Cause-Specific Stillbirth and Neonatal Death According to Prepregnancy Obesity and Early Gestational Weight Gain: A Study in the Danish National Birth Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Stillbirth and Neonatal Death
2.2. Cause of Death Classification
2.3. Exposures and Covariates
2.4. Statistical Analyses
3. Results
3.1. Causes of Stillbirth or Neonatal Death
3.2. Description of the Study Population
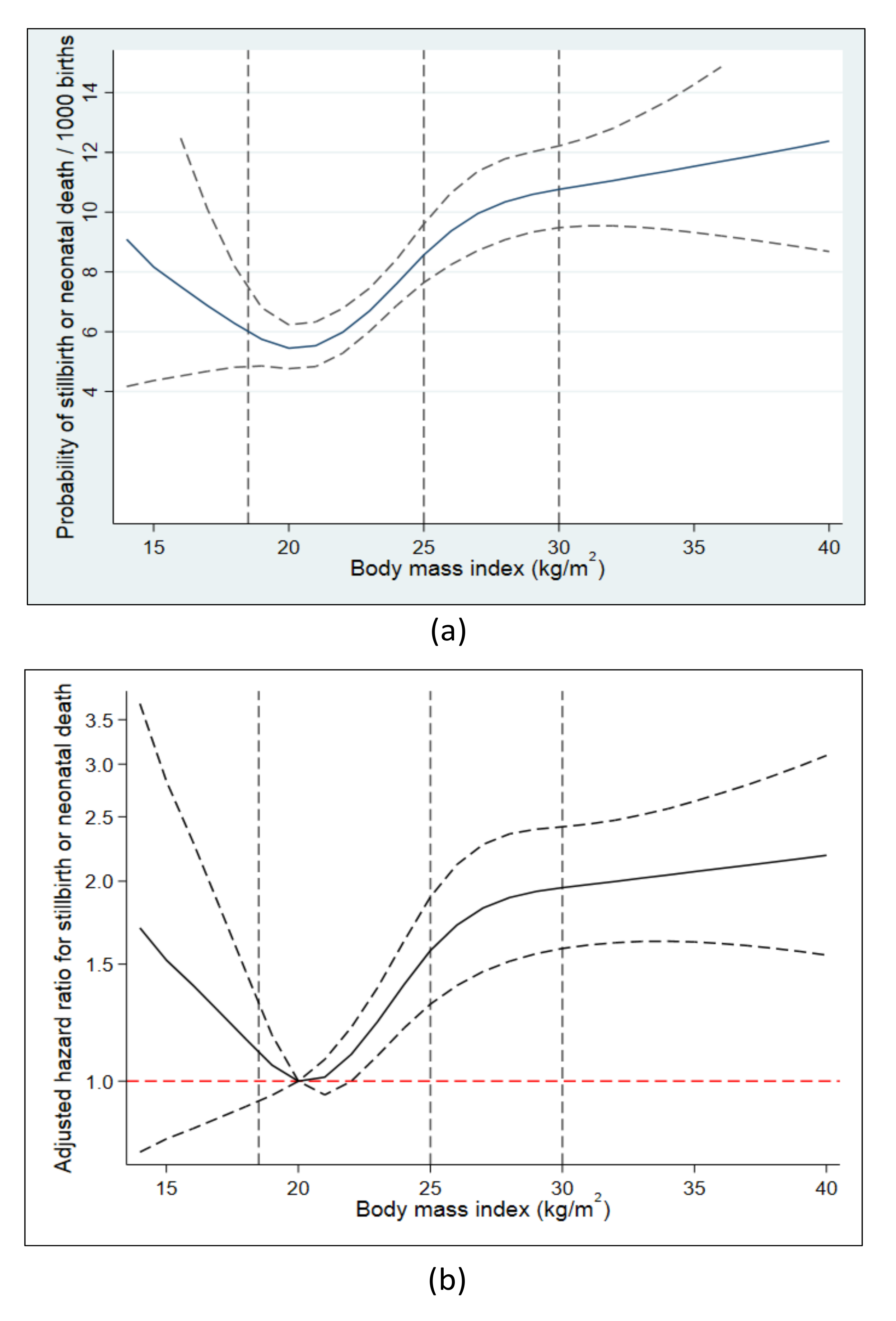
3.3. Prepregnancy Obesity and Risk of Stillbirth or Neonatal Death
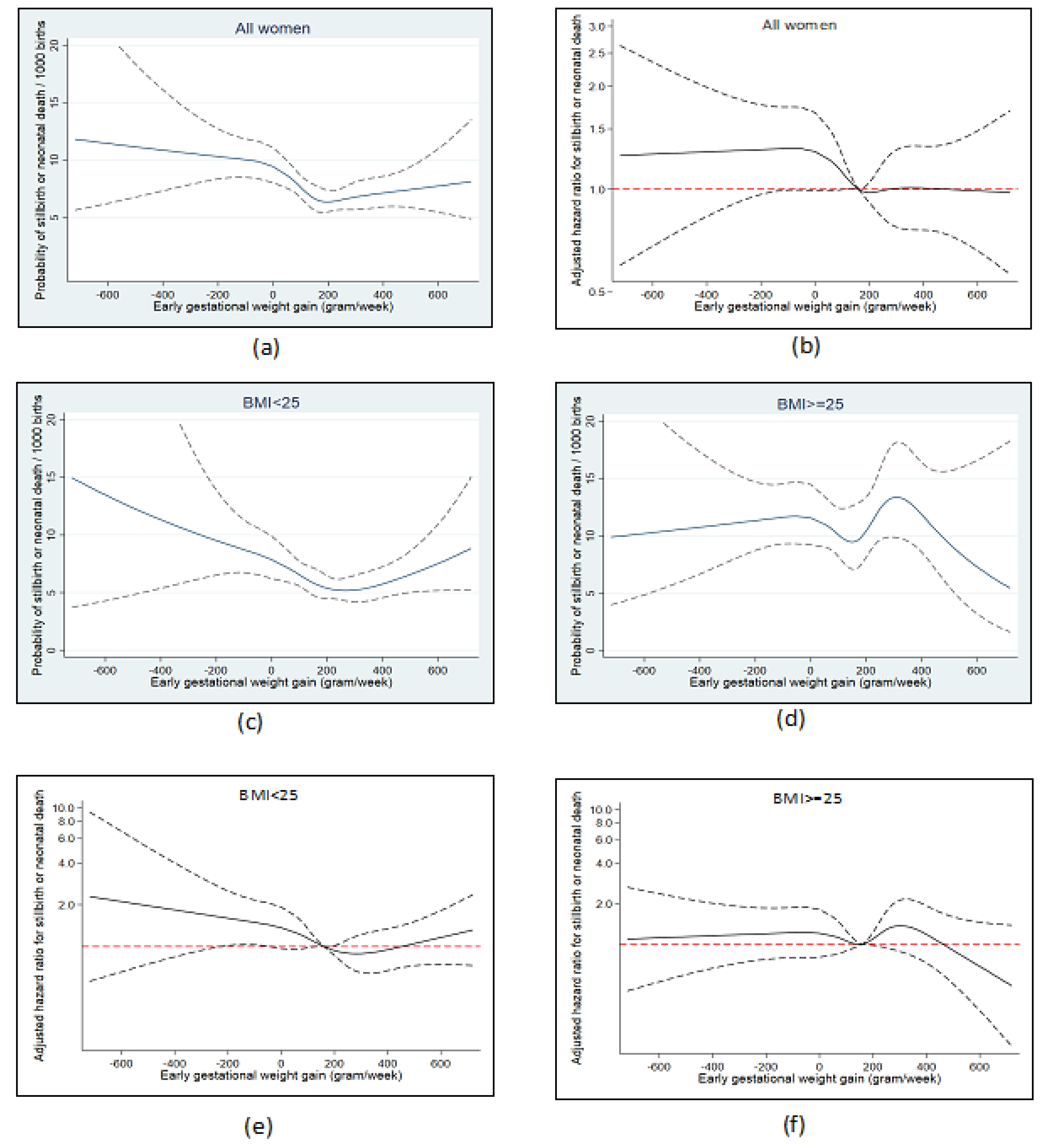
3.4. Early Gestational Weight Gain and Stillbirth or Neonatal Death
3.5. Supplementary Analyses
4. Discussion
4.1. Main Findings
4.2. Prepregnancy Obesity
4.2.1. Comparison with Other Studies
4.2.2. Interpretation
4.3. Early Gestational Weight Gain
4.3.1. Comparison with Other Studies
4.3.2. Interpretation
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: A systematic review and meta-analysis. JAMA 2014, 311, 1536–1546. [Google Scholar] [CrossRef]
- Yao, R.; Ananth, C.V.; Park, B.Y.; Pereira, L.; Plante, L.A.; Perinatal Research, C. Obesity and the risk of stillbirth: A population-based cohort study. Am. J. Obstet. Gynecol. 2014, 210, 457.e1–457.e9. [Google Scholar] [CrossRef]
- Nohr, E.A.; Bech, B.H.; Davies, M.J.; Frydenberg, M.; Henriksen, T.B.; Olsen, J. Prepregnancy Obesity and Fetal Death: A Study Within the Danish National Birth Cohort. Obstet. Gynecol. 2005, 106, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Parks, W.T.; Perkins, K.; Pugh, S.J.; Platt, R.W.; Feghali, M.; Florio, K.; Young, O.; Bernstein, S.; Simhan, H.N. Maternal prepregnancy obesity and cause-specific stillbirth. Am. J. Clin. Nutr. 2015, 102, 858–864. [Google Scholar] [CrossRef]
