Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups, Diets, and Procedures
2.3. Quantitative Reverse Transcription Polymerase Chain Reaction
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Western Blotting
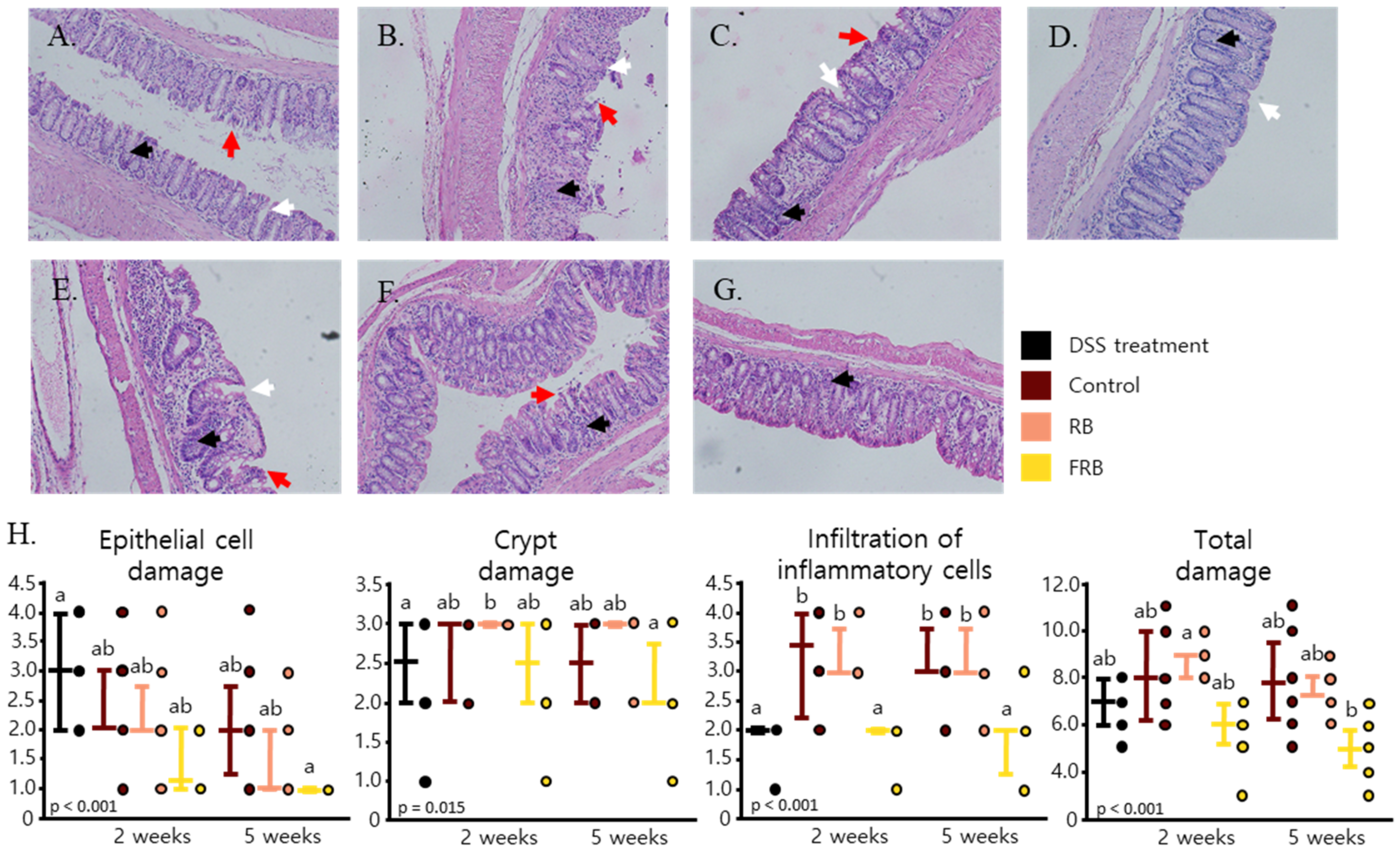
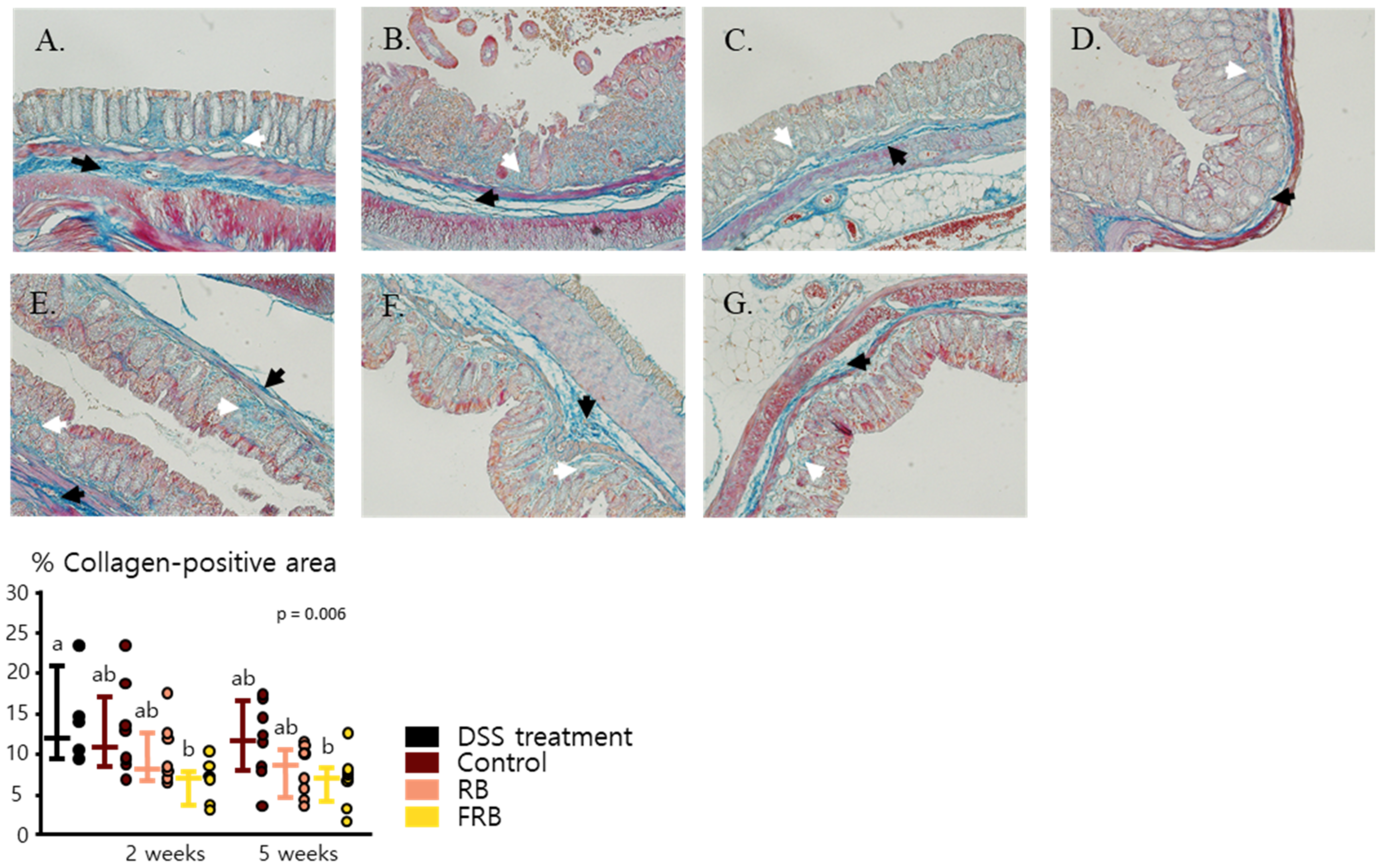
2.6. Histological Analysis
2.7. Statistical Analysis
3. Results
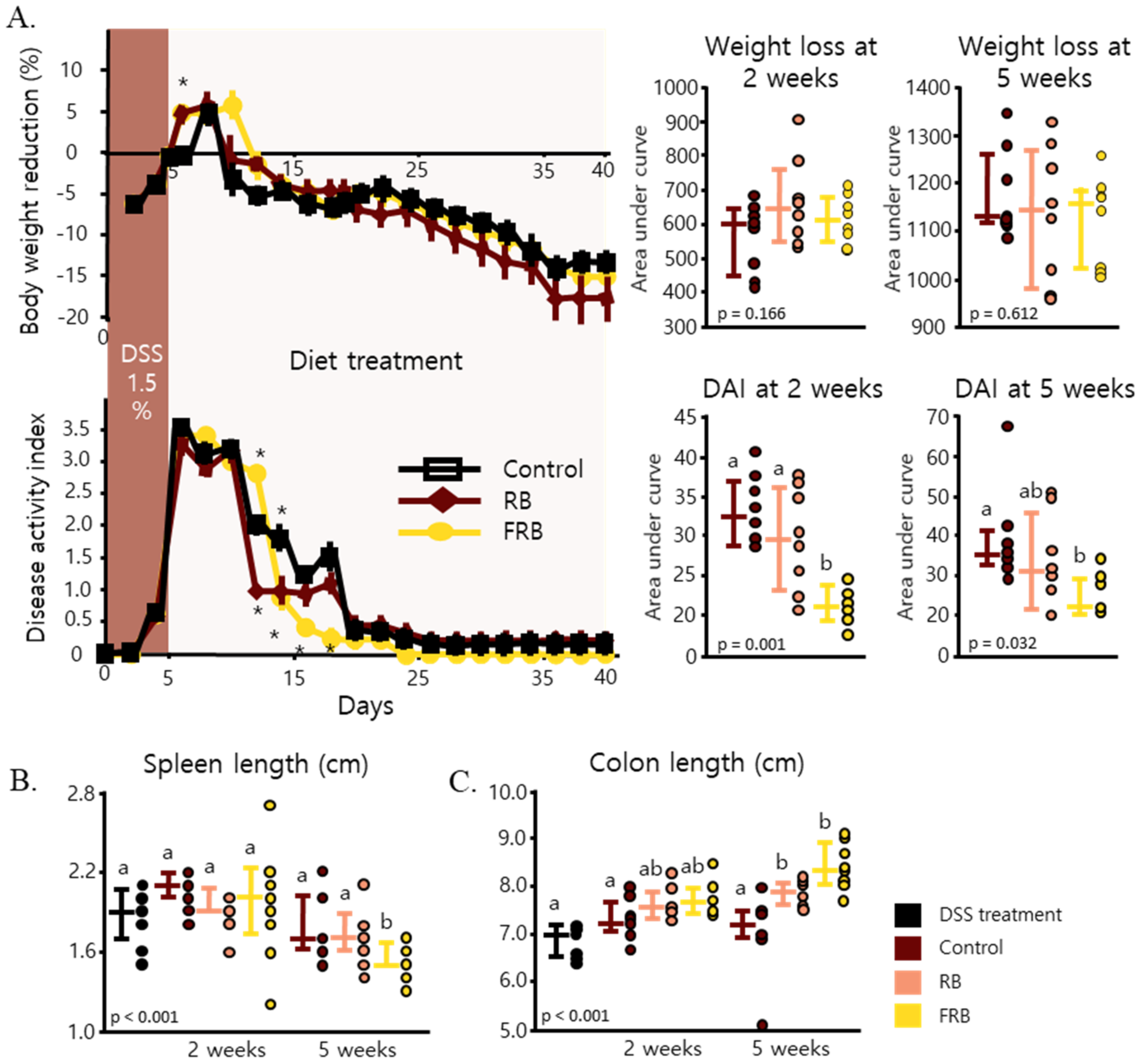
3.1. General Observation of Colitis
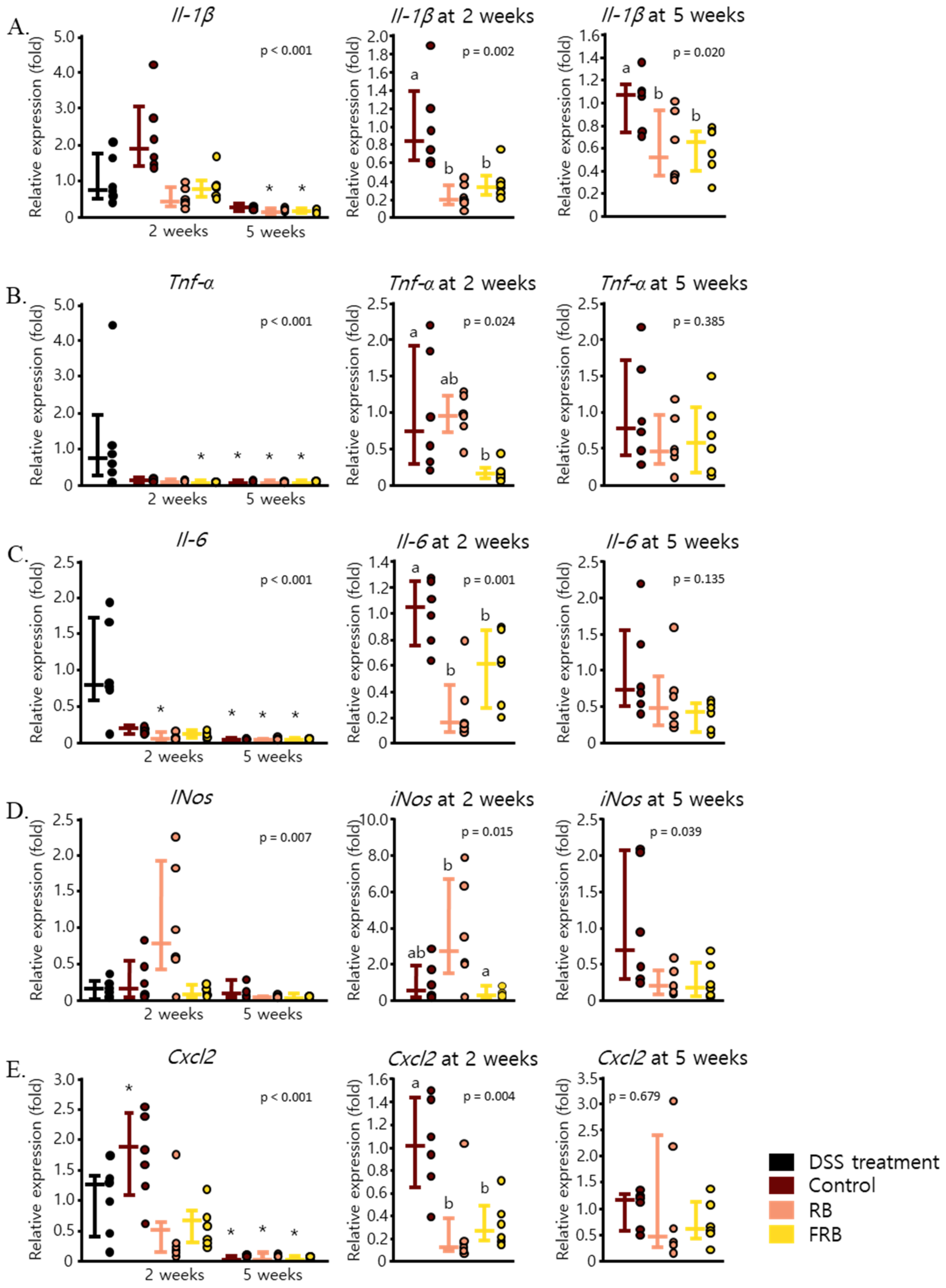
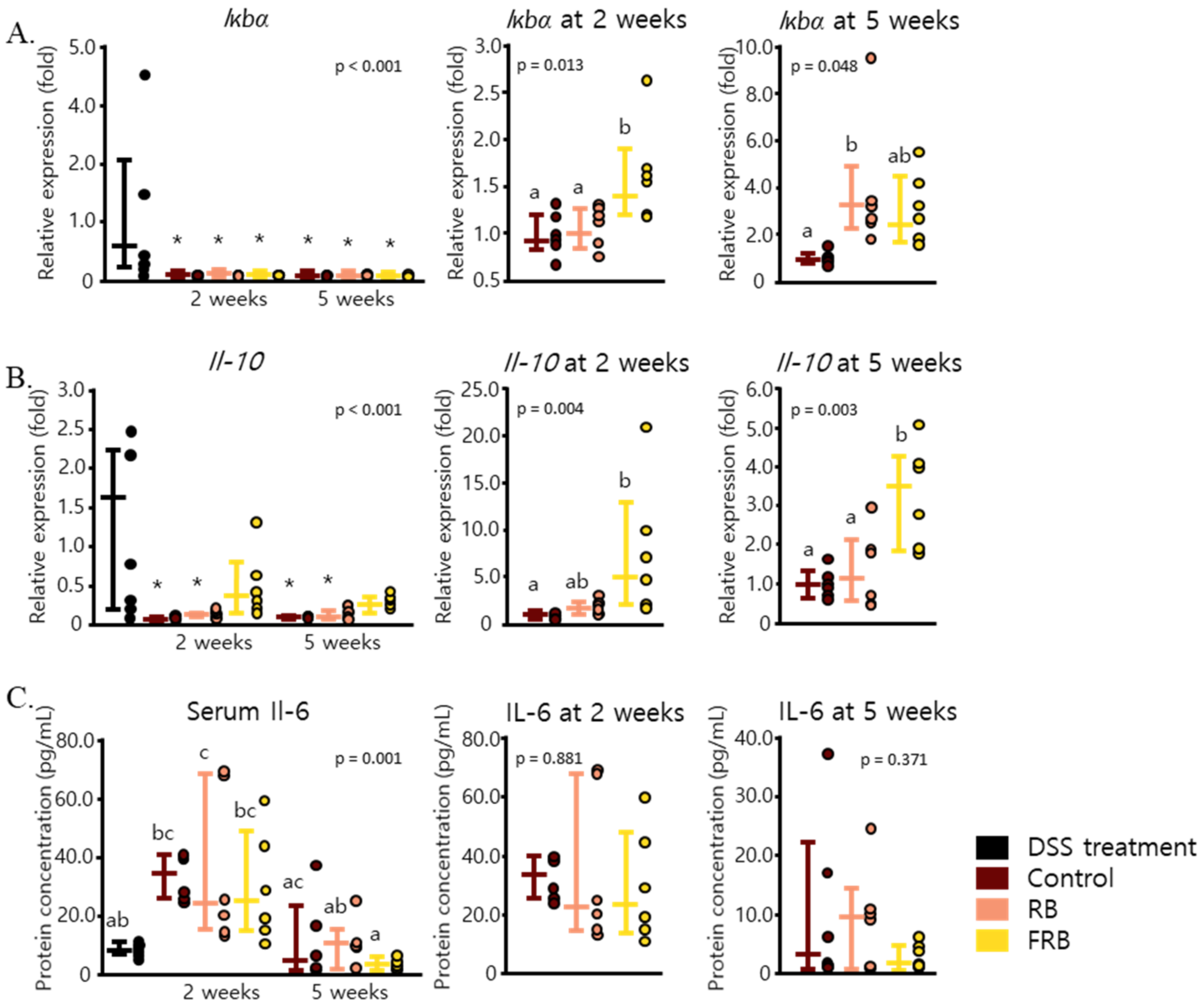
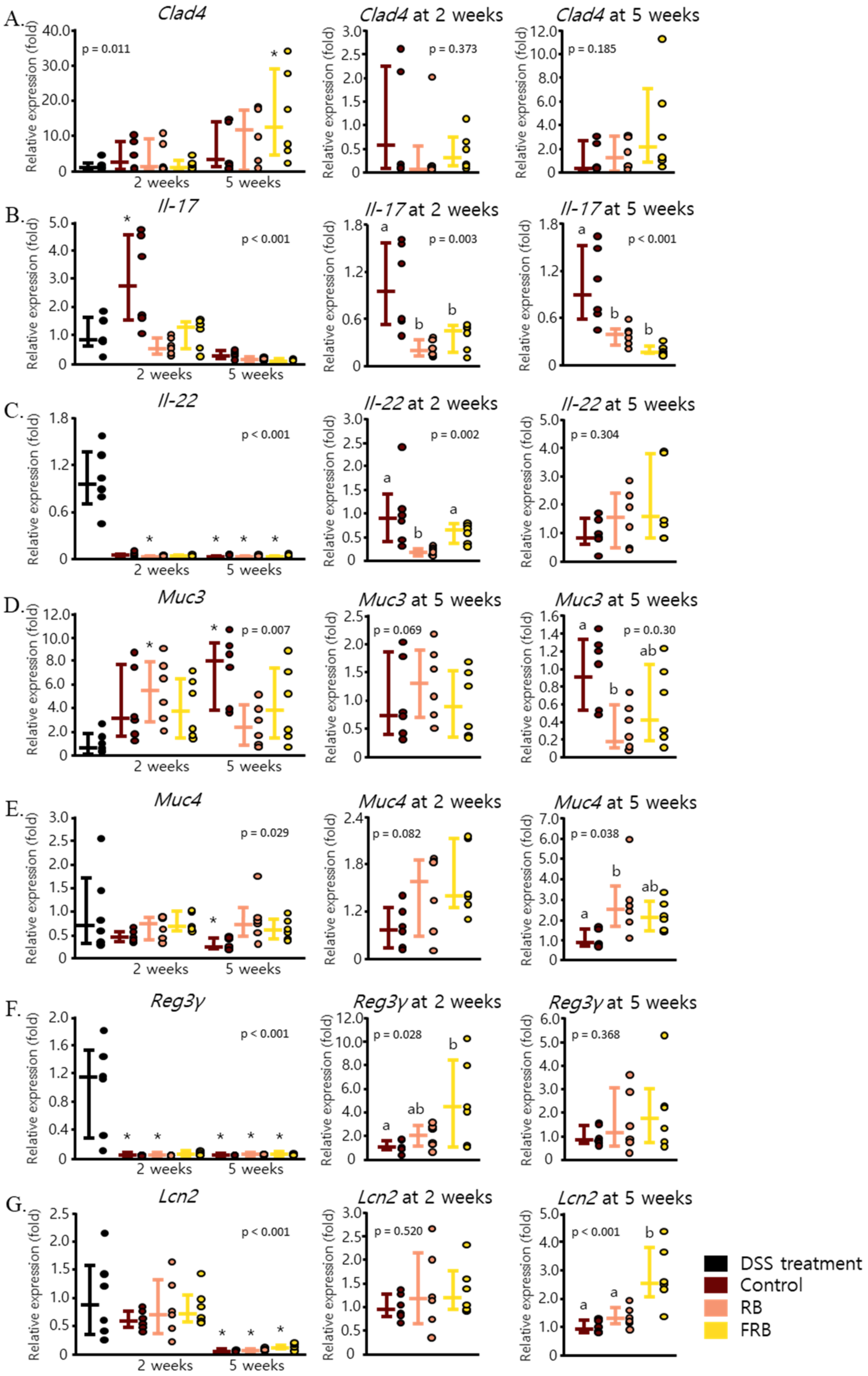
3.2. RB and FRB Supplementation Reduces Pro-Inflammatory Cytokine Levels and Increases Anti-Inflammatory Cytokine Levels in Mice Intestines
3.3. Effect of RB and FRB Supplementation on Intestinal Barrier Function
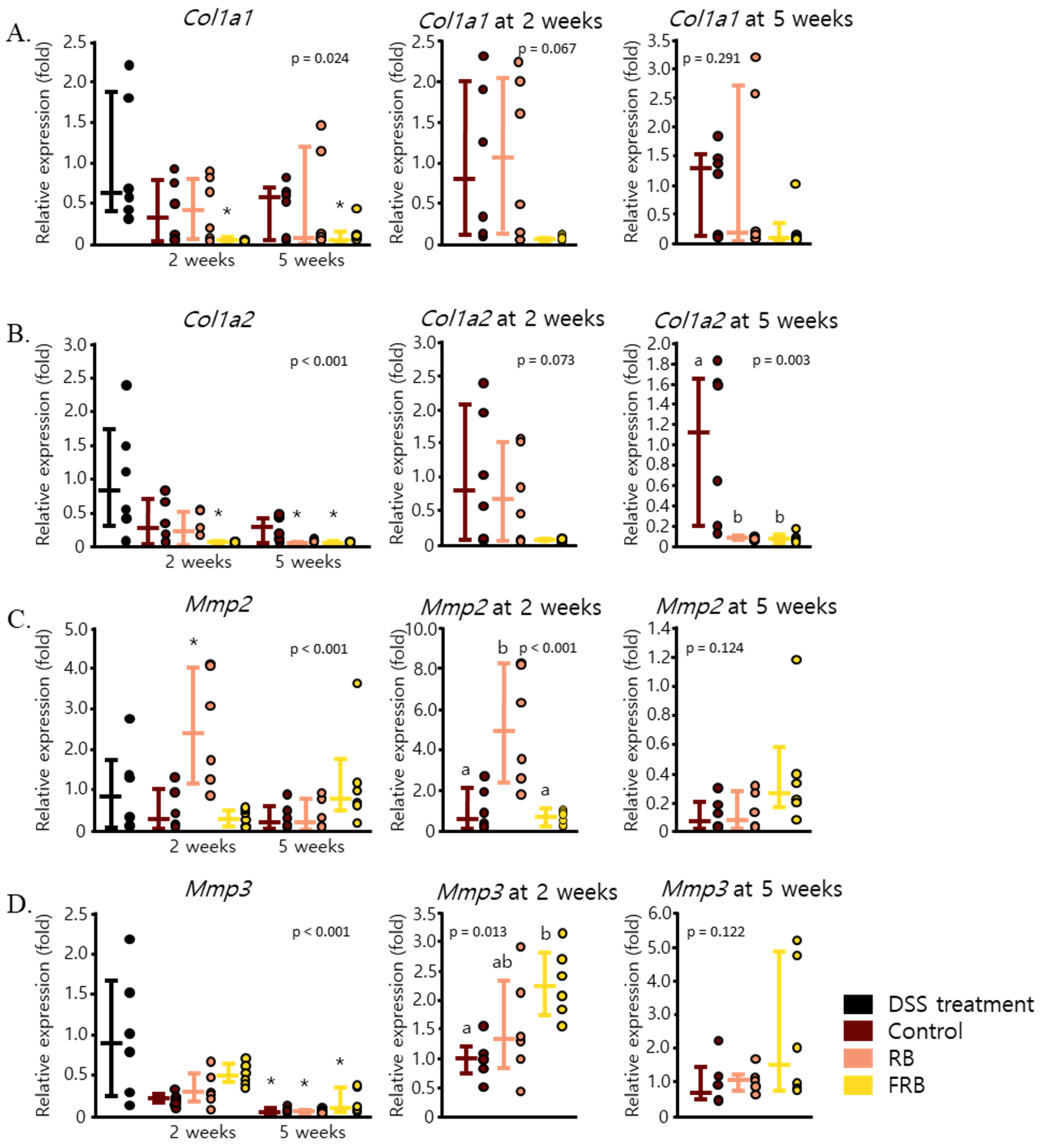
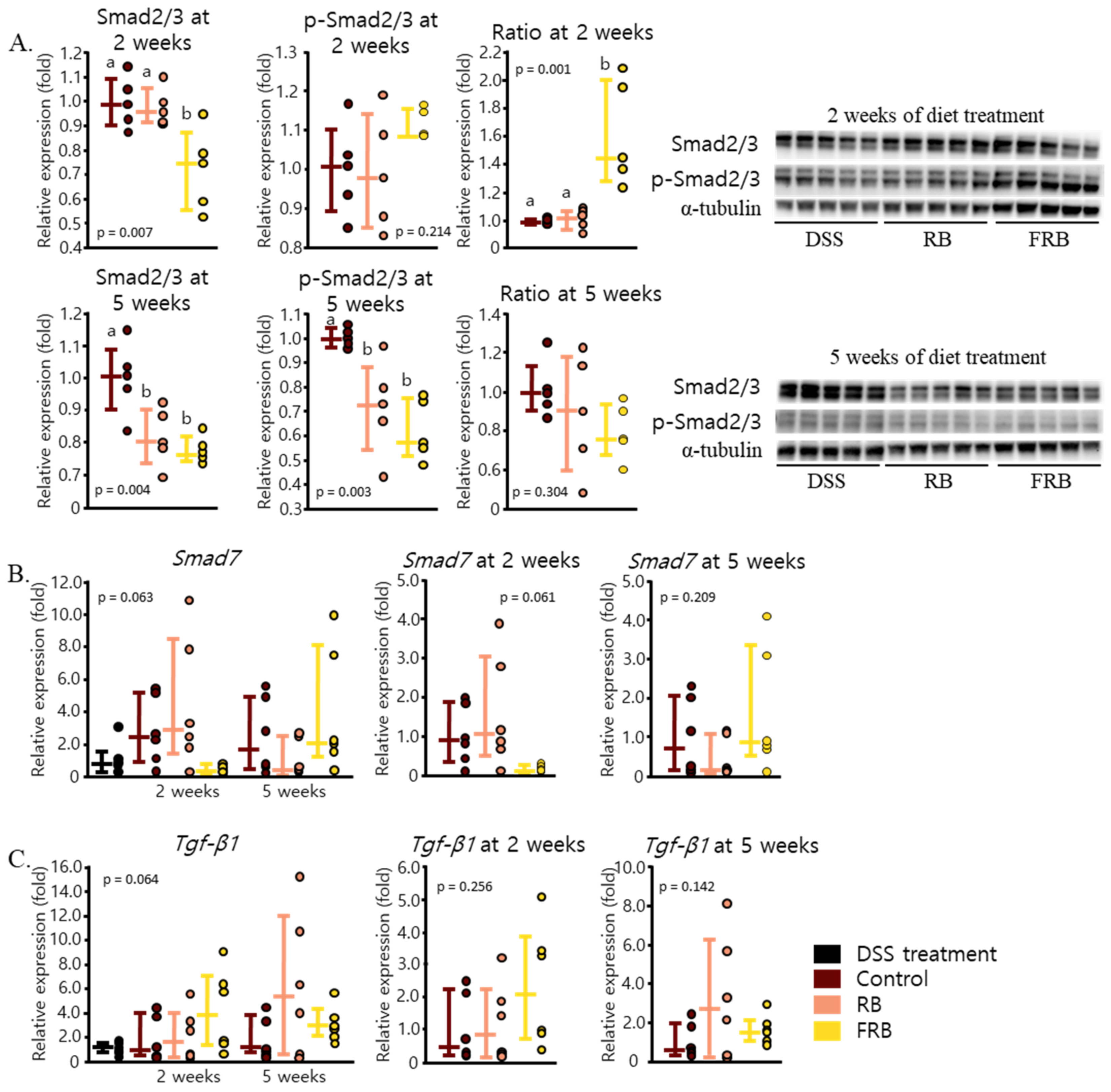
3.4. RB and FRB Supplementation Regulates Extracellular Matrix Deposition in the Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamabe, K.; Liebert, R.; Flores, N.; Pashos, C.L. Health-related quality of life outcomes and economic burden of inflammatory bowel disease in japan. Clin. Outcomes Res. 2019, 11, 221–232. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Tanaka, H.; Sakurai, Y.; Tange, K.; Nakai, Y.; Ohkawara, T.; Takeda, H.; Harashima, H.; Akita, H. Effect of particle size on their accumulation in an inflammatory lesion in a dextran sulfate sodium (DSS)-induced colitis model. Int. J. Pharm. 2016, 509, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary supplementation of fermented rice bran effectively alleviates dextran sodium sulfate-induced colitis in mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah; Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gottfries, J.; Melgar, S.; Michaëlsson, E. Modelling of mouse experimental colitis by global property screens: A holistic approach to assess drug effects in inflammatory bowel disease. PLoS ONE 2012, 7, e30005. [Google Scholar] [CrossRef] [PubMed]
