Circulating Alpha-Tocopherol Levels, Bone Mineral Density, and Fracture: Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
2.1. Selection of Genetic Variants
2.2. Summary-Level Data for Outcomes
2.3. Two-Sample Summary-Level MR Analysis
2.4. Pleiotropy Assessment
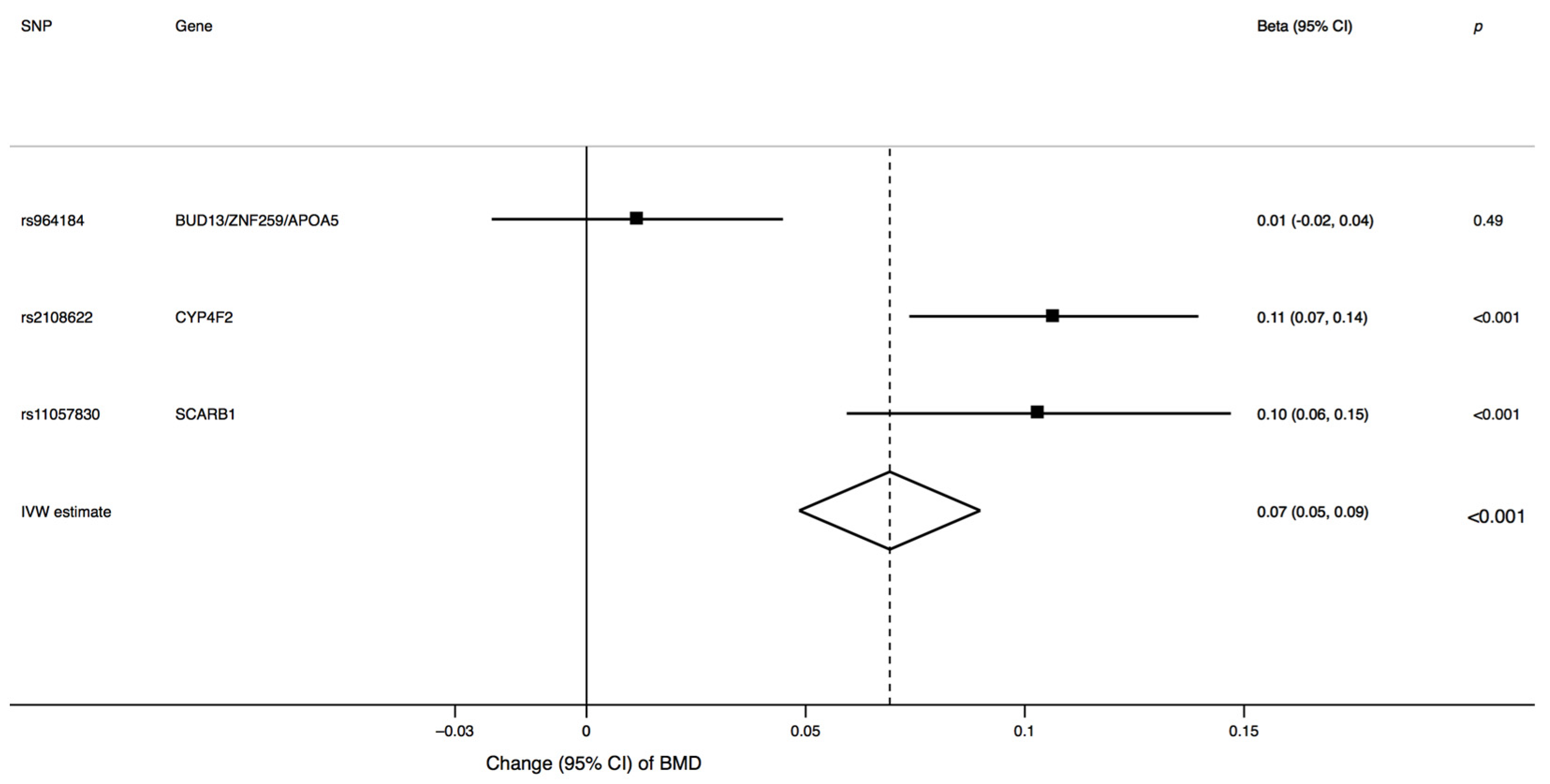
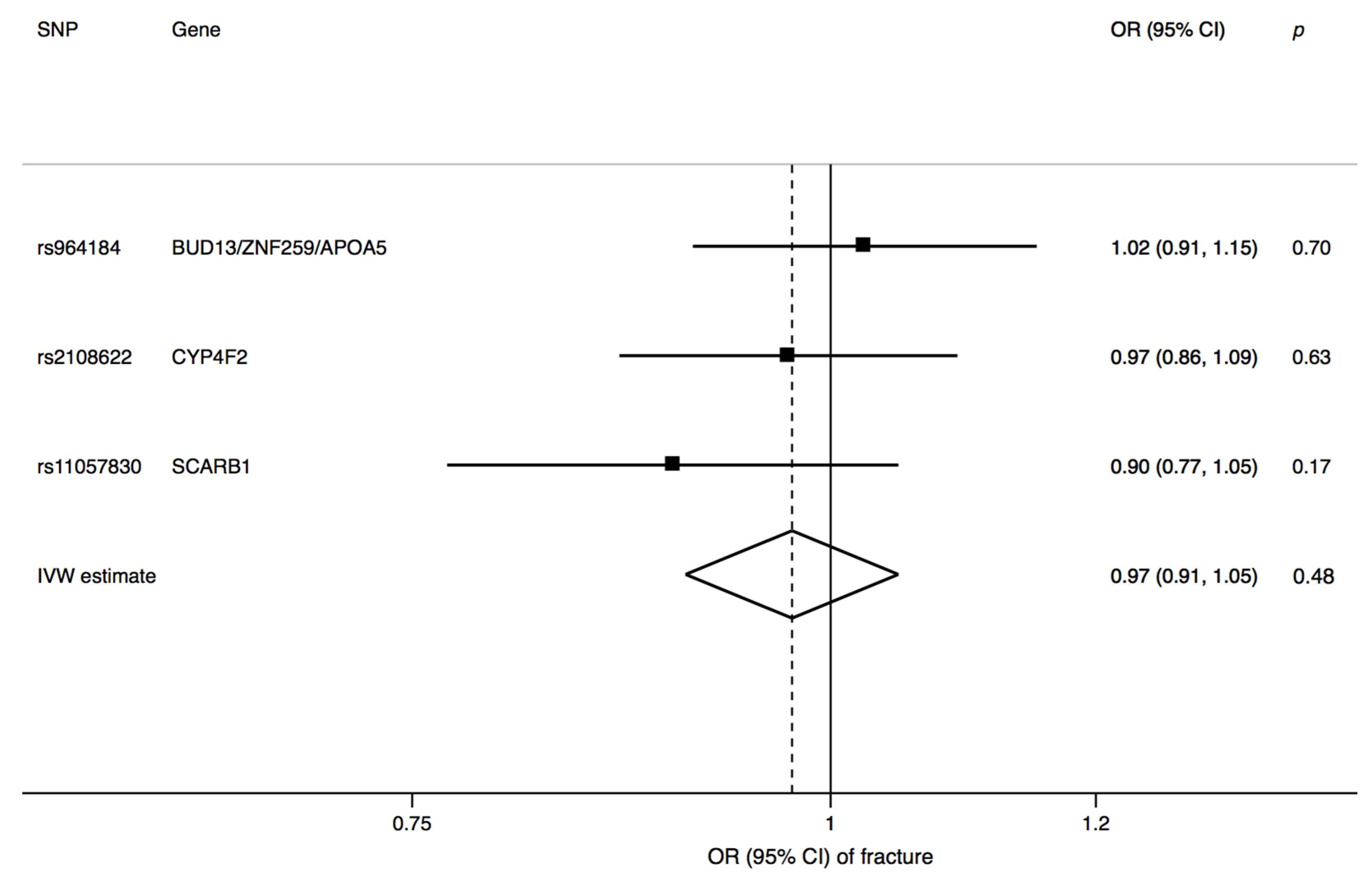
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riggs, B.L.; Melton, L.J.D. The prevention and treatment of osteoporosis. N. Engl. J. Med. 1992, 327, 620–627. [Google Scholar]
- Michaelsson, K.; Nordstrom, P.; Nordstrom, A.; Garmo, H.; Byberg, L.; Pedersen, N.L.; Melhus, H. Impact of hip fracture on mortality: A cohort study in hip fracture discordant identical twins. J. Bone Miner. Res. 2014, 29, 424–431. [Google Scholar] [CrossRef]
- Jarvinen, T.L.; Michaelsson, K.; Jokihaara, J.; Collins, G.S.; Perry, T.L.; Mintzes, B.; Musini, V.; Erviti, J.; Gorricho, J.; Wright, J.M.; et al. Overdiagnosis of bone fragility in the quest to prevent hip fracture. BMJ 2015, 350, h2088. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; Oden, A.; Jonsson, B.; De Laet, C.; Dawson, A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 2000, 27, 585–590. [Google Scholar] [CrossRef]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Seeley, D.G.; Lui, L.Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R. Osteoporotic Fractures Research, G. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef]
- Michaelsson, K.; Melhus, H.; Ferm, H.; Ahlbom, A.; Pedersen, N.L. Genetic liability to fractures in the elderly. Arch. Intern. Med. 2005, 165, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Moayyeri, A.; Hammond, C.J.; Hart, D.J.; Spector, T.D. Effects of age on genetic influence on bone loss over 17 years in women: The Healthy Ageing Twin Study (HATS). J. Bone Miner. Res. 2012, 27, 2170–2178. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. TEM 2010, 21, 369–374. [Google Scholar] [CrossRef]
- Corrado, A.; Cici, D.; Rotondo, C.; Maruotti, N.; Cantatore, F.P. Molecular Basis of Bone Aging. Int. J. Mol. Sci. 2020, 21, 3679. [Google Scholar] [CrossRef]