- Chen, A.; Feresu, S.A.; Fernandez, C.; Rogan, W.J. Maternal obesity and the risk of infant death in the United States. Epidemiology 2009, 20, 74–81. [Google Scholar] [CrossRef]
- Johansson, S.; Villamor, E.; Altman, M.; Bonamy, A.K.; Granath, F.; Cnattingius, S. Maternal overweight and obesity in early pregnancy and risk of infant mortality: A population based cohort study in Sweden. BMJ 2014, 349, g6572. [Google Scholar] [CrossRef] [PubMed]
- Frias, A.E.; Morgan, T.K.; Evans, A.E.; Rasanen, J.; Oh, K.Y.; Thornburg, K.L.; Grove, K.L. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011, 152, 2456–2464. [Google Scholar] [CrossRef]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef]
- Yao, R.; Park, B.Y.; Foster, S.E.; Caughey, A.B. The association between gestational weight gain and risk of stillbirth: A population-based cohort study. Ann. Epidemiol 2017, 27, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Ukah, U.V.; Bayrampour, H.; Sabr, Y.; Razaz, N.; Chan, W.S.; Lim, K.I.; Lisonkova, S. Association between gestational weight gain and severe adverse birth outcomes in Washington State, US: A population-based retrospective cohort study, 2004–2013. PLoS Med. 2019, 16, e1003009. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chauhan, S.P. Association between Gestational Weight Gain Adequacy and Adverse Maternal and Neonatal Outcomes. Am. J. Perinatol. 