- Iraporda, C.; Romanin, D.E.; Bengoa, A.A.; Errea, A.J.; Cayet, D.; Foligné, B.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Local treatment with lactate prevents intestinal inflammation in the TNBS-induced colitis model. Front. Immunol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.V.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, P.; Sun, Y.; Sun, J.; Dong, J.N.; Wang, H.G.; Zuo, L.G.; Gong, J.F.; Li, Y.; Gu, L.L.; et al. DHA protects against experimental colitis in IL-10-deficient mice associated with the modulation of intestinal epithelial barrier function. Br. J. Nutr. 2015, 114, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Shailubhai, K. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Melgar, S.; Karlsson, A.; Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Walton, K.L.W.; Blue, R.E.; MacNaughton, K.; Magness, S.T.; Lund, P.K. Mucosal healing and fibrosis after acute or chronic inflammation in wild type FVB-N mice and C57BL6 procollagen α1(I)-promoter-GFP reporter mice. PLoS ONE 2012, 7, e42568. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.; Sans, M.; Fiocchi, C. Animal models of intestinal fibrosis: New tools for the understanding of pathogenesis and therapy of human disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 786–801. [Google Scholar] [CrossRef]
- Hefnawy, H.T.M.; El-shourbagy, G.A. Chemical Analysis and Antioxidant Activity of Polysaccharide Extracted from Rice Bran. World J. Dairy Food Sci. 2014, 9, 95–104. [Google Scholar] [CrossRef]
- Park, H.Y.; Yu, A.R.; Choi, I.W.; Do Hong, H.; Lee, K.W.; Choi, H.D. Immunostimulatory effects and characterization of a glycoprotein fraction from rice bran. Int. Immunopharmacol. 2013, 17, 191–197. [Google Scholar] [CrossRef]
