Investigating Sex Differences in Rates and Correlates of Food Addiction Status in Women and Men with PTSD
Abstract
1. Introduction
Study Aims
2. Materials and Methods
2.1. Participants and Procedure
2.2. Measures
2.2.1. Predictor Variables
Food Addiction (FA)
Sex
2.2.2. Proposed Correlates
Body Mass Index (BMI)
Loss of Control (LOC) Overeating and Compensatory Behaviors
PTSD and Depression Symptom Severity
Alcohol Use Severity and Frequency
2.2.3. Demographics and Covariates
Demographics
Covariates
2.3. Data Analyses
3. Results
3.1. Participant Chracterstics
3.2. Rates of Food Addiction
3.3. Body Mass Index (BMI)
3.4. LOC Eating and Compensatory Behaviors Frequency
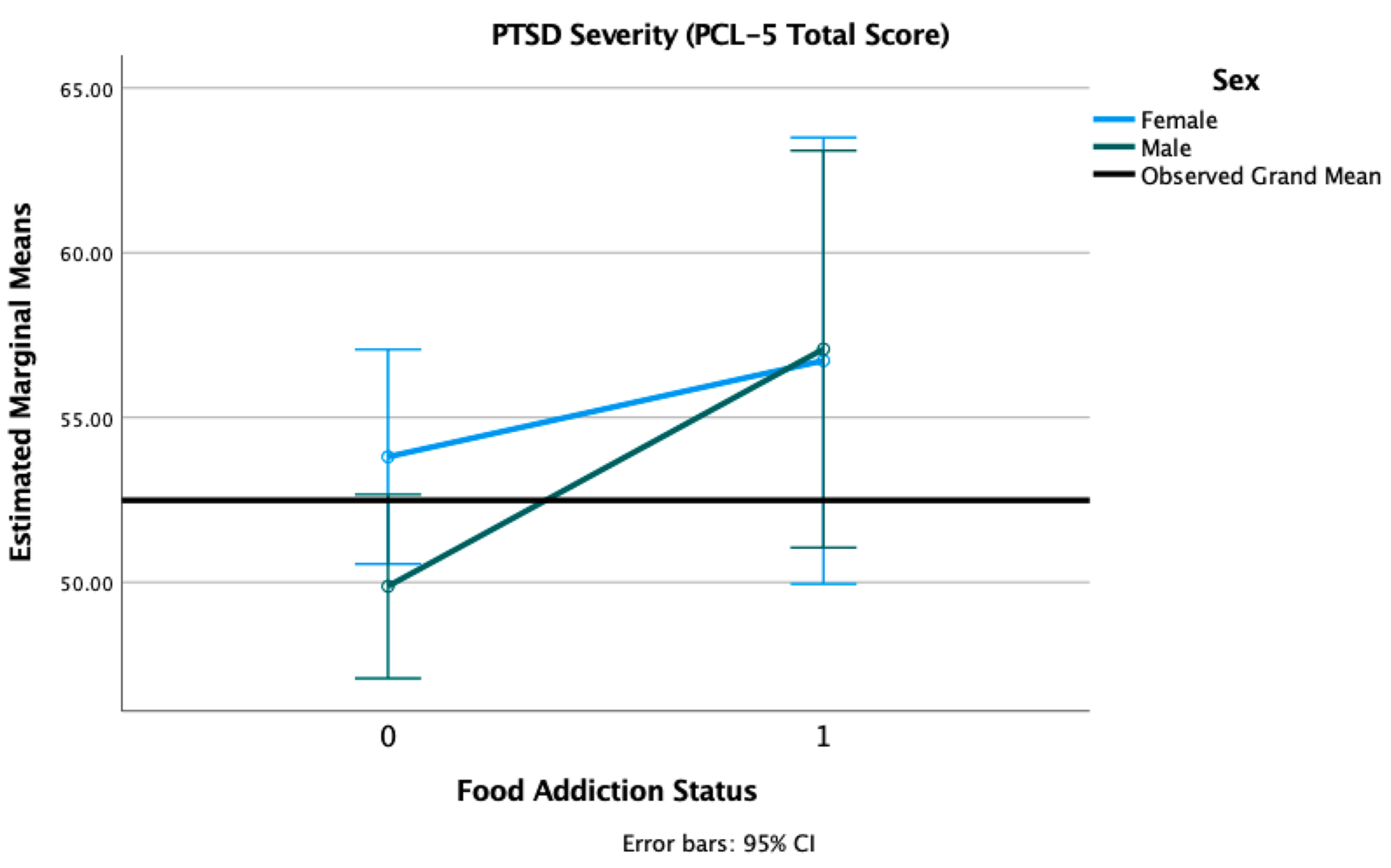
3.5. PTSD and Depression Symptom Severity
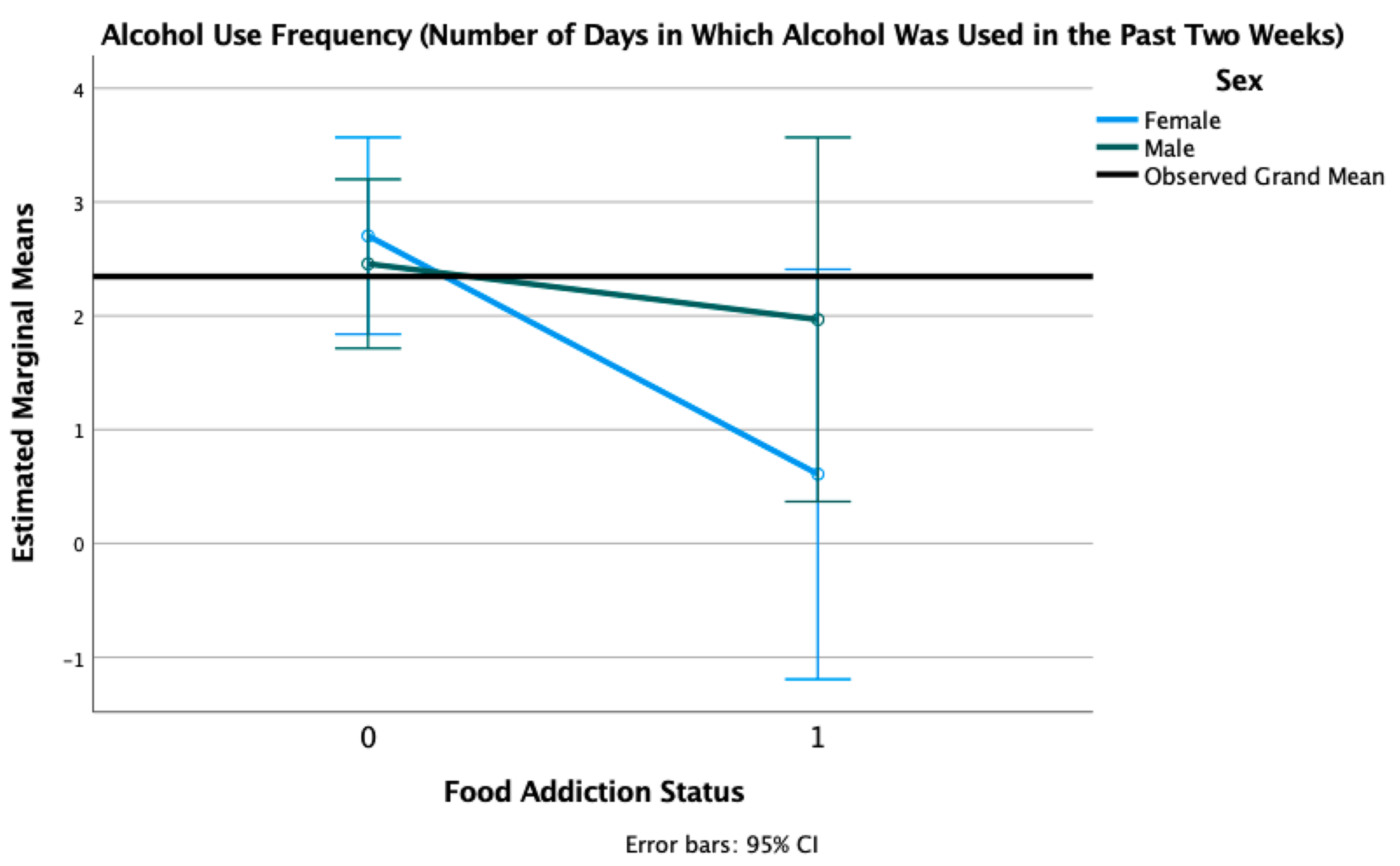
3.6. Alcohol Use Severity and Frequency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.M.; Gearhardt, A.N. Development of the Modified Yale Food Addiction Scale Version 2.0. Eur. Eat. Disord. Rev. 2017, 25, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef]
- Fletcher, P.C.; Kenny, P.J. Food addiction: A valid concept? Neuropsychopharmacology 2018, 43, 2506–2513. [Google Scholar] [CrossRef]
- Hebebrand, J.; Gearhardt, A.N. The concept of “food addiction” helps inform the understanding of over-eating and obesity: NO. Am. J. Clin. Nutr. 2021, 113, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Cassin, S.; Sockalingam, S. Advances and Future Directions in the Clinical Utility of Food Addiction. Nutrition 2021, 13, 708. [Google Scholar] [CrossRef]
- Meule, A.; Gearhardt, A.N. Five years of the Yale Food Addiction Scale: Taking stock and moving forward. Curr. Addict. Rep. 2014, 1, 193–205. [Google Scholar] [CrossRef]
- Penzenstadler, L.; Soares, C.; Karila, L.; Khazaal, Y. Systematic Review of Food Addiction as Measured with the Yale Food Addiction Scale: Implications for the Food Addiction Construct. Curr. Neuropharmacol. 2019, 17, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Eichen, D.M.; Lent, M.R.; Goldbacher, E.; Foster, G.D. Exploration of “Food Addiction” in overweight and obese treatment-seeking adults. Appetite 2013, 67, 22–24. [Google Scholar] [CrossRef]
- Burmeister, J.M.; Hinman, N.; Koball, A.; Hoffmann, D.A.; Carels, R.A. Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite 2013, 60, 103–110. [Google Scholar] [CrossRef]
