Vitamin D Boosts Alendronate Tail Effect on Bone Mineral Density in Postmenopausal Women with Osteoporosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kataoka, Y.; Luo, Y.; Chaimani, A.; Onishi, A.; Kimachi, M.; Tsujimoto, Y.; Murad, M.H.; Li, T.; Cipriani, A.; Furukawa, T.A. Cumulative network meta-analyses, practice guidelines, and actual prescriptions for postmenopausal osteoporosis: A meta-epidemiological study. Arch. Osteoporos. 2020, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Cummings, S.R.; Karpf, D.B.; Cauley, J.A.; Thompson, D.E.; Nevitt, M.C.; Bauer, D.C.; Genant, H.K.; Haskell, W.L.; Marcus, R.; et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996, 348, 1535–1541. [Google Scholar] [CrossRef]
- Cummings, S.R.; Black, D.M.; Thompson, D.E.; Applegate, W.B.; Barrett-Connor, E.; Musliner, T.A.; Palermo, L.; Prineas, R.; Rubin, S.M.; Scott, J.C.; et al. Effect of Alendronate on Risk of Fracture in Women With Low Bone Density but Without Vertebral FracturesResults From the Fracture Intervention Trial. JAMA 1998, 280, 2077–2082. [Google Scholar] [CrossRef]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; McKeever, C.D.; Hangartner, T.; Keller, M.; Iii, C.H.C.; Brown, J.; Eriksen, E.F.; Hoseyni, M.S.; et al. Effects of Risedronate Treatment on Vertebral and Nonvertebral Fractures in Women With Postmenopausal OsteoporosisA Randomized Controlled Trial. JAMA 1999, 282, 1344–1352. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Minne, H.W.; Sorensen, O.H.; Hooper, M.; Roux, C.; Brandi, M.L.; Lund, B.; Ethgen, D.; Pack, S.; Roumagnac, I.; et al. Randomized Trial of the Effects of Risedronate on Vertebral Fractures in Women with Established Postmenopausal Osteoporosis. Osteoporos. Int. 2000, 11, 83–91. [Google Scholar] [CrossRef]
- McClung, M.R.; Geusens, P.; Miller, P.D.; Zippel, H.; Bensen, W.G.; Roux, C.; Adami, S.; Fogelman, I.; Diamond, T.; Eastell, R.; et al. Effect of Risedronate on the Risk of Hip Fracture in Elderly Women. N. Engl. J. Med. 2001, 344, 333–340. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.L.; Leung, P.C.; Man, Z.; et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef]
