Characterization of an Agarophyton chilense Oleoresin Containing PPARγ Natural Ligands with Insulin-Sensitizing Effects in a C57Bl/6J Mouse Model of Diet-Induced Obesity and Antioxidant Activity in Caenorhabditis elegans
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Sampling of Biomass
2.3. Oleoresin Analysis
2.3.1. A. Chilense (Gracilex®) Oleoresin Production
2.3.2. Determination of Total Antioxidant Capacity of Gracilex® Using a Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
2.3.3. Analysis of Lipid and Antioxidant Content of Gracilex®
2.4. Cellular Studies
2.4.1. Cell Lines
2.4.2. Cellular Transfection
2.4.3. Adipocyte Differentiation
2.4.4. MTT Viability Assay
2.4.5. RT-qPCR
2.5. Mouse Studies
2.5.1. Mouse Treatments
2.5.2. Measurement of Plasma Metabolic Parameters
2.5.3. Histopathological Studies
2.6. C. Elegans Studies
2.7. Statistical Analysis
3. Results
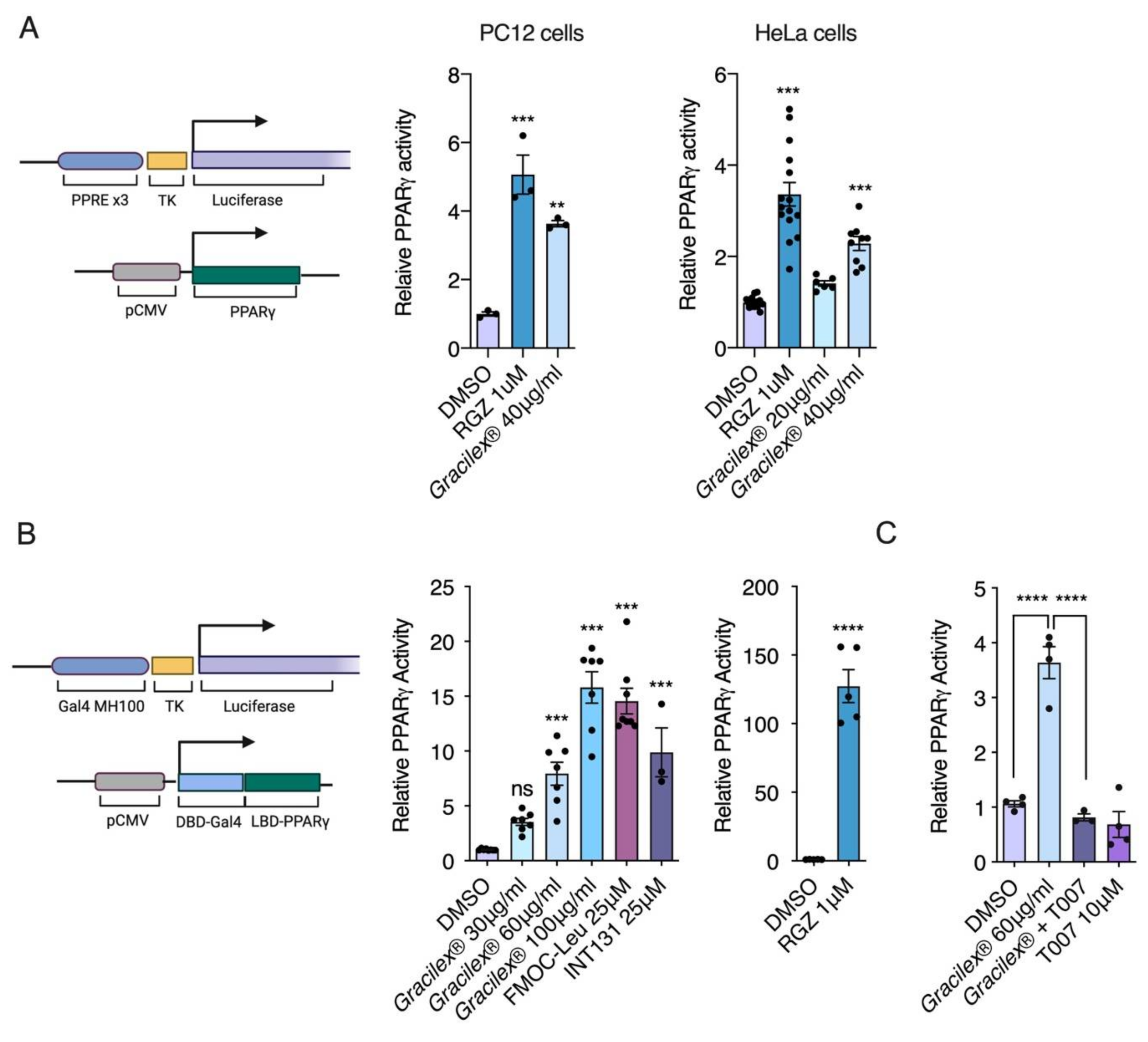
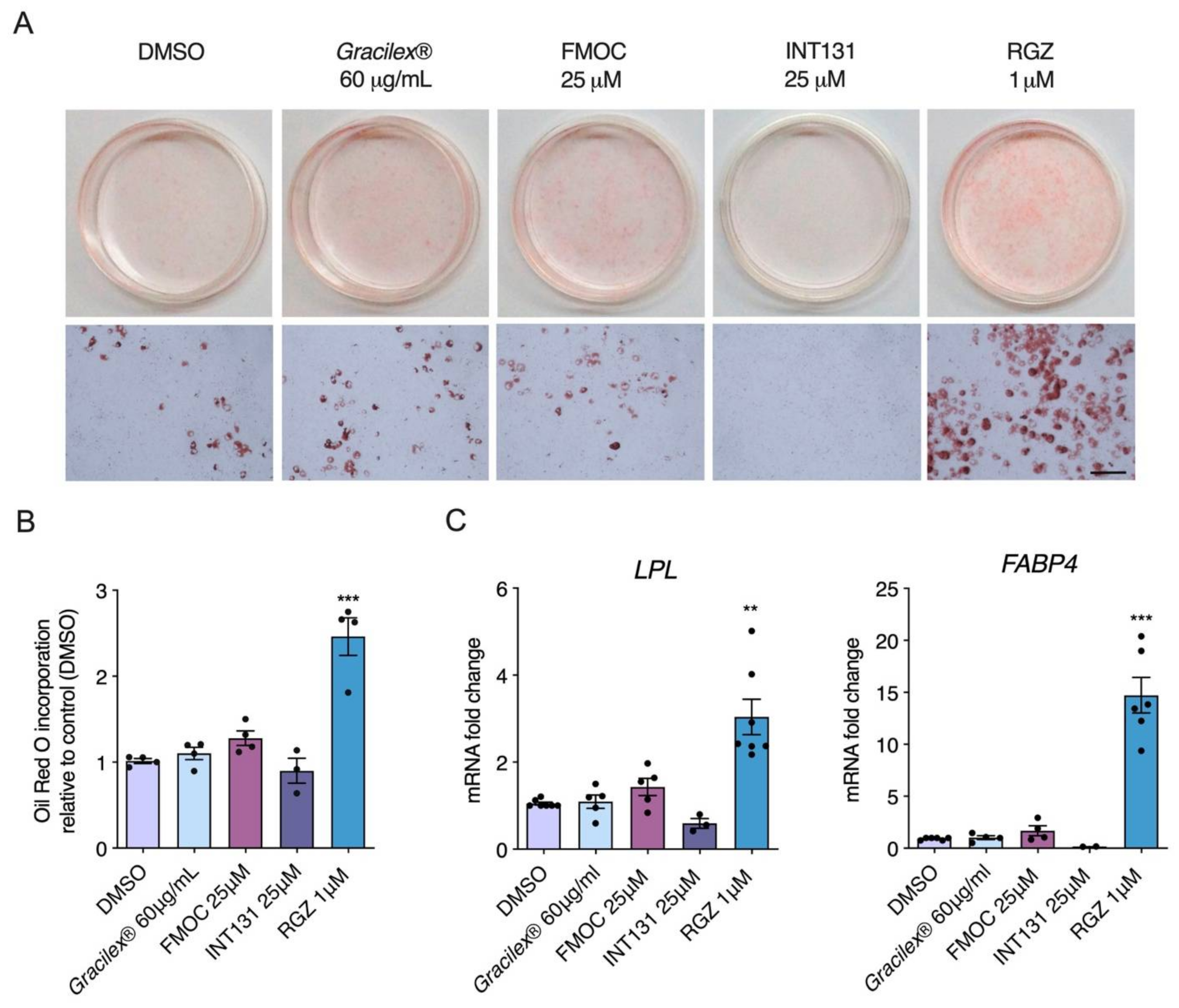
3.1. Effect of Gracilex® on PPARγ Transcriptional Activity
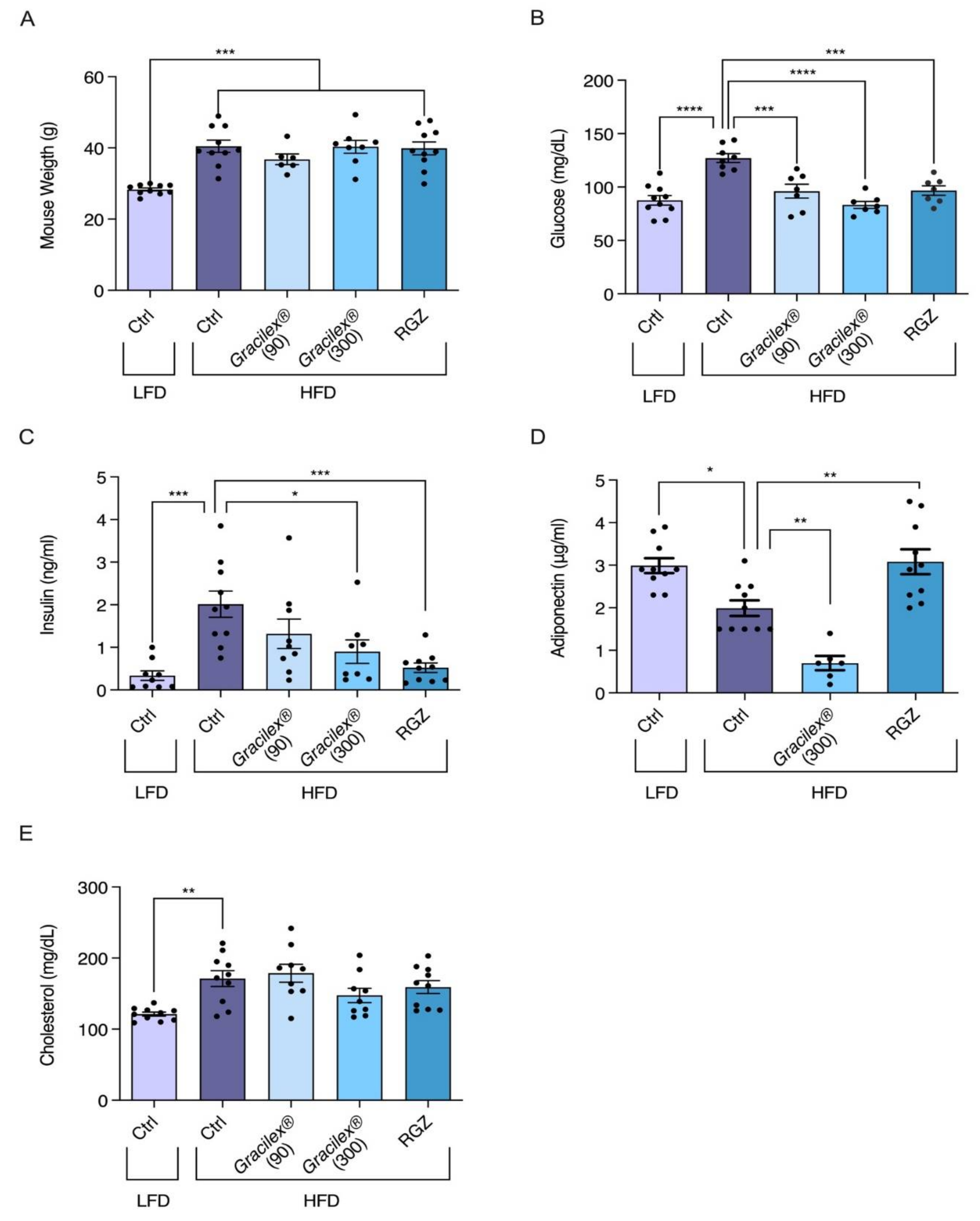
3.2. Effect of A. Chilense Oleoresin on Metabolic Dysfunction Caused by High-Fat Diet (HFD)-Induced Obesity in Male Mice
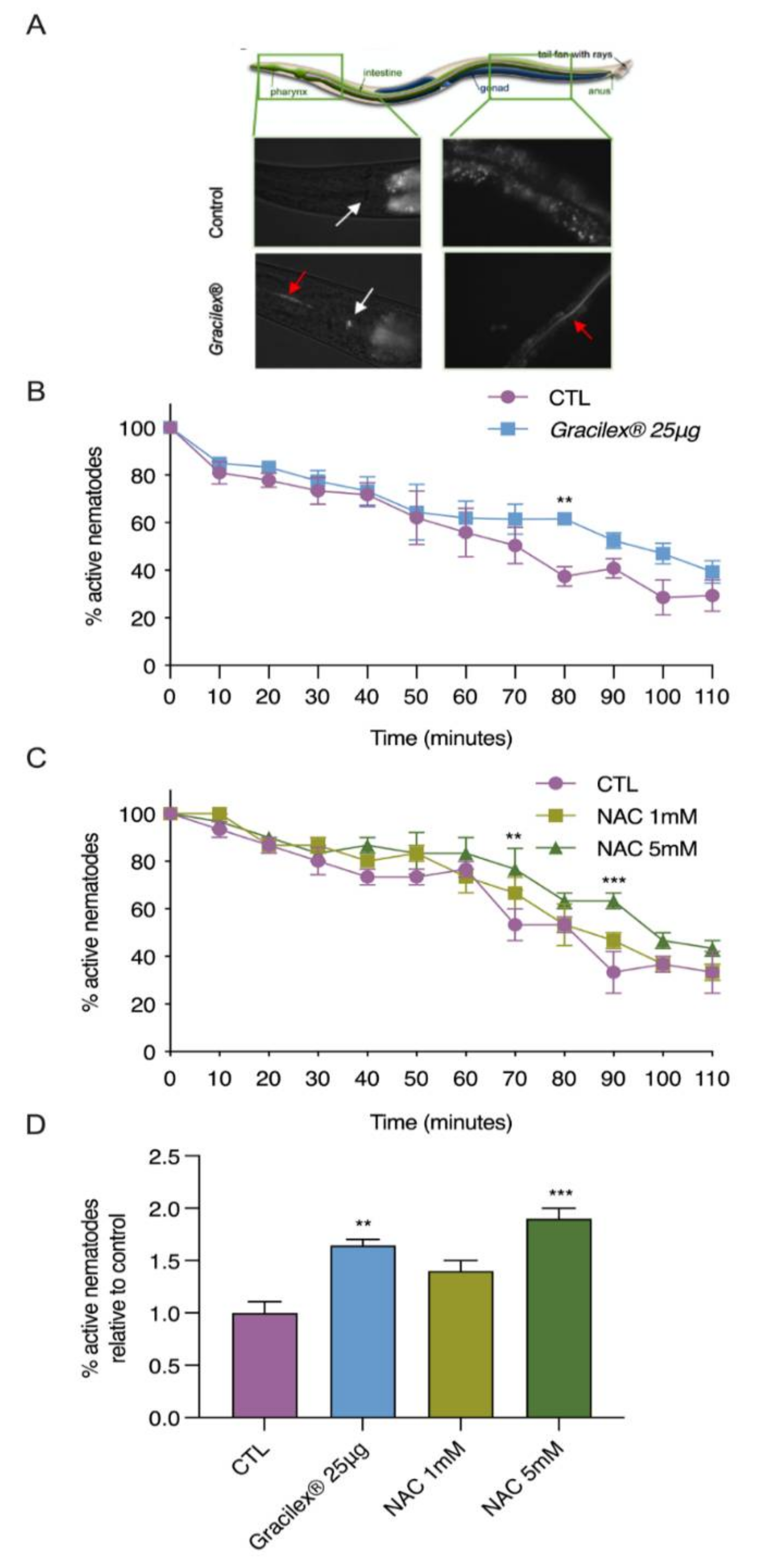
3.3. Antioxidant Properties of Gracilex®
4. Discussion
5. Conclusions
Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurgel, C.F.D.; Norris, J.N.; Schmidt, W.E.; Le, H.N.; Fredericq, S. Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera, and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa 2018, 374, 1–23. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Ramirez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef]
- Bird, C.J.; McLachlan, J.; De Oliveira, E.C. Gracilaria chilensis sp.nov. (Rhodophyta, Gigartinales), from Pacific South America. Can. J. Bot. 1986, 64, 2928–2934. [Google Scholar] [CrossRef]