- Justo, M.L.; Candiracci, M.; Dantas, A.P.; de Sotomayor, M.A.; Parrado, J.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Rice bran enzymatic extract restores endothelial function and vascular contractility in obese rats by reducing vascular inflammation and oxidative stress. J. Nutr. Biochem. 2013, 24, 1453–1461. [Google Scholar] [CrossRef]
- Rashid, N.Y.A.; Razak, D.L.A.; Jamaluddin, A.; Sharifuddin, S.A.; Long, K. Bioactive compounds and antioxidant activity of rice bran fermented with lactic acid bacteria. Malays. J. Microbiol. 2015, 11, 156–162. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Ardiansyah; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Kang, C.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Chen, J.; Mitra, S.; Sarna, S.K. Impaired Integrity of DNA after Recovery from Inflammation Causes Persistent Dysfunction of Colonic Smooth Muscle. Gastroenterology 2008, 141, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Billioud, V.; Sachar, D.B.; Peyrin-biroulet, L. Ulcerative Colitis as a Progressive Disease: The Forgotten Evidence. Inflamm. Bowel Dis. 2012, 18, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Kersten, C.; Schmied, A.; Vieth, M.; Primbs, T.; Carlé, B.; Knieling, F.; Claussen, J.; Klimowicz, A.C.; Zheng, J.; et al. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice With Chronic Intestinal Inflammation. Gastroenterology 2019, 156, 1082–1097.e11. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Di Sabatino, A. Intestinal fibrosis. Mol. Asp. Med. 2019, 65, 100–109. [Google Scholar] [CrossRef]
- Latella, G.; Rieder, F. Intestinal fibrosis. Curr. Opin. Gastroenterol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef]
- Van Halsema, E.E.; Van Hooft, J.E.; Small, A.J.; Baron, T.H.; García-Cano, J.; Cheon, J.H.; Lee, M.S.; Kwon, S.H.; Mucci-Hennekinne, S.; Fockens, P.; et al. Perforation in colorectal stenting: A meta-analysis and a search for risk factors. Gastrointest. Endosc. 2014, 79, 970–982.e7. [Google Scholar] [CrossRef]