- Pursey, K.M.; Stanwell, P.; Gearhardt, A.N.; Collins, C.E.; Burrows, T.L. The Prevalence of Food Addiction as Assessed by the Yale Food Addiction Scale: A Systematic Review. Nutrition 2014, 6, 4552–4590. [Google Scholar] [CrossRef]
- Meadows, A.; Nolan, L.J.; Higgs, S. Self-perceived food addiction: Prevalence, predictors, and prognosis. Appetite 2017, 114, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Bourdier, L.; Orri, M.; Carre, A.; Gearhardt, A.; Romo, L.; Dantzer, C.; Berthoz, S. Are emotionally driven and addictive-like eating behaviors the missing links between psychological distress and greater body weight? Appetite 2018, 120, 536–546. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Pardue, M.L.; Wizemann, T.M. Exploring the Biological Contributions to Human Health. Does Sex Matter? National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders: DSM 5; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Betts, K.S.; Williams, G.M.; Najman, J.M.; Alati, R. Exploring the Female Specific Risk to Partial and Full PTSD Following Physical Assault. J. Trauma. Stress 2013, 26, 86–93. [Google Scholar] [CrossRef]
- Kline, A.; Ciccone, D.S.; Weiner, M.; Interian, A.; Hill, L.S.; Falca-Dodson, M.; Black, C.M.; Losonczy, M. Gender Differences in the Risk and Protective Factors Associated With PTSD: A Prospective Study of National Guard Troops Deployed to Iraq. Psychiatry 2013, 76, 256–272. [Google Scholar] [CrossRef]
- Tolin, D.F.; Foa, E.B. Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychol. Bull. 2006, 132, 959–992. [Google Scholar] [CrossRef]
- Hoerster, K.D.; Jakupcak, M.; Hanson, R.; McFall, M.; Reiber, G.; Hall, K.S.; Nelson, K.M. PTSD and depression symptoms are associated with binge eating among US Iraq and Afghanistan veterans. Eat. Behav. 2015, 17, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; El-Gabalawy, R.; Sommer, J.L.; Pietrzak, R.H.; Mitchell, K.; Mota, N. Trauma Exposure, DSM-5 Posttraumatic Stress, and Binge Eating Symptoms. J. Clin. Psychiatry 2019, 80. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.S.; Mazzeo, S.E.; Ba, M.R.S.; Brewerton, T.D.; Smith, B.N. Comorbidity of partial and subthreshold ptsd among men and women with eating disorders in the national comorbidity survey-replication study. Int. J. Eat. Disord. 2011, 45, 307–315. [Google Scholar] [CrossRef]