- Guillemin, M.-L.; Faugeron, S.; Destombe, C.; Viard, F.; Correa, J.A.; Valero, M. Genetic variation in wild and cultivated populations of the haploid—Diploid red alga Gracilaria chilensis: How farming practices favor asexual reproduction and heterozigosity. Evolution 2008, 62, 1500–1519. [Google Scholar] [CrossRef]
- Huanel, O.R.; Nelson, W.A.; Robitzch, V.; Mauger, S.; Faugeron, S.; Preuss, M.; Zuccarello, G.C.; Guillemin, M. Comparative phylogeography of two Agarophyton species in the New Zealand archipelago. J. Phycol. 2020, 56, 1575–1590. [Google Scholar] [CrossRef] [PubMed]
- Robitzch, V.; Arakaki, N.; Mauger, S.; Rojas, J.C.Z.; Guillemin, M.-L. Stranded alone: The first reported Peruvian population of Agarophyton chilensis is a single-male’s clone. Algal Res. 2019, 41, 101527. [Google Scholar] [CrossRef]
- Armisen, R. World-wide use and importance of Gracilaria. Environ. Boil. Fishes 1995, 7, 231–243. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Weinberger, F.; Lion, U.; Delage, L.; Kloareg, B.; Potin, P.; Beltrán, J.; Flores, V.; Faugeron, S.; Correa, J.; Pohnert, G. Up-regulation of lipoxygenase, phospholipase, and pxylipin-production in the induced chemical defense of the red alga Gracilaria chilensis against epiphytes. J. Chem. Ecol. 2011, 37, 677–686. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-inflammatory activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Ishimaru, T.; Itabashi, Y.; Vyssotski, M. Glycerolipid composition of the red macroalga Agarophyton chilensis and comparison to the closely related Agarophyton vermiculophyllum producing different types of eicosanoids. Mar. Drugs 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Pan, B.S. Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 2012, 60, 8404–8410. [Google Scholar] [CrossRef]
- Lion, U.; Wiesemeier, T.; Weinberger, F.; Beltrán, J.; Flores, V.; Faugeron, S.; Correa, J.; Pohnert, G. Phospholipases and galactolipases trigger oxylipin-mediated wound-activated defence in the red alga Gracilaria chilensis against epiphytes. ChemBioChem 2006, 7, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Biologically active oxylipins from enzymatic and nonenzymatic routes in macroalgae. Mar. Drugs 2016, 14, 23. [Google Scholar] [CrossRef]