- Chesnut, C.H.; Skag, A.; Christiansen, C.; Recker, R.; Stakkestad, J.A.; Hoiseth, A.; Felsenberg, D.; Huss, H.; Gilbride, J.; Schimmer, R.C.; et al. Effects of Oral Ibandronate Administered Daily or Intermittently on Fracture Risk in Postmenopausal Osteoporosis. J. Bone Miner. Res. 2004, 19, 1241–1249. [Google Scholar] [CrossRef]
- Bone, H.G.; Hosking, D.; Devogelaer, J.-P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C.; et al. Ten Years’ Experience with Alendronate for Osteoporosis in Postmenopausal Women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Schwartz, A.V.; Ensrud, K.; Cauley, J.A.; Levis, S.; Quandt, S.A.; Satterfield, S.; Wallace, R.B.; Bauer, D.C.; Palermo, L.; et al. Effects of Continuing or Stopping Alendronate After 5 Years of Treatment. JAMA 2006, 296, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Reid, I.R.; Cauley, J.A.; Cosman, F.; Leung, P.C.; Lakatos, P.; Lippuner, K.; Cummings, S.R.; Hue, T.F.; Mukhopadhyay, A.; et al. The Effect of 6 versus 9 Years of Zoledronic Acid Treatment in Osteoporosis: A Randomized Second Extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2015, 30, 934–944. [Google Scholar] [CrossRef]
- Mellström, D.D.; Sorensen, O.H.; Goemaere, S.; Roux, C.; Johnson, T.D.; Chines, A.A. Seven Years of Treatment with Risedronate in Women with Postmenopausal Osteoporosis. Calcif. Tissue Int. 2004, 75, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the american society for bone and mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef] [PubMed]
- Gatti, D.; Adami, S.; Viapiana, O.; Rossini, M. The use of bisphosphonates in women: When to use and when to stop. Expert Opin. Pharmacother. 