- Wagnerova, A.; Babickova, J.; Liptak, R.; Vlkova, B.; Celec, P.; Gardlik, R. Sex differences in the effect of resveratrol on DSS-induced colitis in mice. Gastroenterol. Res. Pract. 2017, 1–12. [Google Scholar] [CrossRef]
- Rusbana, T.B.; Agista, A.Z.; Saputra, W.D.; Ohsaki, Y.; Watanabe, K.; Ardiansyah; Budijanto, S.; Koseki, T.; Aso, H.; Komai, M.; et al. Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model. Nutrients 2020, 12, 2409. [Google Scholar] [CrossRef] [PubMed]
- Bylund-Fellenius, A.C.; Landström, E.; Axelsson, L.G.; Midtvedt, T. Experimental colitis induced by dextran sulphate in normal and germfree mice. Microb. Ecol. Health Dis. 1994, 7, 207–215. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, H.T.; Mu, H.X.; Huang, T.; Lin, Z.S.; Zhong, L.L.D.; Zeng, G.Z.; Fan, B.M.; Lin, C.Y.; Bian, Z.X. Magnolol, a Natural Polyphenol, Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice. Molecules 2017, 22, 1218. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef]
- Zou, Y.; Lin, J.; Li, W.; Wu, Z.; He, Z.; Huang, G.; Wang, J.; Ye, C.; Cheng, X.; Ding, C.; et al. Huangqin-tang ameliorates dextran sodium sulphate-induced colitis by regulating intestinal epithelial cell homeostasis, inflammation and immune response. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Luo, H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef]
- Ginzel, M.; Feng, X.; Kuebler, J.F.; Klemann, C.; Yu, Y.; von Wasielewski, R.; Park, J.K.; Hornef, M.W.; Vieten, G.; Ure, B.M.; et al. Dextran sodium sulfate (DSS) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS ONE 2017, 12, e0182732. [Google Scholar] [CrossRef] [PubMed]
- MacMaster, J.F.; Dambach, D.M.; Lee, D.B.; Berry, K.K.; Qiu, Y.; Zusi, F.C.; Burke, J.R. An inhibitor of IκB kinase, BMS-345541, blocks endothelial cell adhesion molecule expression and reduces the severity of dextran sulfate sodium-induced colitis in mice. Inflamm. Res. 2003, 52, 508–511. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.H.; Lau, L. The matricellular protein CCN1 promotes mucosal healing in murine colitis through IL-6. Mucosal. Immunol. 2015, 8, 1285–1296. [Google Scholar] [CrossRef]