- Marie, P.J. Bone cell senescence: Mechanisms and perspectives. J. Bone Miner. Res. 2014, 29, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Trajanoska, K.; Morris, J.A.; Oei, L.; Zheng, H.F.; Evans, D.M.; Kiel, D.P.; Ohlsson, C.; Richards, J.B.; Rivadeneira, F.; Consortium, G.G.; et al. Assessment of the genetic and clinical determinants of fracture risk: Genome wide association and mendelian randomisation study. BMJ 2018, 362, k3225. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, F.; Gambassi, G.; Cesari, M. Rationale for antioxidant supplementation in sarcopenia. J. Aging Res. 2012, 2012, 316943. [Google Scholar] [CrossRef]
- Michaelsson, K.; Wolk, A.; Byberg, L.; Arnlov, J.; Melhus, H. Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am. J. Clin. Nutr. 2014, 99, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Mohamad, S.; Muhammad, N.; Fadzilah, F.M.; Mokhtar, S.A.; Mohamed, N.; Soelaiman, I.N. Effects of alpha-tocopherol on the early phase of osteoporotic fracture healing. J. Orthop. Res. 2011, 29, 1732–1738. [Google Scholar] [CrossRef]
- Mehat, M.Z.; Shuid, A.N.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J. Bone Miner. Metab. 2010, 28, 503–509. [Google Scholar] [CrossRef]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594. [Google Scholar] [CrossRef]
- Kasai, S.; Ito, A.; Shindo, K.; Toyoshi, T.; Bando, M. High-Dose alpha-Tocopherol Supplementation Does Not Induce Bone Loss in Normal Rats. PLoS ONE 2015, 10, e0132059. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar] [CrossRef]
- Holvik, K.; Gjesdal, C.G.; Tell, G.S.; Grimnes, G.; Schei, B.; Apalset, E.M.; Samuelsen, S.O.; Blomhoff, R.; Michaelsson, K.; Meyer, H.E. Low serum concentrations of alpha-tocopherol are associated with increased risk of hip fracture. A NOREPOS study. Osteoporos. Int. 2014, 25, 2545–2554. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Hayhoe, R.P.G.; Luben, R.N.; Welch, A.A. Positive Associations of Dietary Intake and Plasma Concentrations of Vitamin E with Skeletal Muscle Mass, Heel Bone Ultrasound Attenuation and Fracture Risk in the EPIC-Norfolk Cohort. Antioxidants 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, C.R.; Shardell, M.D.; Hicks, G.E.; Orwig, D.L.; Hochberg, M.C.; Semba, R.D.; Yu-Yahiro, J.A.; Ferrucci, L.; Magaziner, J.S.; Miller, R.R. Serum vitamin E concentrations among highly functioning hip fracture patients are higher than in nonfracture controls. Nutr. Res. 2011, 31, 205–214. [Google Scholar] [CrossRef]