2015, 16, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Wong, J.B.; Giovannucci, E.; Dietrich, T.; Dawson-Hughes, B. Fracture Prevention With Vitamin D Supplementation. JAMA 2005, 293, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Orav, E.J.; Lips, P.; Meunier, P.J.; Lyons, R.A.; Flicker, L.; Wark, J.; Jackson, R.D.; Cauley, J.A.; et al. A Pooled Analysis of Vitamin D Dose Requirements for Fracture Prevention. N. Engl. J. Med. 2012, 367, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Wong, J.B.; Stuck, A.E.; Staehelin, H.B.; Orav, E.J.; Thoma, A.; Kiel, D.; Henschkowski, J. Prevention of Nonvertebral Fractures With Oral Vitamin D and Dose Dependency. Arch. Intern. Med. 2009, 169, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Mirarchi, L.; Venanzi-Rullo, E.; Santoro, D.; Iaria, C.; Catalano, A.; Lasco, A.; Arcoraci, V.; Gullo, A.L.; Bitto, A.; et al. Vitamin D Status and the Relationship with Bone Fragility Fractures in HIV-Infected Patients: A Case Control Study. Int. J. Mol. Sci. 2018, 19, 119. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53. [Google Scholar]
- Cesari, M.; Incalzi, R.A.; Zamboni, V.; Pahor, M. Vitamin D hormone: A multitude of actions potentially influencing the physical function decline in older persons. Geriatr. Gerontol. Int. 2010, 11, 133–142. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; Fuleihan, G.E.-H.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- Holick, M.F.; Siris, E.S.; Binkley, N.; Beard, M.K.; Khan, A.; Katzer, J.T.; Petruschke, R.A.; Chen, E.; de Papp, A.E. Prevalence of Vitamin D Inadequacy among Postmenopausal North American Women Receiving Osteoporosis Therapy. J. Clin. Endocrinol. Metab. 2005, 90, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Isaia, G.; Luisetto, G.; Minisola, S.; Sinigaglia, L.; Gentilella, R.; Agnusdei, D.; Iori, N.; Nuti, R. Fracture Incidence and Characterization in Patients on Osteoporosis Treatment: The ICARO Study. J. Bone Miner. Res. 2006, 21, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Giannini, S.; Bianchi, G.; Sinigaglia, L.; Di Munno, O.; Fiore, C.E.; Minisola, S.; Rossini, M. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos. Int. 2008, 20, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, M.; Sakamoto, Y.; Yamanaka, M.; Tokita, A.; Kitahara, K.; Kaneko, H.; Kurosawa, H. Minimum Required Vitamin D Level for Optimal Increase in Bone Mineral Density with Alendronate Treatment in Osteoporotic Women. Calcif. Tissue Int. 2009, 85, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Morabito, N.; Di Stefano, A.; Morini, E.; Basile, G.; Faraci, B.; Loddo, S.; Ientile, R.; Lasco, A. Vitamin D and bone mineral density changes in postmenopausal women treated with strontium ranelate. J. Endocrinol. Investig. 2015, 38, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Bauer, D.C.; Vittinghoff, E.; Lui, L.-Y.; Grauer, A.; Marin, F.; Khosla, S.; de Papp, A.; Mitlak, B.; Cauley, J.A.; et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: Meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 672–682. [Google Scholar] [CrossRef]
- Calabria, S.; Cinconze, E.; Rossini, M.; Rossi, E.; Maggioni, A.P.; Pedrini, A.; De Rosa, M. Adherence to alendronic or risedronic acid treatment, combined or not to calcium and vitamin D, and related determinants in Italian patients with osteoporosis. Patient Prefer. Adherence 2016, 10, 523–530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minisola, S.; Colangelo, L.; Pepe, J.; Occhiuto, M.; Piazzolla, V.; Renella, M.; Biamonte, F.; Sonato, C.; Cilli, M.; Cipriani, C. Vitamin D screening. J. Endocrinol. Investig. 2020, 43, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Giusti, A.; Pioli, G.; Girasole, G.; Razzano, M.; Pizzonia, M.; Palummeri, E.; Bianchi, G. Secondary Hyperparathyroidism Due to Hypovitaminosis D Affects Bone Mineral Density Response to Alendronate in Elderly Women with Osteoporosis: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2007, 55, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Morabito, N.; Atteritano, M.; Basile, G.; Cucinotta, D.; Lasco, A. Vitamin D Reduces Musculoskeletal Pain After Infusion of Zoledronic Acid for Postmenopausal Osteoporosis. Calcif. Tissue Int. 2012, 90, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Giusti, A.; Barone, A.; Razzano, M.; Pizzonia, M.; Oliveri, M.; Palummeri, E.; Pioli, G. High prevalence of secondary hyperparathyroidism due to hypovitaminosis D in hospitalized elderly with and without hip fracture. J. Endocrinol. Investig. 2006, 29, 809–813. [Google Scholar] [CrossRef]
