; Zakaria, Z. Histological changes in the heart and the proximal aorta in expe rimental diabetic rats fed with Piper sarmentsoum. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 396–404. [Google Scholar]
- Cosyns, B.; Droogmans, S.; Weytjens, C.; Lahoutte, T.; Van Camp, G.; Schoors, D.; Franken, P.R. Effect of streptozotocin-induced dia-betes on left ventricular function in adult rats: An in vivo Pinhole Gated SPECT study. Cardiovasc. Diabetol. 2007, 6, 30. [Google Scholar] [CrossRef]
- Jemai, H.; Sayadi, S. Heart Histopathology and Oxidative Features in Diabetic Rats and Protective Effects of Oleuropein. Adv. Biosci. Biotechnol. 2015, 06, 383–389. [Google Scholar] [CrossRef][Green Version]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuro-pein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Lepore, S.M.; Maggisano, V.; Bulotta, S.; Mignogna, C.; Arcidiacono, B.; Procopio, A.; Brunetti, A.; Russo, D.; Celano, M. Oleacein Prevents High Fat Diet-Induced A Diposity and Ameliorates Some Biochemical Parameters of Insulin Sensitivity in Mice. Nutrients 2019, 11, 1829. [Google Scholar] [CrossRef]
- Lombardo, G.E.; Lepore, S.M.; Morittu, V.M.; Arcidiacono, B.; Colica, C.; Procopio, A.; Maggisano, V.; Bulotta, S.; Costa, N.; Mignogna, C.; et al. Effects of Oleacein on High-Fat Diet-Dependent Steatosis, Weight Gain, and Insulin Resistance in Mice. Front. Endocrinol. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed]

| Target Genes | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| Fbp1 | GTGTCAACTGCTTCATGCTG | GAGATACTCATTGATGGCAGGG |
| G6pase | CGACTCGCTATCTCCAAGTGA | GTTGAACCAGTCTCCGACCA |
| PTP1B | CCATCACCTCCTGGAAGAACA | TGCTGGCTTCTCTGGGTAAA |
| Pepck | CCATCACCTCCTGGAAGAACA | ACCCTCAATGGGTACTCCTTCTG |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| m/m | db/db | db/db + OP | |
|---|---|---|---|
| ALT (U/L) | 49.60 ± 4.11 | 190.43 ± 38.27 # | 171.90 ± 36.91 |
| AST (U/L) | 151.80 ± 24.68 | 282.11 ± 60.08 | 209.80 ± 24.68 |
| TC (mmol/L) | 2.41 ± 0.07 | 2.39 ± 0.34 | 3.39 ± 0.39 |
| TG (mmol/L) | 0.90 ± 0.06 | 1.28 ± 0.17 | 0.98 ± 0.09 |
| LDL-C (mmol/L) | 0.28 ± 0.03 | 0.47 ± 0.09 | 0.46 ± 0.08 |
| HDL-C (mmol/L) | 1.94 ± 0.06 | 1.96 ± 0.28 | 2.82 ± 0.32 |
| Tissues | m/m | db/db | db/db + OP |
|---|---|---|---|
| Pancreas (g) | 0.30 ± 0.01 | 0.25 ± 0.02 | 0.28 ± 0.02 |
| Pancreas % | 1.08 ± 0.04 | 0.54 ± 0.04 ## | 0.59 ± 0.09 |
| Liver (g) | 1.36 ± 0.04 | 2.66 ± 0.29 ## | 3.04 ± 0.35 |
| Liver % | 4.98 ± 0.09 | 5.88 ± 0.48 | 6.14 ± 0.37 |
| Epididymal fat (g) | 0.28 ± 0.03 | 2.73 ± 0.24 ## | 1.99 ± 0.36 |
| Epididymal fat % | 1.00 ± 0.08 | 6.00 ± 0.34 ## | 3.81 ± 0.56 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Wang, Y.; Fang, J.; Geng, R.; Li, M.; Zhao, Y.; Kang, S.-G.; Huang, K.; Tong, T. Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/db Mice by Regulating Gut Microbiota. Nutrients 2021, 13, 2131. https://doi.org/10.3390/nu13072131
Zheng S, Wang Y, Fang J, Geng R, Li M, Zhao Y, Kang S-G, Huang K, Tong T. Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/db Mice by Regulating Gut Microbiota. Nutrients. 2021; 13(7):2131. https://doi.org/10.3390/nu13072131
Chicago/Turabian StyleZheng, Shujuan, Yanan Wang, Jingjing Fang, Ruixuan Geng, Mengjie Li, Yuhan Zhao, Seong-Gook Kang, Kunlun Huang, and Tao Tong. 2021. "Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/db Mice by Regulating Gut Microbiota" Nutrients 13, no. 7: 2131. https://doi.org/10.3390/nu13072131
APA StyleZheng, S., Wang, Y., Fang, J., Geng, R., Li, M., Zhao, Y., Kang, S.-G., Huang, K., & Tong, T. (2021). Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/db Mice by Regulating Gut Microbiota. Nutrients, 13(7), 2131. https://doi.org/10.3390/nu13072131