- Yang, T.C.; Duthie, G.G.; Aucott, L.S.; Macdonald, H.M. Vitamin E homologues alpha- and gamma-tocopherol are not associated with bone turnover markers or bone mineral density in peri-menopausal and post-menopausal women. Osteoporos. Int. 2016, 27, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.L.; Cauley, J.A.; Pettinger, M.; Jackson, R.; Lacroix, A.; Leboff, M.S.; Lewis, C.E.; Nevitt, M.C.; Simon, J.A.; Stone, K.L.; et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the Women’s Health Initiative. Am. J. Clin. Nutr. 2005, 82, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Salvini, S.; van Gils, C.H.; Brustad, M.; Shakya-Shrestha, S.; Buijsse, B.; Verhagen, H.; Touvier, M.; Biessy, C.; Wallstrom, P.; et al. Dietary intakes of retinol, beta-carotene, vitamin D and vitamin E in the European Prospective Investigation into Cancer and Nutrition cohort. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S150–S178. [Google Scholar] [CrossRef]
- Larsson, S.C.; Michaelsson, K.; Burgess, S. Mendelian randomization in the bone field. Bone 2019, 126, 51–58. [Google Scholar] [CrossRef]
- Larsson, S.C.; Melhus, H.; Michaelsson, K. Circulating Serum 25-Hydroxyvitamin D Levels and Bone Mineral Density: Mendelian Randomization Study. J. Bone Miner. Res. 2018, 33, 840–844. [Google Scholar] [CrossRef]
- Larsson, S.C.; Burgess, S.; Michaelsson, K. Association of Genetic Variants Related to Serum Calcium Levels With Coronary Artery Disease and Myocardial Infarction. JAMA 2017, 318, 371–380. [Google Scholar] [CrossRef]
- Stephen Burgess, S.G.T. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation; Chapman and Hall/CRC: Boca Raton, FL, USA, 2015. [Google Scholar]
- Major, J.M.; Yu, K.; Wheeler, W.; Zhang, H.; Cornelis, M.C.; Wright, M.E.; Yeager, M.; Snyder, K.; Weinstein, S.J.; Mondul, A.; et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 2011, 20, 3876–3883. [Google Scholar] [CrossRef]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef]
- Kim, S.K. Identification of 613 new loci associated with heel bone mineral density and a polygenic risk score for bone mineral density, osteoporosis and fracture. PLoS ONE 2018, 13, e0200785. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, C.R.; Miller, R.R.; Shardell, M.D.; Orwig, D.L.; Hochberg, M.C.; Ferrucci, L.; Semba, R.D.; Yu-Yahiro, J.A.; Magaziner, J.; Hicks, G.E. Higher serum concentrations of dietary antioxidants are associated with lower levels of inflammatory biomarkers during the year after hip fracture. Clin. Nutr. 2012. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, C.R.; Miller, R.R.; Hicks, G.E.; Orwig, D.L.; Hochberg, M.C.; Semba, R.D.; Yu-Yahiro, J.A.; Ferrucci, L.; Magaziner, J.; Shardell, M.D. Serum vitamin E concentrations and recovery of physical function during the year after hip fracture. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 784–793. [Google Scholar] [CrossRef]