2019, 36, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; Bodnar, L.M. Good Practices for Observational Studies of Maternal Weight and Weight Gain in Pregnancy. Paediatr. Perinat. Epidemiol. 2018, 32, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Carreno, C.A.; Clifton, R.G.; Hauth, J.C.; Myatt, L.; Roberts, J.M.; Spong, C.Y.; Varner, M.W.; Thorp, J.M., Jr.; Mercer, B.M.; Peaceman, A.M.; et al. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstet. Gynecol. 2012, 119, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Hey, E.N.; Lloyd, D.J.; Wigglesworth, J.S. Classifying perinatal death: Fetal and neonatal factors. Br. J. Obstet. Gynaecol. 1986, 93, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.K.; Hey, E.N.; Thomson, A.M. Classifying perinatal death: An obstetric approach. Br. J. Obstet. Gynaecol. 1986, 93, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.V.; Helweg-Larsen, K.; Lange, A.P. Classification of perinatal and neonatal deaths. Fetal, obstetrical and neonatal causes. Ugeskr. Laeger 1991, 153, 1494–1497. [Google Scholar]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sorensen, T.I.; Aaby, P.; Andersen, A.M.; Taxbol, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort--its background, structure and aim. Scand J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef]
- World Health Organization. Neonatal and perinatal mortality: Country, regional and global estimates. WHO Libr. 2006. Available online: https://apps.who.int/iris/bitstream/handle/10665/43444/9241563206_eng.pdf?sequence=1&isAllowed=y (accessed on 12 April 2021).