- Rempt, M.; Weinberger, F.; Grosser, K.; Pohnert, G. Conserved and species-specific oxylipin pathways in the wound-activated chemical defense of the noninvasive red alga Gracilaria chilensis and the invasive Gracilaria vermiculophylla. Beilstein J. Org. Chem. 2012, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in marine organisms: A review. Mar. Drugs 2019, 17, 428. [Google Scholar] [CrossRef]
- Itoh, T.; Fairall, L.; Amin, K.; Inaba, Y.; Szanto, A.; Bálint, B.L.; Nagy, L.; Yamamoto, K.; Schwabe, J.W.R. Structural basis for the activation of PPARγ by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008, 15, 924–931. [Google Scholar] [CrossRef]
- Keller, H.; Dreyer, C.; Medin, J.; Mahfoudi, A.; Ozato, K.; Wahli, W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. USA 1993, 90, 2160–2164. [Google Scholar] [CrossRef]
- Yu, K.; Bayona, W.; Kallen, C.B.; Harding, H.P.; Ravera, C.P.; McMahon, G.; Brown, M.; Lazar, M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995, 270, 23975–23983. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Huang, P.; Hamuro, Y.; Raghuram, S.; Wang, Y.; Burris, T.P.; Rastinejad, F. Structure of the intact PPAR-γ–RXR-α nuclear receptor complex on DNA. Nat. Cell Biol. 2008, 456, 350–356. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Hong, F.; Xu, P.; Zhai, Y. The opportunities and challenges of peroxisome proliferator-activated receptors ligands in clinical drug discovery and development. Int. J. Mol. Sci. 2018, 19, 2189. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Yamada, H.; Oshiro, E.; Kikuchi, S.; Hakozaki, M.; Takahashi, H.; Kimura, K.-I. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J. Lipid Res. 2014, 55, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Brash, A.R.; Kehrer, J.P.; Stimmel, J.B.; Leesnitzer, L.M.; Yang, P.; Newman, R.A.; Fischer, S.M. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem. J. 2002, 366, 901–910. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dougherty, E.J.; Danner, R.L. PPARγ signaling and emerging opportunities for improved therapeutics. Pharmacol. Res. 2016, 111, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Ouellet-Hellstrom, R.; MaCurdy, T.E.; Ali, F.; Sholley, C.; Worrall, C.; Kelman, J.A. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. JAMA 2010, 304, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E. Perspective: Effect of rosiglitazone on cardiovascular outcomes. Curr. Cardiol. Rep. 2007, 9, 343–344. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency Recommended Suspention of Avandia, A.a.A. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/09/news_detail_001119.jsp. (accessed on 23 September 2010).
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Li, J.; Lian, H. Recent development of single preparations and fixed-dose combination tablets for the treatment of non-insulin-dependent diabetes mellitus. Arch. Pharmacal Res. 2016, 39, 731–746. [Google Scholar] [CrossRef]
- Steffes, M.W.; Sibley, S.; Jackson, M.; Thomas, W. β-Cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003, 26, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.O.; Lau, E.S.; So, W.-Y.; Ma, R.; Kong, A.P.; Ozaki, R.; Chow, F.C.; Chan, J.C. Prospective study on the incidences of cardiovascular-renal complications in chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care 2013, 37, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Day, C. The future of new drugs for diabetes management. Diabetes Res. Clin. Pr. 2019, 155, 107785. [Google Scholar] [CrossRef]