- Durak, K.; Sonmez, G.; Sarisozen, B.; Ozkan, S.; Kaya, M.; Ozturk, C. Histological assessment of the effect of alpha-tocopherol on fracture healing in rabbits. J. Int. Med. Res. 2003, 31, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Turk, C.; Halici, M.; Guney, A.; Akgun, H.; Sahin, V.; Muhtaroglu, S. Promotion of fracture healing by vitamin E in rats. J. Int. Med. Res. 2004, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kurklu, M.; Yildiz, C.; Kose, O.; Yurttas, Y.; Karacalioglu, O.; Serdar, M.; Deveci, S. Effect of alpha-tocopherol on bone formation during distraction osteogenesis: A rabbit model. J. Orthop. Traumatol. 2011, 12, 153–158. [Google Scholar] [CrossRef]
- Savvidis, M.; Papavasiliou, K.; Taitzoglou, I.; Giannakopoulou, A.; Kitridis, D.; Galanis, N.; Vrabas, I.; Tsiridis, E. Postoperative Administration of Alpha-tocopherol Enhances Osseointegration of Stainless Steel Implants: An In Vivo Rat Model. Clin. Orthop. Relat. Res. 2020, 478, 406–419. [Google Scholar] [CrossRef]
- National Research Council (U.S.). Subcommittee on Laboratory Animal Nutrition. In Nutrient Requirements of Laboratory Animals, 4th rev. ed.; National Academy of Sciences: Washington, DC, USA, 1995; p. xii, 173p. [Google Scholar]
- Yasunaga, T.; Kato, H.; Ohgaki, K.; Inamoto, T.; Hikasa, Y. Effect of vitamin E as an immunopotentiation agent for mice at optimal dosage and its toxicity at high dosage. J. Nutr. 1982, 112, 1075–1084. [Google Scholar] [CrossRef]
- Aburto, A.; Britton, W.M. Effects of different levels of vitamins A and E on the utilization of cholecalciferol by broiler chickens. Poult. Sci. 1998, 77, 570–577. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.V.; Ibrahim, N.; Chin, K.Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef]
- Melhus, H.; Michaelsson, K.; Holmberg, L.; Wolk, A.; Ljunghall, S. Smoking, antioxidant vitamins, and the risk of hip fracture. J. Bone Miner. Res. 1999, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Michaelsson, K.; Olofsson, H.; Johansson, S.; Melhus, H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001, 288, 275–279. [Google Scholar] [CrossRef]