- Marsal, K.; Persson, P.H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. Available online: https://apps.who.int/iris/handle/10665/42330 (accessed on 12 April 2021).
- Greenland, S. Dose-response and trend analysis in epidemiology: Alternatives to categorical analysis. Epidemiology 1995, 6, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.S.; Kramer, M.S. The fetuses-at-risk approach: Survival analysis from a fetal perspective. Acta Obstet. Gynecol. Scand. 2018, 97, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.W.; Rankin, J.; Bell, R. Maternal body mass index and the risk of fetal and infant death: A cohort study from the North of England. Hum. Reprod. 2011, 26, 1501–1511. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Siminerio, L.L.; Himes, K.P.; Hutcheon, J.A.; Lash, T.L.; Parisi, S.M.; Abrams, B. Maternal obesity and gestational weight gain are risk factors for infant death. Obesity 2016, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Cedergren, M.I. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet. Gynecol. 2004, 103, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Nohr, E.A.; Villamor, E.; Vaeth, M.; Olsen, J.; Cnattingius, S. Mortality in infants of obese mothers: Is risk modified by mode of delivery? Acta Obstet. Gynecol. Scand. 2012, 91, 363–371. [Google Scholar] [CrossRef]
- Kristensen, J.; Vestergaard, M.; Wisborg, K.; Kesmodel, U.; Secher, N.J. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 2005, 112, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, J.A.; Campbell, O.M.; De Silva, M.J.; Filippi, V. Effect of maternal obesity on neonatal death in sub-Saharan Africa: Multivariable analysis of 27 national datasets. Lancet 2012, 380, 1325–1330. [Google Scholar] [CrossRef]
- Avagliano, L.; Monari, F.; Po, G.; Salerno, C.; Mascherpa, M.; Maiorana, A.; Facchinetti, F.; Bulfamante, G.P. The Burden of Placental Histopathology in Stillbirths Associated With Maternal Obesity. Am. J. Clin. Pathol. 2020, 154, 225–235. [Google Scholar] [CrossRef]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 42–49. [Google Scholar] [CrossRef]
- Ramsay, J.E.; Ferrell, W.R.; Crawford, L.; Wallace, A.M.; Greer, I.A.; Sattar, N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J. Clin. Endocrinol. Metab. 2002, 87, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.C.; Davis, J.M.; Craig, W.Y.; Collins, M.; Allan, W.; Quinn, R.; Dammann, O. Maternal obesity and markers of inflammation in pregnancy. Cytokine 2009, 47, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, S.; Zamorano, M.; Naveiro-Fuentes, M.; Pineda, A.; Rodriguez-Granger, J.; Puertas, A. Maternal obesity and the risk of group B streptococcal colonisation in pregnant women. J. Obstet. Gynaecol. 2019, 39, 628–632. [Google Scholar] [CrossRef]
- Villamor, E.; Norman, M.; Johansson, S.; Cnattingius, S. Maternal Obesity and Risk of Early-onset Neonatal Bacterial Sepsis: Nationwide Cohort and Sibling-controlled Studies. Clin. Infect. Dis. 2020, ciaa78. [Google Scholar] [CrossRef]
- Cnattingius, S.; Villamor, E.; Johansson, S.; Edstedt Bonamy, A.K.; Persson, M.; Wikstrom, A.K.; Granath, F. Maternal obesity and risk of preterm delivery. JAMA 2013, 309, 2362–2370. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Hayes, L.; Crowe, L.; Jones, D.; Robalino, S.; Slack, E.; Rankin, J. Maternal body mass index and post-term birth: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 293–308. [Google Scholar] [CrossRef]
- Slack, E.; Best, K.E.; Rankin, J.; Heslehurst, N. Maternal obesity classes, preterm and post-term birth: A retrospective analysis of 479,864 births in England. BMC Pregnancy Childbirth 2019, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.X.; He, X.J.; Hu, C.L. Maternal pre-pregnancy obesity and the risk of macrosomia: A meta-analysis. Arch. Gynecol. Obstet. 2018, 297, 139–145. [Google Scholar] [CrossRef]
- Blomberg, M. Maternal obesity, mode of delivery, and neonatal outcome. Obstet. Gynecol. 2013, 122, 50–55. [Google Scholar] [CrossRef]