- Mitchell, K.S.; Wolf, E.J. PTSD, food addiction, and disordered eating in a sample of primarily older veterans: The mediating role of emotion regulation. Psychiatry Res. 2016, 243, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Masheb, R.M.; Ruser, C.B.; Min, K.M.; Bullock, A.J.; Dorflinger, L.M. Does food addiction contribute to excess weight among clinic patients seeking weight reduction? Examination of the Modified Yale Food Addiction Survey. Compr. Psychiatry 2018, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.M.; Flint, A.J.; Roberts, A.L.; Agnew-Blais, J.; Koenen, K.C.; Rich-Edwards, J.W. Posttraumatic Stress Disorder Symptoms and Food Addiction in Women by Timing and Type of Trauma Exposure. JAMA Psychiatry 2014, 71, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Stojek, M.M.; Maples-Keller, J.L.; Dixon, H.D.; Umpierrez, G.E.; Gillespie, C.F.; Michopoulos, V. Associations of childhood trauma with food addiction and insulin resistance in African-American women with diabetes mellitus. Appetite 2019, 141, 104317. [Google Scholar] [CrossRef]
- Hardy, R.; Fani, N.; Jovanovic, T.; Michopoulos, V. Food addiction and substance addiction in women: Common clinical characteristics. Appetite 2018, 120, 367–373. [Google Scholar] [CrossRef]
- Meule, A.; Gearhardt, A.N. Ten Years of the Yale Food Addiction Scale: A Review of Version 2.0. Curr. Addict. Rep. 2019, 6, 218–228. [Google Scholar] [CrossRef]
- Scott, K.M.; McGee, M.A.; Wells, J.E.; Browne, M.A.O. Obesity and mental disorders in the adult general population. J. Psychosom. Res. 2008, 64, 97–105. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Stubbs, B.; Ward, P.B.; Steel, Z.; Lederman, O.; Vancampfort, D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: A systematic review and meta-analysis. Metab. Clin. Exp. 2015, 64, 926–933. [Google Scholar] [CrossRef]
- Michopoulos, V.; Vester, A.; Neigh, G. Posttraumatic stress disorder: A metabolic disorder in disguise? Exp. Neurol. 2016, 284, 220–229. [Google Scholar] [CrossRef]