- Samak, G.; Chaudhry, K.K.; Gangwar, R.; Narayanan, D.; Jaggar, J.H.; Rao, R. Calcium-Ask1-MKK7-JNK2-c-Src Signaling Cascade Mediates Disruption of Intestinal Epithelial Tight Junctions by Dextran Sulfate Sodium. Biochem. J. 2015, 465, 503–515. [Google Scholar] [CrossRef]
- Sasaoka, T.; Ito, M.; Yamashita, J.; Nakajima, K.; Tanaka, I.; Narita, M.; Hara, Y.; Hada, K.; Takahashi, M.; Ohno, Y.; et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 568–576. [Google Scholar] [CrossRef]
- Feng, T.; Qin, H.; Wang, L.; Benveniste, E.N.; Elson, C.O.; Cong, Y. Th17 Cells Induce Colitis and Promote Th1 Cell Responses through IL-17 Induction of Innate IL-12 and IL-23 Production. J. Immunol. 2011, 186, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Bériou, G.; Heslan, M.; Bossard, C.; Jarry, A.; Abidi, A.; Hulin, P.; Ménoret, S.; Thinard, R.; Anegon, I.; et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 2016, 9, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cella, M.; Mcdonald, K.; Garlanda, C.; Kennedy, G.D.; Nukaya, M.; Mantovani, A.; Kopan, R.; Bradfield, C.A. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2012, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sun, X.; Nagata, M.; Kawase, T.; Yamaguchi, H.; Sukumaran, V.; Kawauchi, Y.; Kawachi, H.; Nishino, T.; Watanabe, K.; et al. Analysis of intestinal fibrosis in chronic colitis in mice induced by dextran sulfate sodium. Pathol. Int. 2011, 61, 228–238. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Chi, P. Pirfenidone suppresses TGF-β1-induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 18, 3907–3913. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhao, Q.; Chen, M. Role of Interleukin-17 in Pathogenesis of Intestinal Fibrosis in Mice. Dig. Dis. Sci. 2020, 65, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Rojas, M.; Ravi, A.; Bockbrader, K.; Epstein, S.; Vijay-Kumar, M.