- Ovesen, P.; Rasmussen, S.; Kesmodel, U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet. Gynecol. 2011, 118, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Helle, E.; Priest, J.R. Maternal Obesity and Diabetes Mellitus as Risk Factors for Congenital Heart Disease in the Offspring. J. Am. Heart Assoc. 2020, 9, e011541. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Razaz, N.; Edstedt Bonamy, A.K.; Villamor, E.; Cnattingius, S. Maternal Overweight and Obesity and Risk of Congenital Heart Defects. J. Am. Coll. Cardiol. 2019, 73, 44–53. [Google Scholar] [CrossRef]
- Stothard, K.J.; Tennant, P.W.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Maffoni, S.; De Giuseppe, R.; Stanford, F.C.; Cena, H. Folate status in women of childbearing age with obesity: A review. Nutr. Res. Rev. 2017, 30, 265–271. [Google Scholar] [CrossRef]
- Morikawa, M.; Yamada, T.; Yamada, T.; Sato, S.; Cho, K.; Minakami, H. Prevalence of hyperglycemia during pregnancy according to maternal age and pre-pregnancy body mass index in Japan, 2007–2009. Int. J. Gynaecol. Obstet. 2012, 118, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. (Eds.) Weight Gain during Pregnancy: Reexamining the Guidelines; Institute of Medicine. National Academies Press: Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32813/ (accessed on 12 April 2021).
- Pickens, C.M.; Hogue, C.J.; Howards, P.P.; Kramer, M.R.; Badell, M.L.; Dudley, D.J.; Silver, R.M.; Goldenberg, R.L.; Pinar, H.; Saade, G.R.; et al. The association between gestational weight gain z-score and stillbirth: A case-control study. BMC Pregnancy Childbirth 2019, 19, 451. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.J.; Oken, E.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Stuebe, A.M.; Kleinman, K.P.; Gillman, M.W. Weight gain in pregnancy and risk of maternal hyperglycemia. Am. J. Obstet. Gynecol. 2009, 201, 61.e1–61.e7. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.C.; Bodnar, L.M.; Himes, K.P.; Hutcheon, J.A. Patterns of Gestational Weight Gain in Early Pregnancy and Risk of Gestational Diabetes Mellitus. Epidemiology 2017, 28, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, H.; Bliddal, M.; Stovring, H.; Rasmussen, K.M.; Gunderson, E.P.; Kober, L.; Sorensen, T.I.A.; Nohr, E.A. Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: A cohort study. PLoS Med. 2021, 18, e1003486. [Google Scholar] [CrossRef]
- Smith, G.C.; Pell, J.P.; Walsh, D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet 2001, 357, 2002–2006. [Google Scholar] [CrossRef]
- Asgharvahedi, F.; Gholizadeh, L.; Siabani, S. The risk of cardiovascular disease in women with a history of miscarriage and/or stillbirth. Health Care Women Int. 2019, 40, 1117–1131. [Google Scholar] [CrossRef]
- Wang, Y.X.; Minguez-Alarcon, L.; Gaskins, A.J.; Missmer, S.A.; Rich-Edwards, J.W.; Manson, J.E.; Pan, A.; Chavarro, J.E. Association of spontaneous abortion with all cause and cause specific premature mortality: Prospective cohort study. BMJ 2021, 372, n530. [Google Scholar] [CrossRef] [PubMed]
- Many, A.; Elad, R.; Yaron, Y.; Eldor, A.; Lessing, J.B.; Kupferminc, M.J. Third-trimester unexplained intrauterine fetal death is associated with inherited thrombophilia. Obstet. Gynecol. 2002, 99, 684–687. [Google Scholar] [CrossRef]
- Muin, D.A.; Kollmann, M.; Blatterer, J.; Hoermann, G.; Husslein, P.W.; Lafer, I.; Petek, E.; Schwarzbraun, T. Cardio-pathogenic variants in unexplained intrauterine fetal death: A retrospective pilot study. Sci. Rep. 2021, 11, 6737. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, P.; Hoilund-Carlsen, P.F.; Quaade, F. Comparison of self-reported height and weight with controlled height and weight in women and men. Int. J. Obes. 1981, 5, 67–76. [Google Scholar] [PubMed]
- Christensen, A.I.; Ekholm, O.; Davidsen, M.; Juel, K. Health and Disease in Denmark 2010 and the Development Since 1987; Danish Institute of Public Health: Copenhagen, Denmark, 2012; Available online: https://www.sdu.dk/da/sif/rapporter/2012/sundhed_og_sygelighed_i_danmark_2010 (accessed on 12 April 2021).