- Burrows, T.; Kay-Lambkin, F.; Pursey, K.; Skinner, J.; Dayas, C. Food addiction and associations with mental health symptoms: A systematic review with meta-analysis. J. Hum. Nutr. Diet. 2018, 31, 544–572. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Curtis, C.; Levitan, R.D.; Carter, J.C.; Kaplan, A.S.; Kennedy, J.L. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 2011, 57, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Crosby, R.D.; Grilo, C.M. An examination of the food addiction construct in obese patients with binge eating disorder. Int. J. Eat. Disord. 2011, 45, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Grilo, C.M. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr. Psychiatry 2013, 54, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Back, S.E.; Jones, J.L. Alcohol Use Disorder and Posttraumatic Stress Disorder: An Introduction. Alcohol. Clin. Exp. Res. 2018, 42, 836–840. [Google Scholar] [CrossRef]
- Clark, S.M.; Saules, K.K. Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eat. Behav. 2013, 14, 216–219. [Google Scholar] [CrossRef]
- Meule, A.; Vögele, C.; Kübler, A. Deutsche Übersetzung und Validierung der Yale Food Addiction Scale. Diagnostica 2012, 58, 115–126. [Google Scholar] [CrossRef]
- Müller, A.; Leukefeld, C.; Hase, C.; Gruner-Labitzke, K.; Mall, J.W.; Köhler, H.; De Zwaan, M. Food addiction and other addictive behaviours in bariatric surgery candidates. Eur. Eat. Disord. Rev. 2018, 26, 585–596. [Google Scholar] [CrossRef]
- Meule, A.; Heckel, D.; Jurowich, C.F.; Vögele, C.; Kübler, A. Correlates of food addiction in obese individuals seeking bariatric surgery. Clin. Obes. 2014, 4. [Google Scholar] [CrossRef]
- Meule, A. The Relation Between Body Mass Index and Substance Use: A True Can of Worms. Innov. Clin. Neurosci. 2014, 11, 11–12. [Google Scholar]
- Blake, D.D.; Weathers, F.W.; Nagy, L.M.; Kaloupek, D.G.; Gusman, F.D.; Charney, D.S.; Keane, T.M. The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress 1995, 8, 75–90. [Google Scholar] [CrossRef]
- Weathers, F.W.; Keane, T.M.; Davidson, J.R.T. Clinician-administered PTSD scale: A review of the first ten years of research. Depress. Anxiety 2001, 13, 132–156. [Google Scholar] [CrossRef]
- McAdams, M.A.; Van Dam, R.M.; Hu, F.B. Comparison of Self-reported and Measured BMI as Correlates of Disease Markers in U.S. Adults. Obesity 2007, 15, 188–196. [Google Scholar] [CrossRef] [PubMed]
- FerroLuzzi, A. Physical Status: The Use and Interpretation of Anthropometry—Introduction; WHO Expert Committee on Physical Status: Geneva, Switzerland, 1995; Available online: https://apps.who.int/iris/handle/10665/37003 (accessed on 8 March 2021).