- Vijan, S. Type 2 diabetes. Ann. Intern. Med. 2015, 162, ITC1. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Papanas, N.; Rizzo, M.; Rizvi, A.A. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expert Rev. Clin. Pharmacol. 2019, 12, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, C.Y.; Afroz, R.; Kamato, D.; Gray, S.; Little, P.J. Animal models for assessing the impact of natural products on the aetiology and metabolic pathophysiology of Type 2 diabetes. Biomed. Pharmacother. 2017, 89, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Bronfman, M.; Bronfman, F.C.; Pinto, C.; Pissani, C.; Paredes Martínes, M.J. Method for the Preparation of an Oleoresin Originating From a Red Alga That Maintains the Capacity to Induce the Transcriptional Activity of the Nuclear Receptor PPAR-Gamma. U.S. Patent WO2014186913A1, 27 November 2014. [Google Scholar]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Fuenzalida, K.M.; Aguilera, M.C.; Piderit, D.G.; Ramos, P.; Contador, D.; Quiñones, V.; Rigotti, A.; Bronfman, F.C.; Bronfman, M. Peroxisome proliferator-activated receptor γ is a novel target of the nerve growth factor signaling pathway in PC12 Cells. J. Biol. Chem. 2005, 280, 9604–9609. [Google Scholar] [CrossRef]
- Bai, C.; Schmidt, A.; Freedman, L.P. Steroid hormone receptors and drug discovery: Therapeutic opportunities and assay designs. ASSAY Drug Dev. Technol. 2003, 1, 843–852. [Google Scholar] [CrossRef]
- Paguio, A.; Stecha, P.; Wood, K.V.; Fan, F. Improved dual-luciferase reporter assays for nuclear receptors. Curr. Chem. Genom. 2010, 4, 43–49. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Almind, K.; Kahn, C.R. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes 2004, 53, 3274–3285. [Google Scholar] [CrossRef]
- Heydemann, A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J. Diabetes Res. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. Elegans. In WormBook: The Online Review of C.elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2005–2018. Available online: https://www.ncbi.nlm.gov/books/NBK19649/ (accessed on 11 February 2006).
