Abstract
Malnutrition, which commonly occurs in perioperative patients with cancer, leads to decreased muscle mass, hypoalbuminemia, and edema, thereby increasing the patient’s risk of various complications. Thus, the nutritional management of perioperative patients with cancer should be focused on to ensure that surgical treatment is safe and effective, postoperative complications are prevented, and mortality is reduced. Pathophysiological and drug-induced factors in elderly patients with cancer are associated with the risk of developing malnutrition. Pathophysiological factors include the effects of tumors, cachexia, and anorexia of aging. Metabolic changes, such as inflammation, excess catabolism, and anabolic resistance in patients with tumor-induced cancer alter the body’s ability to use essential nutrients. Drug-induced factors include the side effects of anticancer drugs and polypharmacy. Drug–drug, drug–disease, drug–nutrient, and drug–food interactions can significantly affect the patient’s nutritional status. Furthermore, malnutrition may affect pharmacokinetics and pharmacodynamics, potentiate drug effects, and cause side effects. This review outlines polypharmacy and malnutrition, the impact of malnutrition on drug efficacy, drug–nutrient and drug–food interactions, and intervention effects on polypharmacy or cancer cachexia in elderly perioperative patients with cancer.
1. Introduction
Cancers are among the leading causes of morbidity and mortality worldwide, and the number of new cases is expected to rise significantly over the next few decades. At the same time, all types of cancer treatment, such as surgery, radiation therapy, and pharmacological therapies, are improving in sophistication, precision, and in the power to target specific characteristics of individual cancers. All of these treatments, however, are impeded by the frequent development of malnutrition and metabolic derangements in cancer patients, induced by the tumor or by its treatment [1]. It was previously suggested that malnutrition increases the risk of cancer patient mortality and the length of hospital stay [2,3,4,5,6]. Therefore, nutritional management of cancer patients is extremely important.
The relationship between polypharmacy and malnutrition is based on several mechanisms. The long-term use of multiple drugs results in anorexia, generally as a minor or more serious impairment of the digestive tract. Additionally, many drugs have the potential to negatively affect nutritional status by altering the sensory perception of taste, intestinal absorption, and metabolism or inducing the elimination of essential vitamins and minerals [7,8]. Conversely, malnutrition often decreases the bioavailability of drugs and alters their pharmacokinetic and pharmacodynamic properties, which increases the patient’s sensitivity to the drug; even at the usual dose, the drug will have a stronger effect and higher incidence rate of side effects. This gives rise to a vicious circle, wherein polypharmacy, particularly in excess, degrades the nutritional status, and the degraded nutritional status demands increased doses of drugs with the increased occurrence of undesirable side effects [9]. Hence, nutritional status needs to be assessed before prescribing medications.
Elderly patients with cancer often take a large number of medications to prevent or reduce side effects in addition to their multimorbidity. Therefore, they are prone to polypharmacy. As mentioned above, polypharmacy is associated with malnutrition. In elderly perioperative patients with cancer, a multidisciplinary team comprising physicians, pharmacists, nurses, dietitians, and other professionals should be aware of the potential effects of individual drugs and polypharmacy on perioperative nutritional status and seek to reduce negative impacts. Polypharmacy has the potential for adverse clinical outcomes, and it is therefore necessary to synthesize the current evidence to provide a practical direction for future research and clinical practice. This review outlines polypharmacy and malnutrition, the impact of malnutrition on drug efficacy, and drug–nutrient interactions in elderly patients with cancer during the perioperative period.
2. Polypharmacy in Elderly Patients with Cancer
Elderly patients with cancer have a higher risk of requiring polypharmacy than patients of the same age without cancer [10]. Most cancer treatments, such as chemotherapy and supportive care regimens, involve the prescription of multiple medications. Furthermore, drugs with anticancer agents are associated with numerous adverse drug reactions, ranging from mild nausea to myelosuppression, which may prompt polypharmacy [11]. Due to these factors, older adults with cancer are at a high risk of requiring polypharmacy. It is estimated that >50% of older patients with cancer are administered at least five medications and that the drug–drug interactions are associated with impaired physical function [12,13].
There are various definitions of polypharmacy, which makes it challenging to understand the scope and impact of the associated problems. A previous review suggested that there are 24 distinct definitions of polypharmacy in general use [14], including concepts ranging from unnecessary or inappropriate medication use to the use of an excessive number of medications [15]. The most common definition of polypharmacy is receiving ≥5 medications daily; however, due to the wide variance in definitions, the appropriateness of medication usage is described only in a minority of the definitions [16]. In Japan, there is no strict definition of how many drugs constitute polypharmacy. The Ministry of Health, Labor and Welfare’s definition of polypharmacy refers to not only the intake of multiple drugs but also to the fact that polypharmacy is associated with conditions that increase the risk of adverse drug reactions, drug errors, and decreased medication adherence. In some cases, adverse drug events may occur when the patient is only administered two or three drugs, as it is equally important to consider the ingredients of the prescription when managing drug–drug interactions. Thus, in defining polypharmacy, the administration of prescriptions must be optimized with the primary aim of ensuring safety rather than focusing only on a uniform number of drugs or types of drugs.
Polypharmacy can lead to various adverse events. Polypharmacy has been associated with increased falls [17], hospitalization [18], decreased physical and cognitive capability [19], impaired activities of daily living (ADL) [20,21], and mortality [22] in older adults without cancer. Hence, elderly patients with cancer are at a greater risk of medication-related events, as they are usually prescribed an extensive number of medicines, both to treat the disease itself and to provide supportive care in treating the side effects of both the disease and the medications used. Therefore, cancer-related therapy contributes to the prevalence of polypharmacy, which can lead to compromised cancer management plans (i.e., postoperative complications, treatment delays, and/or premature treatment discontinuation) [23]. Additionally, in an oncology setting, polypharmacy with inappropriate medications is likely to contribute to the patient’s worsened condition, frailty syndrome, poor physical function, poor survival, and a higher number of comorbidities [24,25]. As is evident from the above descriptions, polypharmacy in elderly patients with cancer is a crucial factor to consider when treating the health conditions of elderly patients with cancer, as it affects the process and outcomes of cancer treatment.
3. Effects of Polypharmacy and Malnutrition on Elderly Perioperative Patients with Cancer
Drugs are also associated with causes of malnutrition. Factors in drug-induced malnutrition are the side effects of anticancer drugs. The common side effects of cytotoxic chemotherapy include anorexia, nausea, and vomiting, which directly limit food intake. Additionally, cytotoxic anticancer drugs such as oxaliplatin, cisplatin, doxorubicin, 5-fluorouracil, and irinotecan are taken up by muscle cells. These drugs suppress protein synthesis and induce atrophy, oxidative damage, cellular energy depletion, and apoptotic or necrotic cell death [26]. Decreased de novo lipogenesis and increased lipolysis are additional effects that cisplatin and doxorubicin induce in the adipose tissue [26]. Thus, anticancer drugs affect the nutritional status. Additionally, since many drugs are often prescribed to prevent the side effects of anticancer drugs, elderly perioperative patients with cancer are prone to polypharmacy.
Polypharmacy and anticholinergic drugs have also been associated with the risk of developing malnutrition [27,28,29]. The long-term use of multiple drugs results in anorexia, which generally manifests clinically because of a minor or serious impairment of the digestive tract, which may result in lower food intake and affect the patient’s nutritional status. The inverse relationship between medication use and nutritional status has been demonstrated previously, with 50% of those taking ≥10 medications found to be malnourished or at risk of malnourishment [30]. Elderly perioperative patients with cancer commonly have concomitant lifestyle-related diseases such as hypertension, diabetes mellitus, and atherosclerosis. Drugs used to treat these diseases are also known to affect the nutritional status (see Table 1).