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Selective Ablation of Matrix Metalloproteinase-2 Exacerbates Experimental Colitis: Contrasting Role of Gelatinases in the Pathogenesis of Colitis. J. Immunol. 2006, 177, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Saarialho-Kere, U.K.; Vaalamo, M.; Puolakkainen, P.; Airola, K.; Parks, W.C.; Karjalainen-Lindsberg, M.L. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am. J. Pathol. 1996, 148, 519–526. [Google Scholar] [PubMed]
- Finnson, K.W.; Almadani, Y.; Philip, A. Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: Mechanisms and targets. Semin. Cell Dev. Biol. 2020, 101, 115–122. [Google Scholar] [CrossRef]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (dss) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, P.; Leung, P.; Chadee, K. Tumor necrosis factor-α and Muc2 mucin play major roles in disease onset and progression in dextran sodium sulphate-induced colitis. PLoS ONE 2011, 6, e25058. [Google Scholar] [CrossRef]
- De Fazio, L.; Cavazza, E.; Spisni, E.; Strillacci, A.; Centanni, M.; Candela, M.; Praticò, C.; Campieri, M.; Ricci, C.; Valerii, M.C. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J. Gastroenterol. 2014, 20, 2051–2061. [Google Scholar] [CrossRef]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-β in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Miller, S.N.; Hofmann, C.; Vogl, D.; Falk, W.; Schölmerich, J.; Rogler, G. Transforming growth factor-β1 induces intestinal myofibroblast differentiation and modulates their migration. World J. Gastroenterol. 2009, 15, 1431–1442. [Google Scholar] [CrossRef][Green Version]
- Tang, L.Y.; Heller, M.; Meng, Z.; Yu, L.R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Mashima, H.; Sakai, T.; Matsuhashi, T.; Jin, M.; Ohnishi, H. Functional roles of TGF-β1 in intestinal epithelial cells through smad-dependent and non-smad pathways. Dig. Dis. Sci. 2013, 58, 1207–1217. [Google Scholar] [CrossRef]