- Atteritano, M.; Lasco, A.; Mazzaferro, S.; Macrì, I.; Catalano, A.; Santangelo, A.; Bagnato, G.; Bagnato, G.; Frisina, N. Bone mineral density, quantitative ultrasound parameters and bone metabolism in postmenopausal women with depression. Intern. Emerg. Med. 2011, 8, 485–491. [Google Scholar] [CrossRef]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.-J.; Rodriguez, E.G.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guañabens, N.; et al. Fracture Risk and Management of Discontinuation of Denosumab Therapy: A Systematic Review and Position Statement by ECTS. J. Clin. Endocrinol. Metab. 2021, 106, 264–281. [Google Scholar] [CrossRef]
- Bandeira, F.; Dantas, W.; Bilezikian, J.P. Controversies in the treatment of postmenopausal osteoporosis: How long to treat with bisphosphonates? Arch. Endocrinol. Metab. 2020, 64, 331–336. [Google Scholar] [CrossRef]
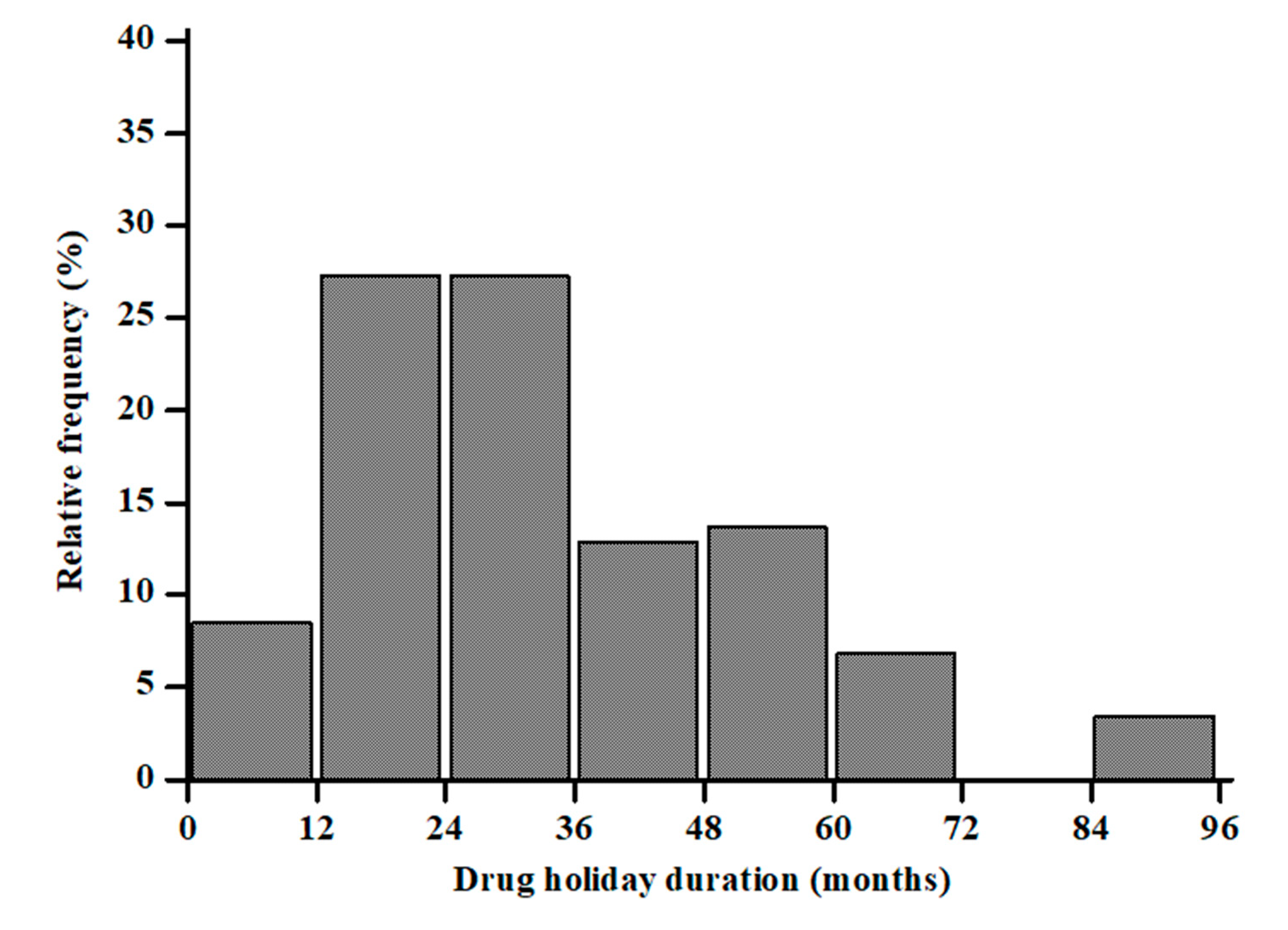
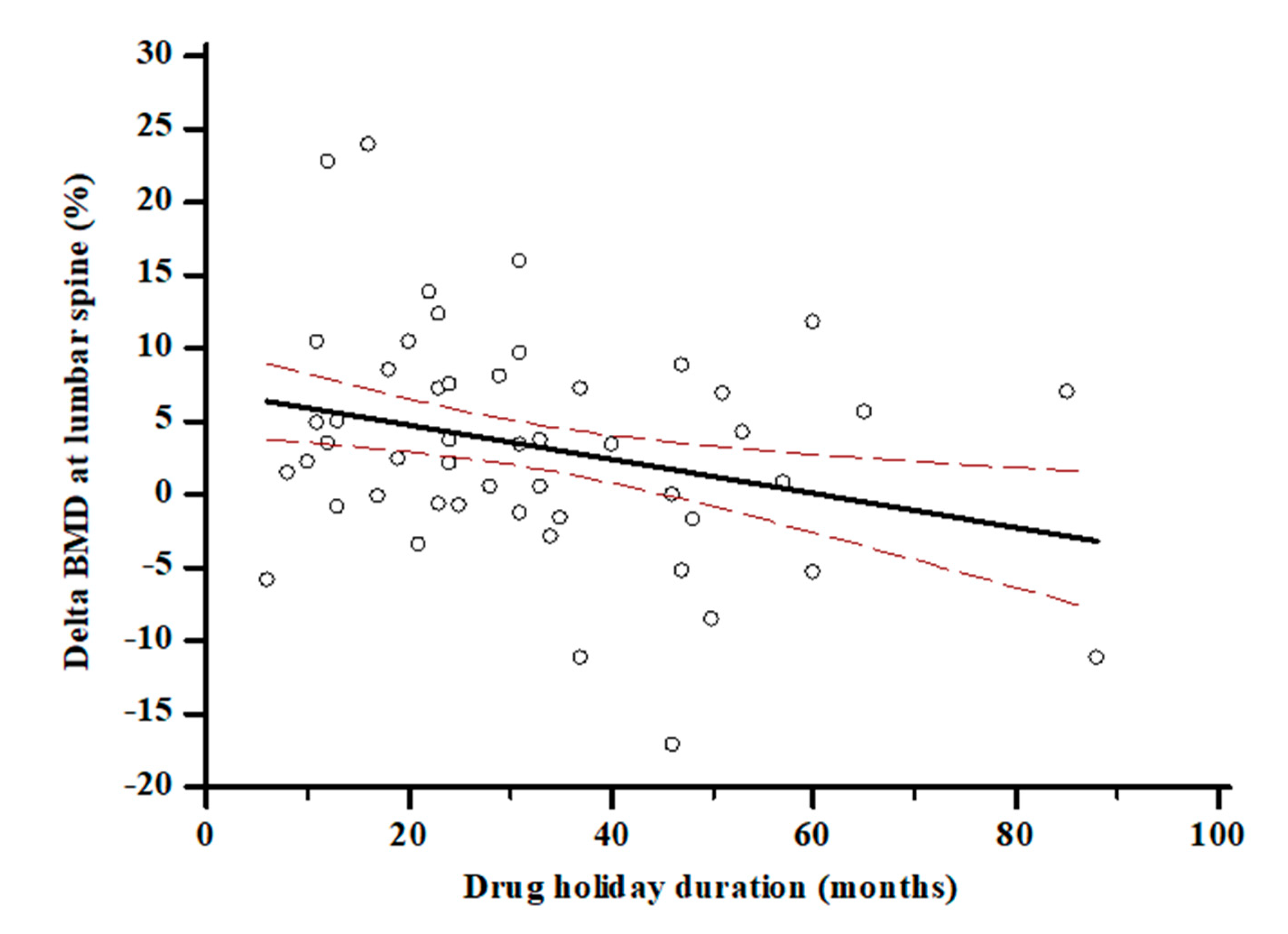
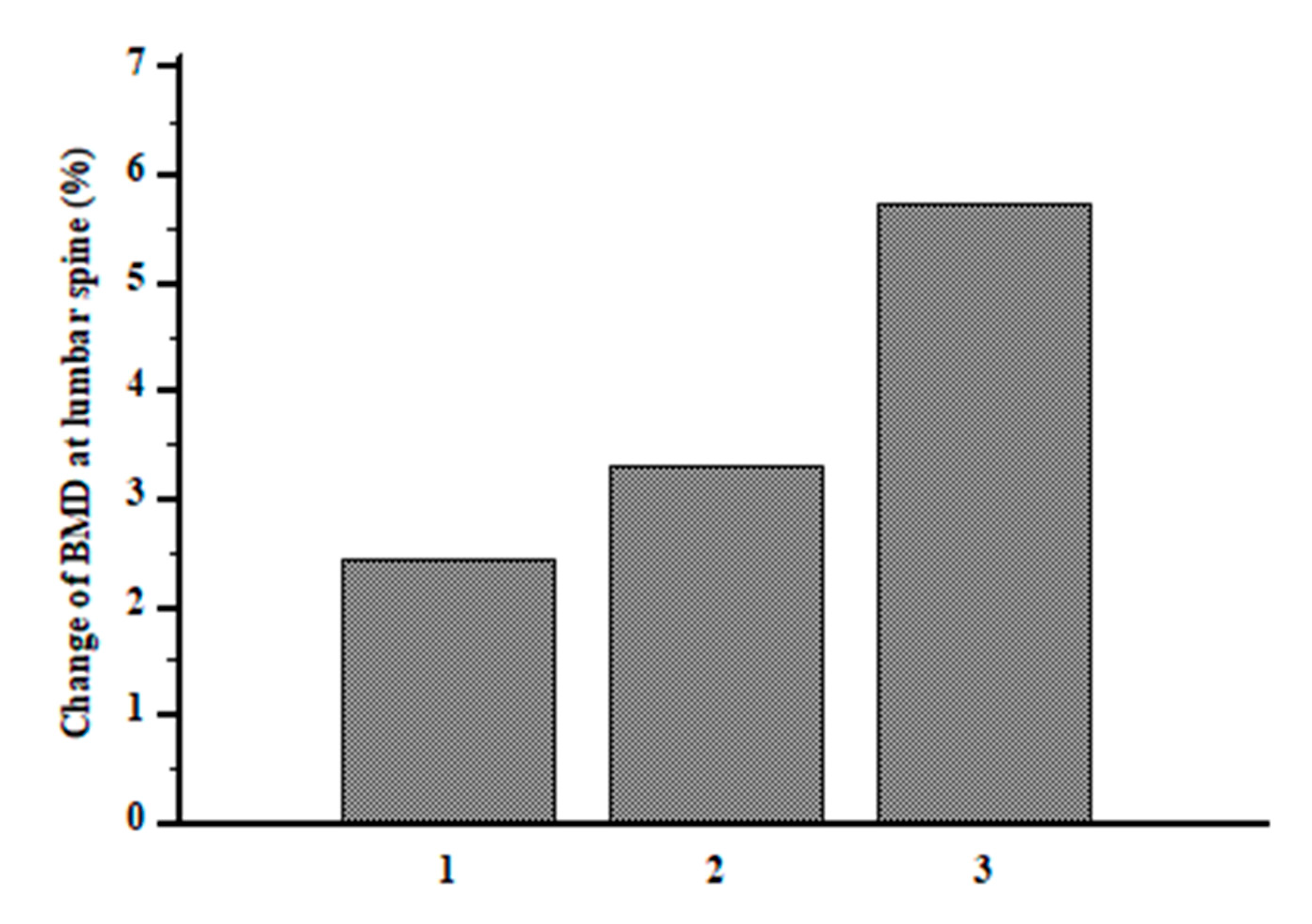
|
Whole Population (n = 96) |
ΔD1 (n = 31) |
ΔD2 (n = 33) |
ΔD3 (n = 32) | |
|---|---|---|---|---|
| Main clinical characteristics | ||||
| Age (years) | 61.1 ± 6.9 | 58.9 ± 6.9 | 62.5 ± 6.6 * | 62.1 ± 7.0 |
| BMI (kg/m2) | 25.7 ± 3.4 | 24.5 ± 2.5 | 26.6 ± 4.5 | 25.6 ± 2.9 |
| Age at menopause (years) | 47.4 ± 4.9 | 47.7 ± 5.1 | 47.3 ± 5.6 | 47.4 ± 4.3 |
| Time since menopause (years) | 13.2 ± 6.8 | 10.6 ± 5.9 | 14.4 ± 6.0 * | 14.2 ± 7.9 # |
| Ten year probability of major fractures (%) | 18.3 ± 11.51 | 16.5 ± 8.9 | 20.9 ± 11.7 | 17.9 ± 13.7 |
| Ten year probability of hip fractures (%) | 8.6 ± 10.5 | 6.2 ± 4.9 | 10.9 ± 10.9 * | 9.0 ± 13.8 |
| ALN treatment duration (months) | 31.2 ± 20.6 | 35.7 ± 23.5 | 31.3 ± 22.0 | 26.1 ± 14.4 # |