Anticholinergic drugs have also been associated with malnutrition. Elderly patients with cancer often take antipsychotic drugs to prevent delirium and improve symptoms of restlessness. Antipsychotic drugs with anticholinergic effects, such as chlorpromazine, haloperidol, and risperidone, block dopaminergic neurons and potentially inhibit the swallowing reflex, which can induce aspiration pneumonia. Additionally, these drugs can cause extrapyramidal disorders, difficulty opening and closing the mouth, and limitations in lingual movement, thus making mastication, the formation of a food bolus, and its passage into the pharynx difficult.
Malnutrition in patients with cancer is based upon multiple factors. As evidenced from the above descriptions, it is difficult to distinguish between drug-induced malnutrition and malnutrition due to disease or other causes. A multidisciplinary team comprising physicians, pharmacists, nurses, dietitians, and other professionals should comprehensively evaluate malnutrition.
4. Effects of Hypoalbuminemia on Drug Efficacy
Elderly patients with cancer may suffer from hypoalbuminemia due to deteriorated swallowing function, loss of dental occlusion, decreased food intake, and the debilitating effects of chronic inflammatory diseases, such as cachexia. When dietary intake is no longer possible and protein and amino acids are not taken into the body as nutrients, the total amount of protein synthesized by the liver reduces, resulting in hypoalbuminemia. If the required amount of energy-producing nutrients is not supplied, amino acids will be used as an energy source and not for body protein synthesis. When protein intake is insufficient, skeletal muscle is broken down and used to sustain life. Hence, when fasting reduces the ability to masticate and swallow, complete loss of the ability to swallow is likely to occur [31]. Additionally, acute inflammation, such as infection or invasion, causes an increase in the serum levels of C-reactive protein. At the same time, albumin synthesis is inhibited, which results in a decrease in the serum levels of albumin. In chronic inflammation, such as cachexia, inflammatory cytokines inhibit albumin synthesis in hepatocytes and promote C-reactive protein production. However, the inhibition of albumin synthesis is usually mild compared with that in acute inflammation.
Drugs sensitive to protein binding affect drug efficacy in hypoalbuminemia. When an administered drug is distributed in the bloodstream, it binds to albumin because of the drug’s pH, electrical charge, steric structure of the molecule, and hydrophilic/hydrophobic properties. Albumin-bound drug molecules are unable to express drug effects. Only free drugs that are not bound to albumin pass through the cell membrane and enter the cell to produce a drug effect. The percentage of drugs that produce a drug effect depends on the type of drug and the amount of albumin and water in the blood. Drug dosage is established by administering the drug to healthy subjects or patients during a clinical trial (dose-finding study). However, there are few data on the appropriate dose for patients with malnutrition and hypoalbuminemia. When albumin in the blood decreases because of undernutrition, the free drugs, which cannot bind to albumin, increase in the blood, resulting in stronger drug effects and more frequent side effects, even at normal doses (Figure 1). Additionally, when the plasma water content is reduced and the blood concentration is increased because of dehydration, the incidence and severity of side effects increase as well. Accordingly, when administering drugs during hypoalbuminemia or dehydration, attention should be given to the changes in the patient’s symptoms.
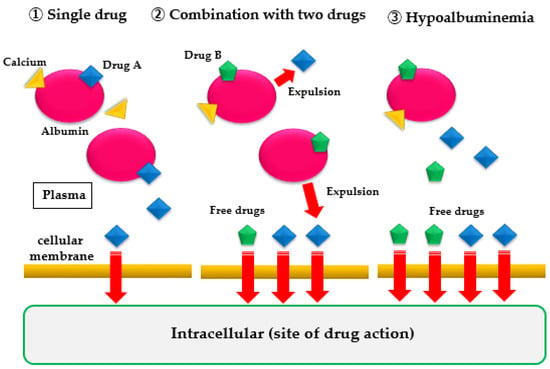
Figure 1.
When a single drug is used alone or in combination with two drugs, or in case of hypoalbuminemia, the amount of free drug that does not bind to plasma albumin increases. Consequently, the amount of drug that passes through the cell membrane increases, and the drug effect is strongly expressed.
Albumin binds to calcium, and the binding is affected by pH and temperature. Approximately 40% of the total blood calcium is bound to plasma proteins, primarily albumin. The remaining 60% includes ionized calcium plus calcium complexed with phosphate and citrate. Total calcium (i.e., protein-bound, complexed, and ionized calcium) is usually determined by clinical laboratory measurement. However, ideally, ionized (or free) calcium should be estimated or measured because it is the physiologically active form of calcium in plasma and because its blood level does not always correlate with total serum calcium.
5. Interaction of Drugs with Nutrients or Diet
5.1. Effects of Drugs on Nutrients
More than 250 drugs have been reported to have adverse effects on the patient’s nutritional status because of drug-induced alterations in taste, intestinal absorption, and metabolism or excretion of essential vitamins and minerals [7,8]. Table 1 shows the effects of major drugs on nutrients.
5.1.1. Antihypertensive Drugs and Zinc
Antihypertensive drugs such as thiazide diuretics, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, and potassium-conserving diuretics decrease zinc levels [7]. Zinc deficiency is a common cause of taste disorders, which can lead to weight loss and malnutrition. Thus, zinc administration may be used to improve or prevent these symptoms. A Cochrane review that examined the improvement of taste perception due to zinc supplementation in patients with idiopathic and zinc-deficient taste disorders found very low-quality evidence that zinc supplementation improves taste perception (relative risk: 1.42, 95% confidence interval: 1.09–1.84; 292 participants, two trials) [32]. Zinc could be useful in the prevention of oral toxicities during irradiation; however, it does not alleviate chemotherapy-induced side effects [33]. Accordingly, several studies have proposed that zinc does not have a positive effect on patient weight or food intake [32].
5.1.2. Acetylcholinesterase Inhibitors
The typical side effects of acetylcholinesterase inhibitors include nausea, vomiting, diarrhea, and anorexia, and each of these symptoms may lead to weight loss. Weight loss is often observed after 3 months of use, but studies have shown that weight loss does not persist over the long-term use of the inhibitors [34]. However, its use in elderly patients with weakness or anorexia should be carefully considered [34].
5.1.3. Proton Pump Inhibitors (PPIs)
Several studies have examined the association between long-term PPI use and the risk of developing vitamin B12 deficiency [35,36]; most, [36] but not all [37], studies reported a 2- to 4-fold increased risk of vitamin B12 deficiency associated with PPI therapy. The complex relationship between PPI use and nutritional status has not been fully elucidated. However, it has been reported that the long-term use of PPIs may improve nutritional status in elderly patients admitted to a long-term care ward, convalescent rehabilitation ward, or community-integrated care ward [35].
Several meta-analyses have reported that PPI use is also associated with the development of hypomagnesemia [38,39]. A dose–response relationship was found between PPI use and the development of hypomagnesemia [38]. Hypomagnesemia increases the risk of developing cardiovascular events [40]. Thus, PPI users should be aware of the risk of developing hypomagnesemia.
PPIs can decrease the absorption of water-insoluble calcium (e.g., calcium carbonate) [41]; however, this effect is not relevant for water-soluble calcium salts [42] or calcium-containing milk or cheese [43]. When calcium supplementation is necessary for patients taking PPIs, calcium supplements that do not require acid for absorption, such as calcium citrate, are recommended. PPI-induced hypochlorhydria can augment osteoclastic activity, thereby decreasing bone density [44,45]. Hence, calcium supplementation is necessary for such patients. Although an association between PPI use and bone fracture is plausible, a causal link has not been established [46].