- Mathur, R.; Alam, M.M.; Zhao, X.F.; Liao, Y.; Shen, J.; Morgan, S.; Huang, T.; Lee, H.J.; Lee, E.; Huang, Y.; et al. Induction of autophagy in Cx3cr1 + mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol. 2019, 12, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, S.; Wang, Y.; Hu, H.; Li, L.; Wu, Y.; Cao, D.; Cai, Y.; Zhang, J.; Zhang, X. Interleukin-22 regulates the homeostasis of the intestinal epithelium during inflammation. Int. J. Mol. Med. 2019, 43, 1657–1668. [Google Scholar] [CrossRef]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; De Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.H.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2013, 144, 357–368.e9. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; Starkey Lewis, P.J.; Pellicoro, A.; Aucott, R.L.; Man, J.; O’Duibhir, E.; Mok, S.E.; Ferreira-Gonzalez, S.; Livingstone, E.; Greenhalgh, S.N.; et al. The STAT3–IL-10–IL-6 Pathway Is a Novel Regulator of Macrophage Efferocytosis and Phenotypic Conversion in Sterile Liver Injury. J. Immunol. 2018, 200, 1169–1187. [Google Scholar] [CrossRef] [PubMed]
- Theiss, A.L.; Simmons, J.G.; Jobin, C.; Lund, P.K. Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J. Biol. Chem. 2005, 280, 36099–36109. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Z.; He, X.; Huang, S.; et al. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bajić, S.S.; Đokić, J.; Dinić, M.; Tomić, S.; Popović, N.; Brdarić, E.; Golić, N.; Tolinački, M. GABA potentiate the immunoregulatory effects of Lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Special section on drug metabolism and the microbiome–Minireview indole and tryptophan metabolism: Endogenous and dietary routes to ah receptor activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef]
| Composition | Control Diet (g) | 10% RB Supplemented Diet (g) | 10% FRB Supplemented Diet (g) |