- Minniti, A.N.; Arriagada, H.; Zúñiga, S.; Bravo-Zehnder, M.; Alfaro, I.E.; Aldunate, R. Temporal pattern of neuronal insulin release during Caenorhabditis elegans aging: Role of redox homeostasis. Aging Cell 2018, 18, e12855. [Google Scholar] [CrossRef]
- Chalfie, M.; Hart, A.C.; Rankin, C.H.; Goodman, M.B. WormBook: The online review of C. Elegans biology [Internet]. WormBook: Pasadena, CA, USA, 2005–2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235860/ (accessed on 31 July 2014).
- Possik, E.; Pause, A. Measuring oxidative stress resistance of Caenorhabditis elegans in 96-well microtiter plates. J. Vis. Exp. 2015, 99, e52746. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Forman, B.M.; Blumberg, B.; Ong, E.S.; Borgmeyer, U.; Mangelsdorf, D.J.; Umesono, K.; Evans, R.M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA 1994, 91, 7355–7359. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Elwood, F.; McNally, J.; Weiszmann, J.; Lindstrom, M.; Amaral, K.; Nakamura, M.; Miao, S.; Cao, P.; Learned, R.M.; et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor γ, functions as an antagonist of biochemical and cellular activities. J. Biol. Chem. 2002, 277, 19649–19657. [Google Scholar] [CrossRef] [PubMed]
- Dunn, F.L.; Higgins, L.S.; Fredrickson, J.; DePaoli, A.M. Selective modulation of PPARγ activity can lower plasma glucose without typical thiazolidinedione side-effects in patients with type 2 diabetes. J. Diabetes Complicat. 2011, 25, 151–158. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 2018, 28, 415–431. [Google Scholar] [CrossRef]
- Toye, A.A.; Lippiat, J.D.; Proks, P.; Shimomura, K.; Bentley, L.; Hugill, A.; Mijat, V.; Goldsworthy, M.; Moir, L.; Haynes, A.; et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetology 2005, 48, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Soccio, R.E.; Li, Z.; Chen, E.R.; Foong, Y.H.; Benson, K.K.; DiSpirito, J.R.; Mullican, S.E.; Emmett, M.J.; Briggs, E.R.; Peed, L.C.; et al. Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. J. Clin. Investig. 2017, 127, 1451–1462. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; Falcão, H.D.S.; Lima, G.R.D.M.; Montenegro, C.D.A.; Lira, N.S.; De Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.D.F.V.D.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Sanz-Pintos, N.; Pérez-Jiménez, J.; Buschmann, A.H.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Saura-Calixto, F. Macromolecular antioxidants and dietary fiber in edible seaweeds. J. Food Sci. 2017, 82, 289–295. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.; Capa-Robles, W.; Olmos-Soto, J.; Gutierrez-Millan, L.E. The carotenogenesis pathway via the isoprenoid-β-carotene interference approach in a new strain of Dunaliella salina isolated from Baja California Mexico. Mar. Drugs 2009, 7, 45–56. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-vitro antioxidant, hypoglycemic activity, and identification of bioactive compounds in phenol-rich extract from the marine red algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef]
- Hoseini, S.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of Spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Sandoval, V.; Femenias, A.; Martínez-Garza, Ú.; Sanz-Lamora, H.; Castagnini, J.M.; Quifer-Rada, P.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Lyophilized Maqui (Aristotelia chilensis) Berry induces browning in the subcutaneous white adipose tissue and ameliorates the insulin resistance in high fat diet-induced obese mice. Antioxidants 2019, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-I.; Park, J.-K.; Park, S.-K. Lifespan extension and increased resistance to environmental stressors by N-Acetyl-L-Cysteine in Caenorhabditis elegans. Clinics 2015, 70, 380–386. [Google Scholar] [CrossRef]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef]