Gastric acid plays a role in the absorption of nonheme iron, and the use of PPIs has been associated with decreased iron absorption [47,48,49,50,51]. However, few studies have specifically evaluated the potential association between PPIs and iron deficiency [52]. In most cases, the decreased absorption of iron does is not clinically significant [52]. In patients with Zollinger–Ellison syndrome, 6 years of taking PPIs was not associated with decreased total body iron levels or iron deficiency [53]. Conversely, PPI use in patients with hereditary hemochromatosis was associated with a significant reduction in the absorption of nonheme iron over the short term as well as a significant reduction in annual phlebotomy requirements over the long term [48]. Such patients may need a higher dose or a longer duration of supplementation.
5.1.4. Statins
Statins decrease the production of coenzyme Q10 (CoQ10) by inhibiting mitochondrial oxidative phosphorylation and inducing mitochondrial apoptosis [7,54]. Decreased CoQ10 results in decreased adenosine triphosphate production and energy deficiency. Consequently, the number of cellular processes decreases, which may induce frailty and sarcopenia.
Mitochondrial function has been associated with myopathy, which is a side effect of taking statins. Since skeletal muscle is highly energy-consuming and deeply dependent on mitochondrial activity, mitochondrial dysfunction is largely associated with the development of statin-induced myopathy [54]. In a recent meta-analysis examining the effects of CoQ10 on statin-induced myopathy, the concomitant use of CoQ10 significantly improved statin-related muscle symptoms, such as muscle pain, muscle cramps, and muscle fatigue [55]. A previous study demonstrated a reduction in CoQ10 after statin treatment, which may have been associated with statin-induced myopathy [56]. As is evident from the above description, the concomitant use of statin with CoQ10 supplements may be a complementary approach to symptom relief of statin-induced myopathy.
5.1.5. Aspirin
The long-term use of high-dose aspirin has been associated with decreased vitamin C levels. It has been suggested that this leads to gastritis, peptic ulcer disease, nausea, anorexia, and thinning of the gastric mucosa with hypotrophy [7]. At this time, there is no evidence suggesting a decrease in vitamin C levels or the need for vitamin C supplementation in patients taking low-dose aspirin for primary or secondary prevention of cardiovascular disease.
5.1.6. Metformin
Metformin causes vitamin B12 deficiency in a dose- and duration-dependent manner [7]. Vitamin B12 deficiency is associated with serious outcomes, such as anemia and cognitive impairment; thus, patients taking metformin should have their vitamin B12 levels measured regularly and consider supplementation if they are deficient.
Metformin inhibits the breakdown of muscle proteins and affects muscle mass and strength. Metformin activates 5′ adenosine monophosphate-activated protein kinase, suppresses inflammatory responses, and inhibits muscle protein degradation. Studies of sarcopenia have revealed numerous health benefits of 5′ adenosine monophosphate-activated protein kinase activation. First, sarcopenia promotes skeletal muscle protein synthesis and cell proliferation. Second, sarcopenia inhibits apoptosis in skeletal muscle. Third, sarcopenia improves dysfunction by promoting mitochondrial biogenesis. Finally, sarcopenia induces the growth of bone marrow-derived muscle progenitor cells in skeletal muscle [57]. Specifically, in a double-blind randomized controlled trial, metformin was found to improve the walking speed of older adults with no diabetes, which indicates that metformin has a positive effect on lower limb muscle strength [58]. In a prospective cohort study of elderly women with diabetes, women who took metformin experienced reduced loss of walking speed compared to the controls [59]. In another cohort study, metformin reduced the age-related loss of lean body mass in elderly male diabetics [60]. These findings suggest that metformin has a positive effect on muscle mass and muscle strength.
5.1.7. Sodium Glucose Transporter 2 Inhibitors
Sodium glucose transporter 2 (SGLT-2) inhibitors may induce sarcopenia by causing protein breakdown, particularly in elderly patients with inadequate dietary intake. SGLT-2 inhibitors cause a decrease in serum insulin levels and an increase in glucagon levels. Consequently, they reduce the uptake of glucose and amino acids into muscle and promote protein breakdown [61]. Dapagliflozin, ipragliflozin, and empagliflozin have been shown to decrease muscle mass and skeletal muscle mass index [62,63,64,65]. However, further studies are needed to determine the frequency of the occurrence of SGLT-2 inhibitor-related sarcopenia and whether SGLT-2 inhibitors cause diabetes-related sarcopenia, as clinical data are limited.

Table 1.
Effects of drugs on nutrients.
Table 1.
Effects of drugs on nutrients.
| Drugs | Effects of Drugs on Nutrients | Symptoms Caused | Countermeasure | References |
|---|---|---|---|---|
| Antihypertensive drugs: thiazide diuretics, ARBs, ACE inhibitors, and potassium-retaining diuretics | Zinc deficiency | Taste disorder, anorexia, lethargy, and delayed wound healing | •Determination of zinc levels in plasma or urine •Blood pressure monitoring to determine the need for continued administration | [7] |
| Acetylcholinesterase inhibitors | Unknown | Nausea, vomiting, diarrhea, and loss of appetite | •Monitoring changes in appetite and weight loss •Assessing the benefits of using medications for the risk of malnutrition | [34] |
| Proton pump inhibitors | Deficiency of VB12, Mg, Ca, and Fe | Clostridium difficile diarrhea, pneumonia, femoral neck fracture, hypomagnesemia, and hypocalcemia | •Measurement of VB12, Mg, Ca, ferritin, and FRAX score •Evaluate the need for continued administration | [35,36,37,38,39,41,53] |
| HMG-CoA reductase inhibitors (stains) | CoQ10 deficiency | Frailty, sarcopenia, and myopathy | •Examining the use of CoQ10 in combination •Evaluate the need for continuous administration in patients over ≥75 years | [7,54] |
| Long-term, high-dose aspirin | VC deficiency | Gastric mucosal thinning | •Long-term use of low-dose aspirin (80–400 mg/day) •VC supplementation if higher doses are needed | [7] |
| Metformin | •VB12 deficiency •Inhibits the breakdown of muscle proteins | •Anemia, fatigue, and cognitive impairment •Improvement of muscle mass and strength | Monitor vitamin B12 and consider switching to another drug if it is low | [7] |
| SGLT-2 inhibitors | Protein degradation | Sarcopenia, decrease in muscle mass, and skeletal muscle mass index | Consider the need for continued administration | [62,63,64,65] |
| Diuretics (loop, thiazide, and osmotic), corticosteroids, kanzo, insulin, β2-adrenergic stimulation | Lower potassium | Vomiting, anorexia, weakness, muscle weakness, tetany | Monitor potassium and consider eating foods rich in potassium or taking supplements | [66,67,68,69,70] |
Abbreviations: Angiotensin-converting enzyme inhibitors, ACE inhibitors; angiotensin II receptor blockers, ARBs; calcium, Ca; coenzyme Q10, CoQ10; hydroxymethylglutaryl-CoA, HMG-CoA; ferrum, Fe; fracture risk assessment tool, FRAX; magnesium, Mg; sodium glucose transporter-2, SGLT-2; vitamin B12, VB12; vitamin C, VC.