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress 2015, 28, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Löwe, B.; Kroenke, K.; Herzog, W.; Gräfe, K. Measuring depression outcome with a brief self-report instrument: Sensitivity to change of the Patient Health Questionnaire (PHQ-9). J. Affect. Disord. 2004, 81, 61–66. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. Can. Med. Assoc. J. 2012, 184, E191–E196. [Google Scholar] [CrossRef]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT Alcohol Consumption Questions (AUDIT-C): An Effective Brief Screening Test for Problem Drinking. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; Bush, K.R.; Epler, A.J.; Dobie, D.J.; Davis, T.M.; Sporleder, J.L.; Maynard, C.; Burman, M.L.; Kivlahan, D.R. Two Brief Alcohol-Screening Tests From the Alcohol Use Disorders Identification Test (AUDIT). Arch. Intern. Med. 2003, 163, 821–829. [Google Scholar] [CrossRef]
- Gray, M.J.; Litz, B.T.; Hsu, J.L.; Lombardo, T.W. Psychometric Properties of the Life Events Checklist. Assessment 2004, 11, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, E.; Norton, C.; Tchanturia, K. Clinicians’ views on treatment adaptations for men with eating disorders: A qualitative study. BMJ Open 2018, 8, e021934. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R. Food Addiction and Diagnostic Criteria for Dependence. In Food and Addiction; Brownell, K.D., Gold, M.S., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 167–171. [Google Scholar]
- Meule, A.; Von Rezori, V.; Blechert, J. Food Addiction and Bulimia Nervosa. Eur. Eat. Disord. Rev. 2014, 22, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Sussman, S. Substance and Behavioral Addictions: Concepts, Causes, and Cures; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Clemmensen, C.; Petersen, M.B.; Sørensen, T.I.A. Will the COVID-19 pandemic worsen the obesity epidemic? Nat. Rev. Endocrinol. 2020, 16, 469–470. [Google Scholar] [CrossRef]
- Cummings, J.R.; Ackerman, J.M.; Wolfson, J.A.; Gearhardt, A.N. COVID-19 stress and eating and drinking behaviors in the United States during the early stages of the pandemic. Appetite 2021, 162, 105163. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.J.; Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D.; Field, A.E.; Rimm, E.B. Food-addiction scale measurement in 2 cohorts of middle-aged and older women. Am. J. Clin. Nutr. 2014, 99, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, Ö.; Föcker, M.; Kliewer, J.; Esber, S.; Peters, T.; de Zwaan, M.; Hebebrand, J. Eating-related psychopathology and food addiction in adolescent psychiatric inpatients. Eur. Eat. Disord. Rev. 2017, 25, 214–220. [Google Scholar] [CrossRef]
- Department of Defense. 2018 Demographics Report: Profile of the Military Community. 2018. Available online: https://tinyurls.com/doddemo18 (accessed on 1 April 2021).