- Corsi, A.K. A Transparent window into biology: A primer on Caenorhabditis elegans. WormBook 2015, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as nuclear receptors for nutrient and energy metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Guasch, L.; Sala, E.; Mulero, M.; Valls, C.; Salvadó, M.J.; Pujadas, G.; Garcia-Vallvé, S. Identification of PPARgamma partial agonists of natural origin (II): In silico prediction in natural extracts with known antidiabetic activity. PLoS ONE 2013, 8, e55889. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Nolte, R.T.; Wisely, G.B.; Westin, S.; Cobb, J.E.; Lambert, M.H.; Kurokawa, R.; Rosenfeld, M.G.; Willson, T.M.; Glass, C.K.; Milburn, M.V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nat. Cell Biol. 1998, 395, 137–143. [Google Scholar] [CrossRef]
- Kroker, A.J.; Bruning, J.B. Review of the structural and dynamic mechanisms of PPARγpartial agonism. PPAR Res. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Uppenberg, J.; Svensson, C.; Jaki, M.; Bertilsson, G.; Jendeberg, L.; Berkenstam, A. Crystal structure of the ligand binding domain of the human nuclear receptor PPARγ. J. Biol. Chem. 1998, 273, 31108–31112. [Google Scholar] [CrossRef]
- Waku, T.; Shiraki, T.; Oyama, T.; Maebara, K.; Nakamori, R.; Morikawa, K. The nuclear receptor PPARγ individually responds to serotonin- and fatty acid-metabolites. EMBO J. 2010, 29, 3395–3407. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, S.; Picard, F.; Vamecq, J.; Gelman, L.; Potier, N.; Zeyer, D.; Dubuquoy, L.; Bac, P.; Champy, M.-F.; Plunket, K.D.; et al. A Unique PPARγ Ligand with Potent Insulin-Sensitizing yet Weak Adipogenic Activity. Mol. Cell 2001, 8, 737–747. [Google Scholar] [CrossRef]
- Kim, S.-N.; Choi, H.Y.; Lee, W.; Park, G.M.; Shin, W.S.; Kim, Y.K. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPARα/γ activation in 3T3-L1 cells. FEBS Lett. 2008, 582, 3465–3472. [Google Scholar] [CrossRef]
- Raskin, P. Why insulin sensitizers but not secretagogues should be retained when initiating insulin in type 2 diabetes. Diabetes Metabol. Res. Rev. 2007, 24, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994, 17, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Jiang, R.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Colditz, G.A.; Hu, F.B. Ethnicity, obesity, and risk of type 2 diabetes in women: A 20-year follow-up study. Diabetes Care 2006, 29, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Banks, A.S.; Estall, J.L.; Kajimura, S.; Boström, P.; Laznik, D.; Ruas, J.L.; Chalmers, M.J.; Kamenecka, T.M.; Blüher, M.; et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk. Nat. Cell Biol. 2010, 466, 451–456. [Google Scholar] [CrossRef]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.-A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef]
- Astapova, O.; Leff, T. Adiponectin and PPARγ. Vitam. Hormon. 2012, 90, 143–162. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Lin, H.-C.T.; Tsai, W.-S.; Chiu, T.-H. Antioxidant properties of seven cultivated and natural edible seaweed extracts from Taiwan. J. Aquat. Food Prod. Technol. 2012, 21, 248–264. [Google Scholar] [CrossRef]
- Batista-Gonzalez, A.E.; Silva, A.M.D.O.E.; Vidal-Novoa, A.; Pinto, J.R.; Mancini, D.A.P.; Mancini-Filho, J. Analysis of in vitro and in vivo antioxidant properties of hydrophilic fractions from the seaweed halimeda monile l. J. Food Biochem. 2011, 36, 189–197. [Google Scholar] [CrossRef]
- Murakami, A.; Ishida, H.; Kobo, K.; Furukawa, I.; Ikeda, Y.; Yonaha, M.; Aniya, Y.; Ohigashi, H. Suppressive effects of Okinawan food items on free radical generation from stimulated leukocytes and identification of some active constituents: Implications for the prevention of inflammation-associated carcinogenesis. Asian Pac. J. Cancer Prev. 2006, 6, 437–448. [Google Scholar]
- Miranda-Delgado, A.M.; Montoya, M.J.; Paz-Araos, M.; Mellado, M.; Villena, J.; Arancibia, P.; Madrid, A.; Jara-Gutierrez, C. Antioxidant and anti-cancer activities of brown and red seaweed extracts from Chilean coasts. Lat. Am. J. Aquat. Res. 2018, 46, 301–313. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 16. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Traber, M. A History of vitamin, E. Ann. Nutr. Metab. 2012, 61, 207–212. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuki, M.; Niki, E. Action of β-Carotene as an antioxidant against lipid peroxidation. Arch. Biochem. Biophys. 1995, 323, 137–147. [Google Scholar] [CrossRef]