- Nohr, E.A.; Frydenberg, M.; Henriksen, T.B.; Olsen, J. Does low participation in cohort studies induce bias? Epidemiology 2006, 17, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.W.; Joseph, K.S.; Ananth, C.V.; Grondines, J.; Abrahamowicz, M.; Kramer, M.S. A proportional hazards model with time-dependent covariates and time-varying effects for analysis of fetal and infant death. Am. J. Epidemiol. 2004, 160, 199–206. [Google Scholar] [CrossRef]
- Smith, G.C. Estimating risks of perinatal death. Am. J. Obstet. Gynecol. 2005, 192, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Herrera, T.T.; Garcia, J.L.; Britton, G.B. Blood-based biomarkers of adverse perinatal outcomes in maternal obesity. J. Matern. Fetal. Neonatal. Med. 2017, 30, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C. Screening and prevention of stillbirth. Best Pract. Res. Clin. Obstet. Gynaecol 2017, 38, 71–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- The Danish National Birth Cohort, website. Available online: https://www.dnbc.dk (accessed on 13 May 2021).
| Stillbirth | Neonatal Death | All Deaths | ||||
|---|---|---|---|---|---|---|
| Cause of Death | n | % | n | % | n | % |
| Congenital anomalies | 28 | 10.3 | 91 | 40.3 | 119 | 23.9 |
| Unexplained intrauterine death (UID) | 79 | 29.0 | 79 | 15.9 | ||
| Placental dysfunction | 79 | 29.0 | 15 | 6.6 | 94 | 18.9 |
| Umbilical cord complications | 36 | 13.2 | 2 | 0.9 | 38 | 7.6 |
| Maternal disease | 9 | 3.3 | 12 | 5.3 | 21 | 4.2 |
| Intrapartum events | 23 | 8.5 | 26 | 11.5 | 49 | 9.8 |
| Preterm birth | 32 | 14.2 | 32 | 6.4 | ||
| Infections | 10 | 3.7 | 23 | 10.2 | 33 | 6.6 |
| Other causes b | 8 | 2.9 | 25 | 11.1 | 33 | 6.6 |
| Total | 272 | 100 | 226 | 100 | 498 | 100 |
| All Pregnancies (n) | Body Mass Index | ||||||
|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | 30+ | Stillbirth b | Neonatal Death c | ||
| Population (n) | 85,822 | 3869 | 58,183 | 16,699 | 7071 | ||
| Stillbirth b (risk/1000) | 407 | 3.1 | 4.0 | 6.7 | 7.5 | 4.7 | |
| Neonatal death c (risk/1000) | 226 | 3.4 | 2.2 | 3.5 | 3.7 | 2.6 | |
| Age at conception | |||||||
| <25 | 11,080 | 18.5 | 11.9 | 13.8 | 16.3 | 4.3 | 3.2 |
| 25–29 | 35,770 | 41.8 | 41.6 | 41.9 | 41.8 | 3.9 | 2.4 |
| 30–34 | 29,131 | 30.2 | 34.6 | 33.3 | 31.8 | 4.8 | 2.5 |
| 35+ | 9841 | 9.5 | 11.9 | 11.1 | 10.1 | 8.0 | 2.9 |