| %/M (SD) | ||||||
|---|---|---|---|---|---|---|
| Whole Sample (n = 214) | Women (n = 91) | Men (n = 123) | χ2/t | p | ||
| Age | 40.88 (9.51) | 39.30 (9.78) | 42.04 (9.18) | 2.09 | 0.037 * | |
| Race | 28.74 | <0.001 ** | ||||
| African American or Black | 28.97 | 45.06 | 17.07 | |||
| Caucasian or White | 62.62 | 43.96 | 76.42 | |||
| Naive Hawaiian or Other Pacific Islander | 0.47 | 0.00 | 0.81 | |||
| Asian | 0.94 | 1.10 | 0.81 | |||
| American Indian or Alaska Native | 1.40 | 2.20 | 0.81 | |||
| Biracial/Multiracial | 2.80 | 5.50 | 0.81 | |||
| Don’t know | 2.80 | 2.20 | 3.25 | |||
| Ethnicity | 4.39 | 0.111 | ||||
| Non-Hispanic or Latino | 85.05 | 86.81 | 83.74 | |||
| Hispanic or Latino | 8.41 | 9.89 | 7.32 | |||
| Missing | 6.54 | 3.30 | 8.94 | |||
| PTSD Status | 15.14 | <0.001 ** | ||||
| Threshold | 78.5% | 91% | 69% | |||
| Subthreshold | 21.5% | 9% | 31% | |||
| BMI | 29.74 (6.00) | 28.86 (5.86) | 30.40 (6.05) | 1.87 | 0.064 | |
| Weight status | 4.41 | 0.220 | ||||
| Normal weight | 20.09 | 24.18 | 17.07 | |||
| Overweight | 35.51 | 32.97 | 37.40 | |||
| Obese | 42.52 | 40.66 | 43.90 | |||
| Underweight | 0.94 | 2.20 | 0.00 | |||
| Missing | 0.94 | 0.00 | 1.63 | |||
| Food Addiction Diagnosis | 0.09 | 0.761 | ||||
| Positive | 17.76 | 18.68 | 17.07 | |||
| Negative | 82.24 | 81.32 | 82.93 | |||
| Food Addiction Symptom Count | Whole Sample | 1.63 (2.54) | 1.87 (2.75) | 1.46 (2.37) | 1.18 | 0.241 |
| Food Addiction Symptom Count | Positive FA Status + | 6.21 (2.39) | 6.88 (2.20) | 5.67 (2.46) | 1.59 | 0.121 |
| Food Addiction Severity + | 1.84 | 0.398 | ||||
| Mild | 13.16 | 5.88 | 19.05 | |||
| Moderate | 26.32 | 23.53 | 28.57 | |||
| Severe | 60.53 | 70.59 | 52.38 | |||
| LOC eating | 0.28 | 0.598 | ||||
| Present | 22.4% | 24.2% | 21.1% | |||
| Absent | 77.6% | 75.8% | 78.9% | |||
| Compensatory Behaviors | 1.56 | 0.211 | ||||
| Present | 21.2% | 25.3% | 18.2% | |||
| Absent | 78.8% | 74.7% | 81.8% | |||
| Missing | 0.9% | 0% | 1.6% | |||
| LOC Eating Frequency | 0.78 (1.77) | 0.82 (1.79) | 0.74 (1.77) | −0.34 | 0.732 | |
| Compensatory Behaviors Frequency | 0.63 (1.49) | 0.80 (1.67) | 0.50 (1.34) | −1.48 | 0.139 | |
| PCL | 52.49 (14.75) | 55.42 (14.31) | 50.33 (14.75) | −2.53 | 0.012 * | |
| LEC | 6.88 (2.63) | 6.69 (2.63) | 7.02 (2.63) | 0.89 | 0.374 | |
| PHQ | 14.69 (5.63) | 14.86 (5.73) | 14.55 (5.58) | −0.37 | 0.710 | |
| Possible Alcohol Use Disorder (AUDIT-C) | 0.08 | 0.775 | ||||
| Positive | 43.93 | 45.05 | 43.09 | |||
| Negative | 56.07 | 54.95 | 56.91 | |||
| Days Used Alcohol | 2.35 (3.72) | 2.35 (3.79) | 2.34 (3.68) | −0.01 | 0.989 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojek, M.M.; Lipka, J.; Maples-Keller, J.M.; Rauch, S.A.M.; Black, K.; Michopoulos, V.; Rothbaum, B.O. Investigating Sex Differences in Rates and Correlates of Food Addiction Status in Women and Men with PTSD. Nutrients 2021, 13, 1840. https://doi.org/10.3390/nu13061840
Stojek MM, Lipka J, Maples-Keller JM, Rauch SAM, Black K, Michopoulos V, Rothbaum BO. Investigating Sex Differences in Rates and Correlates of Food Addiction Status in Women and Men with PTSD. Nutrients. 2021; 13(6):1840. https://doi.org/10.3390/nu13061840
Chicago/Turabian StyleStojek, Monika M., Justyna Lipka, Jessica M. Maples-Keller, Sheila A. M. Rauch, Kathryn Black, Vasiliki Michopoulos, and Barbara O. Rothbaum. 2021. "Investigating Sex Differences in Rates and Correlates of Food Addiction Status in Women and Men with PTSD" Nutrients 13, no. 6: 1840. https://doi.org/10.3390/nu13061840
APA StyleStojek, M. M., Lipka, J., Maples-Keller, J. M., Rauch, S. A. M., Black, K., Michopoulos, V., & Rothbaum, B. O. (2021). Investigating Sex Differences in Rates and Correlates of Food Addiction Status in Women and Men with PTSD. Nutrients, 13(6), 1840. https://doi.org/10.3390/nu13061840