5.1.8. Diuretics, Corticosteroids, Kanzo (Kampo), Insulin, and β2-Adrenergic Stimulation
Loop, thiazides, and osmotic diuretics cause hypokalemia [66]. These drugs act on the renal tubules to inhibit the reabsorption of water along with sodium, thereby producing a diuretic effect. At the same time, they promote the excretion of potassium, resulting in hypokalemia. Hypokalemia may be accompanied by symptoms such as vomiting, loss of appetite, and weakness. The diuretic effect also causes dry mouth. These symptoms may affect food intake. Steroids cause hypokalemia via their mineralocorticoid action [67]. The main ingredient in kanzo is glycyrrhizic acid. Glycyrrhizic acid acts on the distal tubules of the kidneys, causing retention of sodium and water in the body, hypokalemia, and increased blood pressure [68]. These effects are referred to as pseudohypoaldosteronism. Both insulin and β2-adrenergic stimulation activate potassium uptake by stimulating activity of the adenosine triphosphatase sodium/potassium pump predominantly in skeletal muscle [69,70].
5.2. Nutrient–Drug and Diet–Drug Interactions
Typical nutrients or diets, including vitamins, calcium, high-fat meals (approximately ≥900–1000 kcal), and high-protein meals (protein accounting for ≥20% of the total caloric content of the meal), and their drug interactions are listed in Table 2. It is important to be aware of these interactions and to keep their risks in mind when managing patient treatment plans. However, nutrient–drug or diet–drug interactions are still in the developmental stage, and there are not many cases in which the management of such interactions has been established. Under these circumstances, careful observation in the clinical setting is essential to assess the frequency and severity of adverse effects due to nutrient–drug or diet–drug interactions and to identify unknown interactions.

Table 2.
Nutrient–drug and diet–drug interactions.
6. Drug and Eating Habits
Changes in eating habits also affect the efficacy of drugs. A previous study has examined the relationship between the time trends of caloric intake and statin use. Caloric and fat intakes increased among statin users over time, which was not true for nonusers. The increase in BMI was faster for statin users than for nonusers. Efforts aimed at dietary control among statin users may be becoming less intensive [103]. Additionally, another study has shown that eating habits affect blood pressure control in outpatients treated with antihypertensive drugs. Habitual intake of foods rich in potassium and magnesium were associated with reduced intensity and cost of medication and with preservation of blood pressure control in elderly hypertensive outpatients [104]. This change in eating habits will also affect the efficacy of the drug. Elderly perioperative patients with cancer often have concomitant lifestyle-related diseases. It should be noted that a change in eating habits may also have a small effect on nutritional status.
7. Intervention Effects on Polypharmacy, Cancer Cachexia, and Rehabilitation Nutrition
7.1. Intervention Effects on Polypharmacy
Effective interventions for polypharmacy have been studied. A systematic review by Hill-Taylor et al. revealed that intervention using the STOPP&START criteria [105] reduced the proportion of potentially inappropriate medications (PIMs) prescribed. PIMs include prescriptions of an incorrect dose, frequency, or mode of administration or duration that are likely to result in clinically significant drug–drug or drug–disease interactions or have no clear evidence-based clinical indication [106,107]. Another intervention study showed that hospital pharmacists reduced the number of PIMs by using the STOPP&START criteria [108]. In this study, out of 651 PIMs, 292 (44.9%) were changed or discontinued [109]. A retrospective cohort study of 569 older adults based on “rehabilitation pharmacotherapy” [110] reported an association between a decrease in the Beers criteria for [111] PIMs and an improvement in the motor ADL at discharge [112].
Alternatively, a recent systematic review of interventions to reduce polypharmacy failed to show a benefit based on clinical evidence such as mortality rates, the number of hospitalizations, and the frequency of falls [113,114,115]. In patients admitted to an acute care ward, the frequency of emergency room visits and readmissions was smaller in the multimodal intervention group, which combined a medication reduction review with motivational interviewing and follow-up by a multidisciplinary team, than in the other group with usual care [116]. However, there was no significant difference in the outcomes between the usual care group and the medication review-only group. Similarly, interventions that combined patient interviews and patient education with a medication review reduced the number of hospital visits and drug-related hospitalizations [117] and the frequency of emergency department visits [117,118]. Thus, a reduction of polypharmacy is expected to improve the frequency of emergency department visits, readmissions, and quality of life. However, if the goal is to improve clinical outcomes such as mortality rates, the number of hospitalizations, and the frequency of falls, patient-centered multimodal interventions such as the combination of a medication review, multidisciplinary collaboration, and patient education may be more effective.
7.2. Intervention Effect on Cancer Cachexia
Cachexia includes “objective” components (e.g., inadequate food intake, weight loss, inactivity, loss of muscle mass, and metabolic derangements inducing catabolism) and “subjective” components (e.g., anorexia, early satiety, taste alterations, chronic nausea, distress, fatigue, and loss of concentration) [119]. Thus, comprehensive treatment requires a multitargeted and multidisciplinary approach, such as nutrition, rehabilitation, and pharmacotherapy aimed at evaluating objective signs and symptom relief.
The European Society of Medical Oncology Clinical Practice Guidelines for cancer cachexia indicated that nutritional interventions for cancer cachexia will meet energy and nutrient requirements and simultaneously normalize metabolic status, including strength training and reduction of systemic inflammation and pain relief. To maintain nutritional status, at least 25–30 kcal/kg/day and at least 1.2 g protein/kg/day is recommended, with adjustments made to the regimen as required. Furthermore, regimens with fat accounting for half of the nonprotein calories are recommended [120]. The American Society of Clinical Oncology guideline for cancer cachexia indicated that the only nutritional intervention recommended was nutritional counseling by a registered dietitian. A high-protein, high-energy, nutrient-dense diet is the recommended dietary guideline [121].
There are a limited number of drugs that can be used in the pharmacotherapy of cancer cachexia. The European Society of Medical Oncology guidelines state that short-term corticosteroids and progestins can be used to increase appetite and weight gain [120]. The use of olanzapine may also be considered for the treatment of appetite and nausea in patients with advanced cancer. The American Society of Clinical Oncology guidelines similarly recommended the use of progesterone derivatives and corticosteroids for appetite improvement and weight gain [121]. Anamorelin was approved in Japan in January 2021 for the first time in the world for cancer cachexia in patients with non-small-cell lung cancer, gastric cancer, pancreatic cancer, or colorectal cancer. However, it has not been approved in Europe based on the findings from the ROMANO studies [122,123], which did not show the improvement in muscle strength that was demonstrated by the Japanese trials. The approval of the use of anamorelin is expected to change the clinical practice of cancer cachexia in Japan [124].
To reverse cancer cachexia, lean body mass should be increased, and muscle function must be restored. Hence, a combination of improved nutrition, physical exercise that can improve muscle function, and pharmacotherapy may be an option for reversing cachexia in patients with cancer.
7.3. Intervention Effect on Rehabilitation Nutrition
The concept of rehabilitation nutrition, which combines rehabilitation and nutritional therapy, has attracted attention in recent years [125]. Rehabilitation nutrition includes holistic assessment by the International Classification of Functioning, Disability, and Health; assessment for nutritional disorders, sarcopenia, and excessive or inadequate intake of nutrients; diagnosis of rehabilitation nutrition; and goal setting. Rehabilitation nutrition improves the nutritional status of sarcopenia patients with disabilities and frail, older people, besides maximizing their functions, activities, participation, and quality of life. This is achieved via “nutrition care management in consideration of rehabilitation” and “rehabilitation in consideration of nutrition” [125]. Rehabilitation nutrition practices based on the Rehabilitation Nutrition Care Process can improve function and ADL in cases of malnutrition and sarcopenia [126]. The clinical practice guidelines of the 2020 rehabilitation nutrition edition weakly recommended the use of enhanced nutritional therapy in cerebrovascular disease, hip fracture, cancer, and acute disease [127]. Furthermore, improving the nutritional status can recover swallowing ability and ADL in malnourished patients. Malnourished patients with stroke, hip fracture, or pneumonia are more likely to achieve improved swallowing function and ADL following nutrition improvement [128,129,130]. Therefore, in the case of malnutrition and sarcopenia, rehabilitation should be combined with aggressive nutritional therapy so that energy and protein are added to the daily energy expenditure, which will improve the nutritional status.