| Parity | |||||||
| Primiparous | 40,238 | 46.0 | 48.0 | 44.3 | 44.2 | 5.2 | 3.1 |
| Multiparous | 45,584 | 54.0 | 52.0 | 55.7 | 55.8 | 4.3 | 2.3 |
| Smoking | |||||||
| Nonsmoker or cessation | 72,914 | 74.8 | 86.0 | 84.4 | 83.5 | 4.4 | 2.6 |
| Smoking at 1st interview | 12,878 | 25.2 | 14.0 | 15.6 | 16.6 | 6.5 | 2.9 |
| Social status | |||||||
| Highest | 45,108 | 47.6 | 57.1 | 46.2 | 35.8 | 4.3 | 2.2 |
| Middle | 32,260 | 39.3 | 35.0 | 42.8 | 47.4 | 4.8 | 2.9 |
| Lowest | 8097 | 13.2 | 7.9 | 11.0 | 16.8 | 6.7 | 4.3 |
| Obesity-related disease | |||||||
| Pre-gestational diabetes | 256 | 0.0 | 0.3 | 0.4 | 0.5 | 3.9 | 7.8 |
| Gestational diabetes | 1025 | 0.4 | 0.6 | 1.7 | 5.0 | 3.9 | 2.0 |
| Preeclampsia | 1905 | 0.9 | 1.7 | 3.0 | 5.4 | 7.3 | 6.8 |
| Other hypertensive disorders | 1359 | 0.6 | 1.1 | 2.2 | 4.4 | 3.7 | 5.2 |
| Early gestational weight gaind(gram/week) | |||||||
| Lost weight or gain < 50 g | 13,903 | 7.2 | 14.6 | 29.3 | 53.7 | 7.1 | 3.0 |
| 50–300 g | 41,598 | 64.4 | 66.0 | 54.7 | 36.5 | 4.1 | 2.8 |
| 300 g+ | 12,446 | 28.4 | 19.4 | 16.0 | 9.8 | 4.3 | 2.7 |
| BMI < 18.5 (3869) | BMI 18.5–24.9 (58,183) | BMI 25–29.9 (16,699) | BMI 30 + (7071) | Per 1 BMI-Unit Increase (Only BMI ≥20) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 85,822 Cause (Number of Deaths) | Risk /1000 | HR b | 95% CI | Risk/1000 | HR Reference | Risk/ 1000 | HR b | 95% CI | Risk/ 1000 | HR b | 95% CI | HR b | 95% CI | |
| All causes (633) | Crude | 6.46 | 1.06 | (0.70–1.58) | 6.15 | 1.0 (ref) | 10.24 | 1.66 | (1.38–1.99) | 11.17 | 1.82 | (1.42–2.32) | 1.05 | (1.03–1.07) |
| Adjusted c | 1.06 | (0.70–1.60) | 1.0 (ref) | 1.66 | (1.38–2.00) | 1.78 | (1.39–2.27) | 1.05 | (1.03–1.06) | |||||
| Early stillbirths (135) | Crude | 1.55 | 1.14 | (0.50–2.62) | 1.36 | 1.0 (ref) | 2.22 | 1.63 | (1.10–2.41) | 1.84 | 1.34 | (0.75–2.42) | 1.03 | (1.00–1.07 |
| Adjusted c | 1.08 | (0.47–2.52) | 1.0 (ref) | 1.60 | (1.08–2.38) | 1.27 | (0.70–2.29) | 1.03 | (0.99–1.07) | |||||
| Congenital | Crude | 2.07 | 1.91 | (0.91–3.98) | 1.10 | 1.0 (ref) | 2.16 | 1.96 | (1.30–2.94) | 1.56 | 1.40 | (0.74–2.65) | 1.04 | (1.01–1.07) |
| anomalies (119) | Adjusted c | 1.90 | (0.90–4.02) | 1.0 (ref) | 1.89 | (1.26–2.86) | 1.34 | (0.71–2.52) | 1.03 | (1.00–1.06) | ||||
| Unexplained intra- | Crude | 0.26 | 0.33 | (0.05–2.38) | 0.79 | 1.0 (ref) | 1.14 | 1.43 | (0.84–2.44) | 1.84 | 2.31 | (1.25–4.27) | 1.05 | (1.01–1.10) |