- Ostman, B.; Michaelsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative stress and bone mineral density in elderly men: Antioxidant activity of alpha-tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Burgess, S.; Sluijs, I.; Weiderpass, E.; Consortium, E.-C.; Beulens, J.W.J.; van der Schouw, Y.T. Circulating phylloquinone, inactive Matrix Gla protein and coronary heart disease risk: A two-sample Mendelian Randomization study. Clin. Nutr. 2020, 39, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Smith, C.; McMurdo, M.E.; Paterson, C.R.; Mole, P.A.; Harvey, J.M.; Fenton, S.T.; Prynne, C.J.; Mishra, G.D.; Shearer, M.J. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J. Bone Miner. Res. 2007, 22, 509–519. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Vieth, R.; Thompson, L.; Jamal, S.; et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): A randomized controlled trial. PLoS Med. 2008, 5, e196. [Google Scholar] [CrossRef]
- Mott, A.; Bradley, T.; Wright, K.; Cockayne, E.S.; Shearer, M.J.; Adamson, J.; Lanham-New, S.A.; Torgerson, D.J. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos. Int. 2019, 30, 1543–1559. [Google Scholar] [CrossRef] [PubMed]
- Siggeirsdottir, K.; Aspelund, T.; Sigurdsson, G.; Mogensen, B.; Chang, M.; Jonsdottir, B.; Eiriksdottir, G.; Launer, L.J.; Harris, T.B.; Jonsson, B.Y.; et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur. J. Epidemiol. 2007, 22, 631–639. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaëlsson, K.; Larsson, S.C. Circulating Alpha-Tocopherol Levels, Bone Mineral Density, and Fracture: Mendelian Randomization Study. Nutrients 2021, 13, 1940. https://doi.org/10.3390/nu13061940
Michaëlsson K, Larsson SC. Circulating Alpha-Tocopherol Levels, Bone Mineral Density, and Fracture: Mendelian Randomization Study. Nutrients. 2021; 13(6):1940. https://doi.org/10.3390/nu13061940
Chicago/Turabian StyleMichaëlsson, Karl, and Susanna C. Larsson. 2021. "Circulating Alpha-Tocopherol Levels, Bone Mineral Density, and Fracture: Mendelian Randomization Study" Nutrients 13, no. 6: 1940. https://doi.org/10.3390/nu13061940
APA StyleMichaëlsson, K., & Larsson, S. C. (2021). Circulating Alpha-Tocopherol Levels, Bone Mineral Density, and Fracture: Mendelian Randomization Study. Nutrients, 13(6), 1940. https://doi.org/10.3390/nu13061940