8. Conclusions
Pathophysiological and drug-induced factors are associated with the risk of developing malnutrition in elderly patients with cancer. Polypharmacy is associated with malnutrition. The long-term use of several drugs, including anticancer drugs, leads to anorexia as a gastrointestinal disorder and induces malnutrition. Furthermore, several drugs are known to interact with nutrients and diet, which may adversely affect the patient’s nutritional status. Conversely, malnutrition may affect pharmacokinetics and pharmacodynamics, potentiate drug effects, and induce or worsen side effects. Therefore, nutritional management of elderly patients with cancer during their perioperative period should consider pathophysiological factors such as tumors or cancer cachexia as well as drug-related factors. In other words, it is necessary to simultaneously evaluate the development of malnutrition from the perspective of drug interactions and the development of adverse drug events from the perspective of malnutrition. To this end, multidisciplinary teams should put forth an effort to recognize and mitigate the potential impact of the patient’s perioperative nutritional status.
Author Contributions
Conceptualization, E.K. and H.W.; methodology, E.K.; investigation, E.K.; writing—original draft preparation, E.K.; writing—review and editing, E.K.; H.W. and N.Y., supervision, N.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: A systematic review and meta-analysis. Gastroenterology 2018, 155, 391–410. [Google Scholar] [CrossRef]
- Hewitt, J.; Long, S.; Carter, B.; Bach, S.; McCarthy, K.; Clegg, A. The prevalence of frailty and its association with clinical outcomes in general surgery: A systematic review and meta-analysis. Age Ageing 2018, 47, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Huddy, J.R.; Huddy, F.M.S.; Markar, S.R.; Tucker, O. Nutritional optimization during neoadjuvant therapy prior to surgical resection of esophageal cancer- a narrative review. Dis. Esophagus 2018, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P.; Rios-Diaz, A.J.; Dalela, D.; Ravi, P.; Sood, A.; Hanske, J.; Chun, F.K.H.; Kibel, A.S.; Lipstiz, S.R.; Sun, M.; et al. The association of hypoalbuminemia with early perioperative outcomes- a comprehensive assessment across 16 major procedures. Am. J. Surg. 2017, 214, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desne, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.; Brook-Barclay, L.; Delaney, C.L.; Spark, J.I.; Miller, M.D. Do medications commonly prescribed to patients with peripheral arterial disease have an effect on nutritional status? A review of the literature. Ann. Vasc. Surg. 2016, 32, 145–175. [Google Scholar] [CrossRef]
- Syed, Q.; Hendler, K.T.; Koncilja, K. The impact of aging and medical status on dysgeusia. Am. J. Med. 2016, 129, 753.e1–753.e6. [Google Scholar] [CrossRef]
- Jyrkka, J.; Mursu, J.; Enlund, H.; Lönnroos, E. Polypharmacy and nutritional status in elderly people. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 1–6. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, S.; Khanal, S. Polypharmacy in elderly cancer patients: Challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019, 2, 42–49. [Google Scholar] [CrossRef]
- Shrestha, S.; Shakya, R.; Shrestha, S.; Shakya, S. Adverse drug reaction due to cancer chemotherapy and its financial burden in different hospitals of Nepal. Int. J. Pharmacovigil. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Turner, J.P.; Shakib, S.; Singhal, N.; Hogan-Doran, J.; Prowse, R.; Johns, S.; Bell, J.S. Prevalence and factors associated with polypharmacy in older people with cancer. Support. Care Cancer 2014, 22, 1727–1734. [Google Scholar] [CrossRef]
- Prithviraj, G.K.; Koroukian, S.; Margevicius, S.; Berger, N.A.; Bagai, R.; Owusu, C. Patient characteristics associated with polypharmacy and inappropriate prescribing of medications among older adults with cancer. J. Geriatr. Oncol. 2012, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Bushardt, R.L.; Massey, E.B.; Simpson, T.W.; Ariail, J.C.; Simpson, K.N. Polypharmacy: Misleading, but manageable. Clin. Interv. Aging 2008, 3, 383–389. [Google Scholar] [CrossRef]
- Mortazavi, S.S.; Shati, M.; Keshtkar, A.; Malakouti, S.K.; Bazargan, M.; Assari, S. Defining polypharmacy in the elderly: A systematic review protocol. BMJ Open 2016, 6, e010989. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Gillian, E. Caughey. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Dhalwani, N.N.; Fahami, R.; Sathanapally, H.; Seidu, S.; Davies, M.J.; Khunti, K. Association between polypharmacy and falls in older adults: A longitudinal study from England. BMJ Open 2017, 7, e016358. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Cross, A.J.; Jokanovic, N.; Wiese, M.D.; George, J.; Johnell, K.; Diug, B.; Bell, J.S. Clinical Outcomes Associated with Medication Regimen Complexity in Older People: A Systematic Review. J. Am. Geriatr. Soc. 2017, 65, 747. [Google Scholar] [CrossRef]
- Rawle, M.J.; Cooper, R.; Kuh, D.; Richards, M. Associations Between Polypharmacy and Cognitive and Physical Capability: A British Birth Cohort Study. J. Am. Geriatr. Soc. 2018, 66, 916. [Google Scholar] [CrossRef]
- Kose, E.; Maruyama, R.; Okazoe, S.; Hayashi, H. Impact of Polypharmacy on the Rehabilitation Outcome of Japanese Stroke Patients in the Convalescent Rehabilitation Ward. J. Aging Res. 2016, 2016, 7957825. [Google Scholar] [CrossRef] [PubMed]
- Kose, E.; Toyoshima, M.; Okazoe, S.; Oka, R.; Shiratsuchi, Y.; Hayashi, H. The relationship between polypharmacy and recovery of activities of daily living among convalescent stroke patients: A propensity score-matched analysis. Eur. Ger. Med. 2017, 8, 250–255. [Google Scholar] [CrossRef]
- Leelakanok, N.; Holcombe, A.L.; Lund, B.C.; Gu, X.; Schweizer, M.L. Association between polypharmacy and death: A systematic review and meta-analysis. J. Am. Pharm. Assoc. 2017, 57, 729–738.e10. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, G.; Skonecki, E.; Boparai, M. The impact of polypharmacy on patient outcomes in older adults with cancer. Cancer J. 2017, 23, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, G.; Hajjar, E.; Swartz, K.; Andrel, S.J.; Chapman, A. Evaluation of a pharmacist led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J. Clin. Oncol. 2015, 33, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Elliot, K.; Tooze, J.A.; Geller, R.; Powell, B.L.; Pardee, T.S.; Ritchie, E.; Kennedy, L.; Callahan, K.E.; Klepin, H.D. The prognostic importance of polypharmacy in older adults treated for acute myelogenous leukemia (AML). Leuk. Res. 2014, 38, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Schiessel, D.L.; Baracos, V.E. Barriers to cancer nutrition therapy: Excess catabolism of muscle and adipose tissues induced by tumour products and chemotherapy. Proc. Nutr. Soc. 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin. Nutr. 2016, 35, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kose, E.; Hirai, T.; Seki, T. Change in number of potentially inappropriate medications impacts on the nutritional status in a convalescent rehabilitation setting. Geriatr. Gerontol. Int. 2019, 19, 44–50. [Google Scholar] [CrossRef]
- Kose, E.; Hirai, T.; Seki, T.; Yasuno, N. Anticholinergic Load and Nutritional Status in Older Individuals. J. Nutr. Health Aging 2020, 24, 20–27. [Google Scholar] [CrossRef]
- Bernstein, M.; Munoz, N. Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: Food and nutrition for older adults: Promoting health and wellness. J. Acad. Nutr. Diet. 2012, 112, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Koga, T.; Akagi, J. Tentative nil per os leads to poor outcomes in older adults with aspiration pneumonia. Clin. Nutr. 2016, 35, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, S.K.; George, R.P.; Shetty, N.; Levenson, D.; Ferraiolo, D.M.; Shrestha, A. Interventions for managing taste disturbances. Cochrane Database Syst. Rev. 2017, 12, CD010470. [Google Scholar] [CrossRef]