| Drug holiday (months) | 33.3 ± 18.9 | 34.1 ± 16.9 | 31.9 ± 20.7 | 34.4 ± 19.4 |
| DXA measurements | ||||
| L1–L4 BMD (g/cm2) | 0.70 ± 0.32 | 0.74 ± 0.28 | 0.69 ± 0.31 | 0.66 ± 0.37 |
| Femoral neck BMD (g/cm2) | 0.53 ± 0.28 | 0.63 ± 0.16 | 0.57 ± 0.26 | 0.45 ± 0.31# |
| Bone metabolism | ||||
| CTX (ng/mL) | 0.57 ± 0.33 | 0.40 ± 0.28 | 0.58 ± 0.32 | 0.66 ± 0.38 |
| ALP (U/L) | 76.23 ± 35.39 | 68.18 ± 19.00 | 63.6 ± 19.3 | 99.76 ± 50.96 *,# |
| 25(OH)D (ng/mL) at the time of ALN discontinuation | 33.75 ± 15.47 | 46.64 ± 17.2 | 33.62 ± 7.18 * | 21.84 ± 8.97 *,# |
| 25(OH)D (ng/mL) at the end of the observation period (†) | 44.81 ± 13.89 | 38.58 ± 10.79 | 43.53 ± 8.68 * | 51.12 ± 11.62 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, A.; Bellone, F.; Santoro, D.; Schwarz, P.; Gaudio, A.; Basile, G.; Sottile, M.C.; Stoian, S.A.; Corica, F.; Morabito, N. Vitamin D Boosts Alendronate Tail Effect on Bone Mineral Density in Postmenopausal Women with Osteoporosis. Nutrients 2021, 13, 1878. https://doi.org/10.3390/nu13061878
Catalano A, Bellone F, Santoro D, Schwarz P, Gaudio A, Basile G, Sottile MC, Stoian SA, Corica F, Morabito N. Vitamin D Boosts Alendronate Tail Effect on Bone Mineral Density in Postmenopausal Women with Osteoporosis. Nutrients. 2021; 13(6):1878. https://doi.org/10.3390/nu13061878
Chicago/Turabian StyleCatalano, Antonino, Federica Bellone, Domenico Santoro, Peter Schwarz, Agostino Gaudio, Giorgio Basile, Maria Carmela Sottile, Sabrina Atena Stoian, Francesco Corica, and Nunziata Morabito. 2021. "Vitamin D Boosts Alendronate Tail Effect on Bone Mineral Density in Postmenopausal Women with Osteoporosis" Nutrients 13, no. 6: 1878. https://doi.org/10.3390/nu13061878
APA StyleCatalano, A., Bellone, F., Santoro, D., Schwarz, P., Gaudio, A., Basile, G., Sottile, M. C., Stoian, S. A., Corica, F., & Morabito, N. (2021). Vitamin D Boosts Alendronate Tail Effect on Bone Mineral Density in Postmenopausal Women with Osteoporosis. Nutrients, 13(6), 1878. https://doi.org/10.3390/nu13061878