|---|---|---|---|
| Tert-butylhydroquinone | 0.008 | 0.0072 | 0.0072 |
| L-Cystine | 1.8 | 1.62 | 1.62 |
| Choline bitartrate | 2.5 | 2.25 | 2.25 |
| Vitamin mixture | 10 | 9 | 9 |
| Mineral mixture | 35 | 31.5 | 31.5 |
| Soybean oil | 40 | 36 | 36 |
| Cellulose | 50 | 45 | 45 |
| Sucrose | 100 | 90 | 90 |
| Casein | 140 | 126 | 126 |
| Cornstarch | 620.7 | 558.6228 | 558.6228 |
| Rice bran | - | 100 | - |
| Fermented rice bran | - | - | 100 |
| Total | 1000 | 1000 | 1000 |
| Calories per 100 g (kcal) | 385 | 378 | 367 |
| Score | Diarrheal Score | Bloody Stool Score |
|---|---|---|
| 0 | Normal stool | Normal colored stool |
| 1 | Mildly soft stool | Brown stool |
| 2 | Very soft stool | Reddish stool |
| 3 | Watery stool | Bloody stool |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agista, A.Z.; Rusbana, T.B.; Islam, J.; Ohsaki, Y.; Sultana, H.; Hirakawa, R.; Watanabe, K.; Nochi, T.; Ardiansyah; Budijanto, S.; et al. Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice. Nutrients 2021, 13, 1869. https://doi.org/10.3390/nu13061869
Agista AZ, Rusbana TB, Islam J, Ohsaki Y, Sultana H, Hirakawa R, Watanabe K, Nochi T, Ardiansyah, Budijanto S, et al. Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice. Nutrients. 2021; 13(6):1869. https://doi.org/10.3390/nu13061869
Chicago/Turabian StyleAgista, Afifah Zahra, Tubagus Bahtiar Rusbana, Jahidul Islam, Yusuke Ohsaki, Halima Sultana, Ryota Hirakawa, Kouichi Watanabe, Tomonori Nochi, Ardiansyah, Slamet Budijanto, and et al. 2021. "Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice" Nutrients 13, no. 6: 1869. https://doi.org/10.3390/nu13061869
APA StyleAgista, A. Z., Rusbana, T. B., Islam, J., Ohsaki, Y., Sultana, H., Hirakawa, R., Watanabe, K., Nochi, T., Ardiansyah, Budijanto, S., Yang, S.-C., Koseki, T., Aso, H., Komai, M., & Shirakawa, H. (2021). Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice. Nutrients, 13(6), 1869. https://doi.org/10.3390/nu13061869