- Hoppe, C.; Kutschan, S.; Dörfler, J.; Büntzel, J.; Büntzel, J.; Huebner, J. Zinc as a complementary treatment for cancer patients: A systematic review. Clin. Exp. Med. 2021. [Google Scholar] [CrossRef]
- Soysal, P.; Isik, A.T. Effects of acetylcholinesterase inhibitors on nutritional status in elderly patients with dementia: A 6-month follow-up study. J. Nutr. Health Aging. 2016, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, M.; Wakabayashi, H. Effect of long-term proton pump inhibitor therapy on nutritional status in elderly hospitalized patients. J. Nutr. Sci. Vitaminol. 2016, 62, 330–334. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef]
- Den Elzen, W.P.J.; Groeneveld, Y.; de Ruijter, W.; Souverijn, J.H.M.; le Cessie, S.; Assendelft, W.J.J.; Gussekloo, J. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol. Ther. 2008, 27, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Srinutta, T.; Chewcharat, A.; Takkavatakarn, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Jaber, B.L.; Susantitaphong, P. Proton pump inhibitors and hypomagnesemia: A meta-analysis of observational studies. Medicine 2019, 98, e17788. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Kittanamongkolchai, W.; Srivali, N.; Edmonds, P.L.; Ungprasert, P.; O’Corragain, O.A.; Korpaisarn, S.; Erickson, S.B. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren. Fail. 2015, 37, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Yilmaz, M.I.; Apetrii, M.; Saglam, M.; Yaman, H.; Unal, H.U.; Gok, M.; Caglar, K.; Oguz, Y.; Yenicesu, M.; et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am. J. Nephrol. 2012, 36, 228–237. [Google Scholar] [CrossRef]
- O’Connell, M.B.; Madden, D.M.; Murray, A.M.; Heaney, R.P.; Kerzner, L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am. J. Med. 2005, 118, 778–781. [Google Scholar] [CrossRef]
- Ivanovich, P.; Fellows, H.; Rich, C. The absorption of calcium carbonate. Ann. Intern. Med. 1967, 66, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Serfaty-Lacrosniere, C.; Wood, R.J.; Voytko, D.; Saltzman, J.R.; Pedrosa, M.; Sepe, T.E.; Russell, R.R. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J. Am. Coll. Nutr. 1995, 14, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Tuukkanen, J.; Väänänen, H.K. Omeprazole, a specific inhibitor of H+-K+-ATPase, inhibits bone resorption in vitro. Calcif. Tissue Int. 1986, 38, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Mizunashi, K.; Furukawa, Y.; Katano, K.; Abe, K. Effect of omeprazole, an inhibitor of H+-K+-ATPase, on bone resorption in humans. Calcif. Tissue Int. 1986, 38, 123–125. [Google Scholar] [CrossRef]
- Khalili, H.; Huang, E.S.; Jacobson, B.C.; Camargo, C.A., Jr.; Feskanich, D.; Chan, A.T. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: A prospective cohort study. BMJ 2012, 344, e372. [Google Scholar] [CrossRef]
- McColl, K.E.L. Effect of proton pump inhibitors on vitamins and iron. Am. J. Gastroenterol. 2009, 104, S5–S9. [Google Scholar] [CrossRef]
- Hutchinson, C.; Geissler, C.A.; Powell, J.J.; Bomford, A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 2007, 56, 1291–1295. [Google Scholar] [CrossRef]
- Sarzynski, E.; Puttarajappa, C.; Xie, Y.; Grover, M.; Laird-Fick, H. Association between proton pump inhibitor use and anemia: A retrospective cohort study. Dig. Dis. Sci. 2011, 56, 2349–2353. [Google Scholar] [CrossRef]
- Ajmera, A.V.; Shastri, G.S.; Gajera, M.J.; Judge, T.A. Suboptimal response to ferrous sulfate in iron-deficient patients taking omeprazole. Am. J. Ther. 2012, 19, 185–189. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Quesenberry, C.P.; Corley, D.A. Proton pump inhibitor and Histamine-2 receptor antagonist use and iron deficiency. Gastroenterology 2017, 152, 821–829. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The risks and benefits of long-term use of proton pump inhibitors: Expert review and best practice advice from the american gastroenterological association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Termanini, B.; Sutliff, V.E.; Serrano, J.; Yu, F.; Gibril, F.; Jensen, R.T. Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol. Ther. 1998, 12, 83–98. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; Haehling, S.V.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of Coenzyme Q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef] [PubMed]
- Marcoff, L.; Thompson, P.D. The role of coenzyme Q10 in statin-associated myopathy: A systematic review. J. Am. Coll. Cardiol. 2007, 49, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Carling, D. “AMP-activated protein kinase: The current landscape for drug development,” Nature Reviews. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Laksmi, P.W.; Setiati, S.; Tamin, T.T.; Soewondo, P.; Rochmah, W.; Nafrialdi, N.; Prihartono, J. Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: A double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Med. Indones. 2017, 49, 118–127. [Google Scholar]
- Lee, C.G.; Schwartz, A.V.; Yaffe, K.; Hillier, T.A.; LeBlanc, E.S.; Cawthon, P.M. Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J. Am. Geriatr. Soc. 2013, 61, 1872–1878. [Google Scholar] [CrossRef]
- Lee, C.G.; Boyko, E.J.; Barrett-Connor, E.; Miljkovic, I.; Hoffman, A.R.; Everson-Rose, S.A.; Lewis, C.E.; Cawthon, P.M.; Strotmeyer, E.S.; Orwoll, E.S. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011, 34, 2381–2386. [Google Scholar] [CrossRef]
- Yabe, D.; Nishikino, R.; Kaneko, M.; Iwasaki, M.; Seino, Y. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: Considerations for their appropriate use to avoid serious adverse events. Expert. Opin. Drug Saf. 2015, 14, 795–800. [Google Scholar] [CrossRef]
- Yasuda, M.; Iizuka, K.; Kato, T.; Liu, Y.; Takao, K.; Nonomura, K.; Mizuno, M.; Yabe, D. Sodium-glucose cotransporter 2 inhibitor and sarcopenia in a lean elderly adult with type 2 diabetes: A case report. J. Diabetes Investig. 2020, 11, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Morino, K.; Ugi, S.; Tanaka-Mizuno, S.; Fuse, K.; Miyazawa, I.; Kondo, K.; Sato, D.; Ohashi, N.; Ida, S.; et al. Ipragliflozin, a sodiumglucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: A randomized clinical trial. J. Diabetes Investig. 2019, 10, 1012–1021. [Google Scholar] [CrossRef]
- Tsurutani, Y.; Nakai, K.; Inoue, K.; Azuma, K.; Mukai, S.; Maruyama, S.; Iizuka, T.; Matsuzawa, Y.; Saito, J.; Omura, M.; et al. Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: A multicentre, randomized, prospective, open-label, active-controlled Journal of Diabetes Research 5 study. Diabetes Obes. Metab. 2018, 20, 2675–2679. [Google Scholar] [CrossRef]
- Gao, F.; Hall, S.; Bach, L.A. Myopathy secondary to empagliflozin therapy in type 2 diabetes. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 20-0017. [Google Scholar] [CrossRef]
- Kardalas, E.; Paschou, S.A.; Anagnostis, P.; Muscogiuri, G.; Siasos, G.; Vryonidou, A. Hypokalemia: A clinical update. Endocr. Connect. 2018, 7, R135–R146. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Sato, N.; Goto, M.; Kobayashi, M.; Takehara, N.; Takeuchi, T.; Talib, A.K.; Sugiyama, E.; Minoshima, A.; Tanabe, Y.; et al. Three cases of corticosteroid therapy triggering ventricular fibrillation in J-wave syndromes. Heart Vessels 2014, 29, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Penninkilampi, R.; Eslick, E.M.; Eslick, G.D. The association between consistent licorice ingestion, hypertension and hypokalaemia: A systematic review and meta-analysis. J. Hum. Hypertens. 2017, 31, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Appel, L.J.; Grams, M.E.; Gutekunst, L.; McCullough, P.A.; Palmer, B.F.; Pitt, B.; Sica, D.A.; Townsend, R.R. Potassium homeostasis in health and disease: A scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J. Am. Soc. Hypertens. 2017, 11, 783–800. [Google Scholar] [CrossRef]
- DynaMed. Available online: https://www.dynamed.com/drug-monograph/paclitaxel (accessed on 5 June 2021).