| Fatty Acid | Chain Length | Mean % | SD | |
|---|---|---|---|---|
| Saturated | Decanoic Acid | 10:00 | 0.760 | 1.1 |
| Dodecanoic Acid | 12:00 | 0.305 | 0.3 | |
| Tridecanoic Acid | 13:00 | 0.983 | 0.3 | |
| Tetradecanoic Acid | 14:00 | 4.438 | 0.9 | |
| Pentadecanoic Acid | 15:00 | 0.440 | 0.3 | |
| Hexadecanoic Acid | 16:00 | 40.005 | 5.4 | |
| Heptadecanoic Acid | 17:00 | 0.750 | 1.3 | |
| Octadecanoic Acid | 18:00 | 2.683 | 3.0 | |
| Eicosanoic Acid | 20:00 | 0.152 | 0.08 | |
| Docosanoic Acid | 22:00 | 0.238 | 0.05 | |
| Tetracosanoic Acid | 24:00 | 0.127 | 0.08 | |
| Mono-Insaturated | 10-Pentadecaenoic Acid | 15:1 n-5 | 1.87 | 1.8 |
| 9-Hexadecaenoic Acid | 16:1 n-7 | 0.46 | 0.3 | |
| 9-Octadecaenoic Acid | 18:1 n-9 | 14.13 | 4.6 | |
| 11-Octadecaenoic Acid | 18:1 n-7 | 4.07 | 1.4 | |
| Omega-6 Polyunsaturated | 9,12-Octadecadienoic Acid | 18:2 n-6 | 2.87 | 0.81 |
| 6,9,12-Octadecatrienoic Acid | 18:3 n-6 | 0.17 | 0.10 | |
| 11,14-Eicosadienoic Acid | 20:2 n-6 | 0.34 | 0.37 | |
| 8,11,14-Eicosatrienoic Acid | 20:3 n-6 | 0.47 | 0.09 | |
| 5,8,11,14-Eicosatetraenoic Acid | 20:4 n-6 | 21.06 | 3.81 | |
| Omega-3 Polyunsaturated | 9,12,15-Octadecatrienoic Acid | 18:3 n-3 | 0.390 | 0.42 |
| 5,8,11,14,17-Eicosapentaenoic Acid | 20:5 n-3 | 0.408 | 0.38 | |
| 7,10,13,16,19-Docosapentaenoic Acid | 22:5 n-3 | 0.27 | 0.12 | |
| 4,7,10,13,16,19-Docosahexaenoic Acid | 22:6 n-3 | 0.125 | 0.04 | |
| Other | Conjugated Fatty Acids | |||
| Fatty Acid | Chain Length | Mean % | SD | |
| c9, t11-octadecadienoic Acid | 18:2 n-cla | 0.187 | 0.06 | |
| Trans Fatty Acids | ||||
| Fatty Acid | Chain Length | Mean % | SD | |
| 10-Transpentadecaenoic Acid | 15:1 n-5t | 0.258 | 0.24 | |
| 9-Octadecaenoic Acid | 18:1 n-9t | 1.037 | 1.30 | |
| 11-TransOctadecaenoic Acid | 18:1 n-7t | 0.540 | 0.30 | |
| 9,12-Octadecadienoic Acid | 18:2 n-6tt | 0.11 | 0.04 | |
| Sample | mg Uric Acid Eq/100mg Oleoresin |
|---|---|
| Mean ± SEM | |
| Gracilex® | 430 ± 58.3 |
| Spirulina oleoresin | 344 ± 90.6 |
| Maqui oleoresin | 305 ± 49.9 |
| Sample | μg/g of Gracilex® |
|---|---|
| Mean ± SEM | |
| α-Tocopherol | 527.7 ± 85.3 |
| γ-Tocopherol | 5332.8 ± 1523.3 |
| δ-Tocopherol | 2660 ± 397.1 |
| Total Tocopherols | 6673 ± 1568.2 |
| β-Carotene | 1538 ± 378.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, C.; Ibáñez, M.R.; Loyola, G.; León, L.; Salvatore, Y.; González, C.; Barraza, V.; Castañeda, F.; Aldunate, R.; Contreras-Porcia, L.; et al. Characterization of an Agarophyton chilense Oleoresin Containing PPARγ Natural Ligands with Insulin-Sensitizing Effects in a C57Bl/6J Mouse Model of Diet-Induced Obesity and Antioxidant Activity in Caenorhabditis elegans. Nutrients 2021, 13, 1828. https://doi.org/10.3390/nu13061828
Pinto C, Ibáñez MR, Loyola G, León L, Salvatore Y, González C, Barraza V, Castañeda F, Aldunate R, Contreras-Porcia L, et al. Characterization of an Agarophyton chilense Oleoresin Containing PPARγ Natural Ligands with Insulin-Sensitizing Effects in a C57Bl/6J Mouse Model of Diet-Induced Obesity and Antioxidant Activity in Caenorhabditis elegans. Nutrients. 2021; 13(6):1828. https://doi.org/10.3390/nu13061828
Chicago/Turabian StylePinto, Claudio, María Raquel Ibáñez, Gloria Loyola, Luisa León, Yasmin Salvatore, Carla González, Víctor Barraza, Francisco Castañeda, Rebeca Aldunate, Loretto Contreras-Porcia, and et al. 2021. "Characterization of an Agarophyton chilense Oleoresin Containing PPARγ Natural Ligands with Insulin-Sensitizing Effects in a C57Bl/6J Mouse Model of Diet-Induced Obesity and Antioxidant Activity in Caenorhabditis elegans" Nutrients 13, no. 6: 1828. https://doi.org/10.3390/nu13061828
APA StylePinto, C., Ibáñez, M. R., Loyola, G., León, L., Salvatore, Y., González, C., Barraza, V., Castañeda, F., Aldunate, R., Contreras-Porcia, L., Fuenzalida, K., & Bronfman, F. C. (2021). Characterization of an Agarophyton chilense Oleoresin Containing PPARγ Natural Ligands with Insulin-Sensitizing Effects in a C57Bl/6J Mouse Model of Diet-Induced Obesity and Antioxidant Activity in Caenorhabditis elegans. Nutrients, 13(6), 1828. https://doi.org/10.3390/nu13061828