- Hansson, O.; Sillanpaa, M. Pyridoxine and serum concentration of phenytoin and phenobarbitone. Lancet 1976, 1, 256. [Google Scholar] [CrossRef]
- Feldman, S.; Hedrick, W. Antacid effects on the gastrointestinal absorption of riboflavin. J. Pharm. Sci. 1983, 72, 121–123. [Google Scholar] [CrossRef]
- Yahr, M.D.; Duvoisin, R.C. Pyridoxine, levodopa, and l-α-methyldopa hydrazine regimen in Parkinsonism. JAMA 1971, 216, 2141. [Google Scholar] [CrossRef]
- Streeter, A.K.; Goulston, K.J.; Bathur, F.A.; Hilmer, R.S.; Crane, G.G.; Pheils, M.T. Cimetidine and malabsorption of cobalamin. Dig. Dis. Sci. 1982, 27, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Brise, H.; Hallberg, L. Effect of ascorbic acid on iron absorption. Acta. Med. Scand. Suppl. 1962, 376, 51–58. [Google Scholar] [CrossRef]
- Parfitt, A.M. Thiazide-induced hypercalcemia in vitamin D-treated hypoparathyroidism. Ann. Intern. Med. 1972, 77, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Up to Date. Available online: https://www.uptodate.com/contents/digoxin-drug-information?search=digoxin&source=panel_search_result&selectedTitle=1~148&usage_type=panel&kp_tab=drug_general&display_rank=1 (accessed on 5 June 2021).
- Corrigan, J.J., Jr.; Marcus, F.I. Coagulopathy associated with vitamin E ingestion. JAMA 1974, 230, 1300–1301. [Google Scholar] [CrossRef] [PubMed]
- Karlson, B.; Leijd, B.; Hellström, K. On the influence of vitamin K-rich vegetables and wine on the effectiveness of warfarin treatment. Acta Med. Scand. 1986, 220, 347–350. [Google Scholar] [CrossRef]
- Odou, P.; Barthélémy, C.; Robert, H. Influence of seven beverages on salicylate disposition in humans. J. Clin. Pharm. Ther. 2001, 26, 187–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leyden, J.J. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and iron. J. Am. Acad. Dermatol. 1985, 12, 308–312. [Google Scholar] [CrossRef]
- Minami, R.; Inotsume, N.; Nakano, M.; Sudo, Y.; Higashi, A.; Matsuda, I. Effect of milk on absorption of norfloxacin in healthy volunteers. J. Clin. Pharmacol. 1993, 33, 1238–1240. [Google Scholar] [CrossRef]
- Boonen, S.; Bouillon, R.; Haentjens, P.; Vanderschueren, D. Optimizing the benefits of bisphosphonates in osteoporosis: The importance of appropriate calcium intake. Treat. Endocrinol. 2006, 5, 375–383. [Google Scholar] [CrossRef]
- Gunnarsson, P.O.; Davidsson, T.; Andersson, S.B.; Backman, C.; Johansson, S.A. Impairment of estramustine phosphate absorption by concurrent intake of milk and food. Eur. J. Clin. Pharmacol. 1999, 38, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; Gerber, T.C.; Hayes, D.L.; Reeder, G.S. Digoxin, hypercalcaemia, and cardiac conduction. Postgrad. Med. J. 1999, 75, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Pepe, J.; Piemonte, S.; Cipriani, C. The diagnosis and management of hypercalcaemia. BMJ 2015, 350, h2723. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.A.; Kovarik, J.M.; van Bree, J.B.; Grevel, J.; Lücker, P.W.; Kutz, K. Influence of a fat-rich meal on the pharmacokinetics of a new oral formulation of cyclosporine in a crossover comparison with the market formulation. Pharm. Res. 1994, 11, 151–155. [Google Scholar] [CrossRef]
- Welling, P.G.; Lyons, L.L.; Craig, W.A.; Trochta, G.A. Influence of diet and fluid on bioavailability of theophylline. Clin. Pharmacol. Ther. 1975, 17, 475–480. [Google Scholar] [CrossRef]
- Ogunbona, F.A.; Smith, I.F.; Olawoye, O.S. Fat contents of meals and bioavailability of griseofulvin in man. J. Pharm. Pharmacol. 1985, 37, 283–284. [Google Scholar] [CrossRef]
- Up to Date. Available online: https://www.uptodate.com/contents/oxycodone-drug-information?search=Oxycodone&source=panel_search_result&selectedTitle=1~148&usage_type=panel&kp_tab=drug_general&display_rank=1 (accessed on 5 June 2021).
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.K.; Lasseter, K. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 2002, 42, 1122–1133. [Google Scholar] [CrossRef]
- Ling, J.; Fettner, S.; Lum, B.L.; Riek, M.; Rakhit, A. Effect of food on the pharmacokinetics of erlotinib, an orally active epidermal growth factor receptor tyrosine-kinase inhibitor, in healthy individuals. Anticancer Drugs 2008, 19, 209–216. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Ferron, G.M.; Lim, H.K.; Parker, V. The effect of a high-fat meal on the oral bioavailability of the immunosuppressant sirolimus (rapamycin). J. Clin. Pharmacol. 1999, 39, 1155–1161. [Google Scholar] [PubMed]
- Product Information: STIVARGA(R) Oral Tablets, Regorafenib Oral Tablets; Bayer HealthCare Pharmaceuticals Inc. (per FDA): Whippany, NJ, USA, 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203085lbl.pdf (accessed on 5 June 2021).
- Product Information: NEXAVAR(R) Oral Tablets, Sorafenib Oral Tablets; Bayer HealthCare Pharmaceuticals Inc. (per FDA): Whippany, NJ, USA, 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021923s020lbl.pdf (accessed on 5 June 2021).
- Product Information: REVLIMID(R) Oral Capsules, Lenalidomide Oral Capsules; Celgene Corporation (per FDA): Summit, NJ, USA, 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021880s034lbl.pdf (accessed on 5 June 2021).
- Cox, D.S.; Papadopoulos, K.; Fang, L.; Bauman, J.; LoRusso, P.; Tolcher, A.; Patnaik, A.; Pendry, C.; Orford, K.; Ouellet, K. Evaluation of the effects of food on the single-dose pharmacokinetics of trametinib, a first-in-class MEK inhibitor, in patients with cancer. J. Clin. Pharmacol. 2013, 53, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, D.; Grossmann, K.F.; Limentani, G.; Nebot, N.; Lan, K.; Knowles, L.; Gordon, M.S.; Sharma, S.; Infante, J.R.; Lorusso, P.M.; et al. Effects of particle size, food, and capsule shell composition on the oral bioavailability of dabrafenib, a BRAF inhibitor, in patients with BRAF mutation-positive tumors. J. Pharm. Sci. 2013, 102, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Fagan, T.C.; Walle, T.; Oexmann, M.J.; Walle, U.K.; Bai, S.A.; Gaffney, T.E. Increased clearance of propranolol and theophylline by high-protein compared with high-carbohydrate diet. Clin. Pharmacol. Ther. 1987, 41, 402–406. [Google Scholar] [CrossRef]
- Nutt, J.G.; Woodward, W.R.; Hammerstad, J.P.; Carter, J.H.; Anderson, J.L. The “on-off” phenomenon in Parkinson’s disease. Relation to levodopa absorption and transport. N. Engl. J. Med. 1984, 310, 483–488. [Google Scholar] [CrossRef]
- Halter, F.; Huber, R.; Häcki, W.H.; Varga, L.; Bachmann, C. Effect of food on antacid neutralizing capacity in man. Eur. J. Clin. Investig. 1982, 12, 209–217. [Google Scholar] [CrossRef]
- Sugiyama, T.; Tsugawa, Y.; Tseng, C.H.; Kobayashi, Y.; Shapiro, M.F. Different time trends of caloric and fat intake between statin users and nonusers among US adults: Gluttony in the time of statins? JAMA Intern. Med. 2014, 174, 1038–1045. [Google Scholar] [CrossRef]
- Ono, A.; Shibaoka, M.; Yano, J.; Asai, Y.; Fujita, T. Eating habits and intensity of medication in elderly hypertensive outpatients. Hypertens. Res. 2000, 23, 195–200. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef]
- O’Connor, M.N.; Gallagher, P.; O’Mahony, D. Inappropriate prescribing: Criteria, detection and prevention. Drugs Aging 2012, 29, 437–452. [Google Scholar] [CrossRef]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- Hill-Taylor, B.; Walsh, K.A.; Stewart, S.; Hayden, J.; Byrne, S.; Sketris, I.S. Effectiveness of the STOPPSTART (Screening Tool of Older Persons’ potentially inappropriate Prescriptions Screening Tool to Alert doctors to the Right Treatment) criteria: Systematic review and meta-analysis of randomized controlled studies. J. Clin. Pharm. Ther. 2016, 41, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Ogura, F.; Yamamoto, K.; Uda, A.; Nishioka, T.; Makimoto, H.; Yano, I.; Hirai, M. Potentially inappropriate medications in elderly Japanese patients: Effects of pharmacists’ assessment and intervention based on Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions criteria ver.2. J. Clin. Pharm. Ther. 2017, 42, 209–214. [Google Scholar] [CrossRef]
- Kose, E.; Wakabayashi, H. Rehabiitation pharmacotherapy: A scoping review. Geriatr. Gerontol. Int. 2020, 20, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Fick, D.M.; Semla, T.P.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.E.; Pezzullo, L.; Epplin, J.J.; Flanagan, N.; et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Kose, E.; Hirai, T.; Seki, T.; Yasuno, N. The impact of decreasing potentially inappropriate medications on activities of daily living in a convalescent rehabilitation setting. Int. J. Clin. Pharm. 2020, 2. [Google Scholar] [CrossRef]
- Johansson, T.; Abuzahra, M.E.; Keller, S.; Mann, E.; Faller, B.; Sommerauer, C.; Höck, J.; Löffler, C.; Köchling, A.; Schuler, J.; et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2016, 532–548. [Google Scholar] [CrossRef]
- Rankin, A.; Cadogan, C.A.; Patterson, S.M.; Kerse, N.; Cardwell, C.R.; Bradley, M.C.; Ryan, C.; Hughes, C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 2018, 9, CD008165. [Google Scholar] [CrossRef]
- Huiskes, V.J.B.; Burger, D.M.; Van Den Ende, C.H.M.; Van Den Bemt, B.J.F. Effectiveness of medication review: A systematic review and meta-analysis of randomized controlled trials. BMC Fam. Pract. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Nielsen, L.V.; Duckert, M.L.; Lund, M.L.; Henriksen, J.P.; Nielsen, M.L.; Eriksen, C.S.; Buck, T.C.; Pottegård, A.; Hansen, M.R.; Hallas, J. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission a randomized clinical trial. JAMA Intern. Med. 2018, 178, 375–382. [Google Scholar] [CrossRef]
- Gillespie, U.; Alassaad, A.; Henrohn, D.; Garmo, H.; Hammarlund-Udenaes, M.; Toss, H.; Kettis-Lindblad, A.; Melhus, H.; Mörlin, C. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: A randomized controlled trial. Arch. Intern. Med. 2009, 169, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Lisby, M.; Bonnerup, D.K.; Brock, B.; Gregersen, P.A.; Jensen, J.; Larsen, M.L.; Rungby, J.; Sonne, J.; Mainz, J. Medication Review and Patient Outcomes in an Orthopedic Department: A Randomized Controlled Study. J. Patient Saf. 2018, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Schcolnik-Cabrera, A.; Chávez-Blanco, A.; Domínguez-Gómez, G.; Dueñas-González, A. Understanding tumor anabolism and patient catabolism in cancerassociated cachexia. Am. J. Cancer Res. 2017, 7, 1107–1135. [Google Scholar] [PubMed]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, C.I.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Fabbro, E.D.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of cancer cachexia: ASCO guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Temel, J.S.; Abernethy, A.P.; Currow, D.C.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomized, double-blind, phase 3 trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef]
- Currow, D.; Temel, J.S.; Abernethy, A.; Milanowski, J.; Friend, J.; Fearon, K.C. ROMANA. 3: A phase 3 safety extension study of anamorelin in advanced nonsmall-cell lung cancer (NSCLC) patients with cachexia. Ann. Oncol. 2017, 28, 1949–1956. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Arai, H.; Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef]
- Wakabayashi, H. Rehabilitation nutrition in general and family medicine. J. Gen. Fam. Med. 2017, 18, 153–154. [Google Scholar] [CrossRef]
- Nishioka, S.; Aragane, H.; Suzuki, N.; Yoshimura, Y.; Fujiwara, D.; Mori, T.; Kanehisa, Y.; Iida, Y.; Higashi, K.; Yoshimura-Yokoi, Y.; et al. Clinical practice guidelines for rehabilitation nutrition in cerebrovascular disease, hip fracture, cancer, and acute illness: 2020 update. Clin. Nutr. ESPEN 2021. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Nishioka, E.; Yoshida, T.; Mori, N.; Watanabe, R. Nutritional improvement correlates with recovery of activities of daily living among malnourished elderly stroke patients in the convalescent stage: A cross-sectional study. J. Acad. Nutr. Diet. 2016, 116, 837–843. [Google Scholar] [CrossRef]
- Uno, C.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ogawa, N.; Okamoto, T.; Hoyano, K.; Momosaki, R. Nutritional status change and activities of daily living in elderly pneumonia patients admitted to acute care hospital: A retrospective cohort study from the Japan Rehabilitation Nutrition Database. Nutrition 2020, 71, 110613. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Momosaki, R. Nutritional status changes and activities of daily living after hip fracture in convalescent rehabilitation units: A retrospective observational cohort study from the Japan rehabilitation nutrition database. J. Acad. Nutr. Diet. 2018, 118, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).