| uterine death (UID) (79) | Adjustedc | 0.35 | (0.05–2.56) | 1.0 (ref) | 1.50 | (0.88–2.56) | 2.42 | (1.32–4.44) | 1.06 | (1.01–1.10) | ||||
| Placental | Crude | 1.03 | 1.25 | (0.45–3.48) | 0.82 | 1.0 (ref) | 1.68 | 2.03 | (1.27–3.24) | 1.98 | 2.40 | (1.33–4.34) | 1.09 | (1.05–1.12) |
| dysfunction (94) | Adjusted c | 1.21 | (0.43–3.43) | 1.0 (ref) | 2.06 | (1.27–3.34) | 2.39 | (1.30–4.39) | 1.09 | (1.05–1.12) | ||||
| Umbilical cord | Crude | 0.00 | NA | NA | 0.36 | 1.0 (ref) | 0.66 | 1.84 | (0.89–3.81) | 0.85 | 2.40 | (0.97–5.94) | 1.08 | (1.01–1.15) |
| complications (38) | Adjusted c | NA | NA | 1.0 (ref) | 1.91 | (0.93–3.93) | 2.55 | (1.02–6.37) | 1.08 | (1.02–1.15) | ||||
| Maternal | Crude | 0.52 | 2.52 | (0.56–11.37) | 0.21 | 1.0 (ref) | 0.36 | 1.72 | (0.64–4.60) | 0.14 | 0.68 | (0.09–5.22) | 0.99 | (0.91–1.08) |
| disease (21) | Adjustedc | 2.81 | (0.64–12.28) | 1.0 (ref) | 1.87 | (0.72–4.83) | 0.75 | (0.10–5.70) | 1.00 | (0.93–1.08) | ||||
| Intrapartum | Crude | 0.00 | NA | NA | 0.50 | 1.0 (ref) | 0.60 | 1.20 | (0.58–2.46) | 1.41 | 2.89 | (1.41–5.93) | 1.07 | (1.02–1.13) |
| events (49) | Adjusted c | NA | NA | 1.0 (ref) | 1.33 | (0.64–2.75) | 3.37 | (1.59–7.12) | 1.08 | (1.03–1.14) | ||||
| Preterm birth (32) | Crude | 0.00 | NA | NA | 0.38 | 1.0 (ref) | 0.36 | 0.94 | (0.38–2.33) | 0.57 | 1.54 | (0.53–4.48) | 1.03 | (0.95–1.11) |
| Adjusted c | NA | NA | 1.0 (ref) | 0.94 | (0.38–2.29) | 1.52 | (0.53–4.36) | 1.03 | (0.95–1.11) | |||||
| Infections (33) | Crude | 0.52 | 2.08 | (0.48–9.04) | 0.26 | 1.0 (ref) | 0.60 | 2.31 | (1.04–5.16) | 0.85 | 3.30 | (1.28–8.50) | 1.08 | (1.03–1.13) |
| Adjusted c | 2.29 | (0.54–9.78) | 1.0 (ref) | 2.43 | (1.08–5.47) | 3.56 | (1.38–9.13) | 1.08 | (1.03–1.13) | |||||
| Other causes d (33) | Crude | 0.52 | 1.37 | (0.32–5.70) | 0.38 | 1.0 (ref) | 0.48 | 1.26 | (0.56–2.85) | 0.14 | 0.38 | (0.05–2.83) | 0.96 | (0.87–1.06) |
| Adjusted c | 1.29 | (0.30–5.50) | 1.0 (ref) | 1.24 | (0.55–2.81) | 0.37 | (0.05–2.70) | 0.96 | (0.86–1.06) | |||||
| <50 g/Week (13,899) | 50–300 g/Week (41,578) | 300 g+/Week (12,430) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 67,947 | Risk/1000 | HRc | 95% CI | Risk/1000 | HR c Reference | Risk/1000 | HRc | 95% CI | |
| Cause (number of deaths) | |||||||||
| All causes (513) | Crude | 10.1 | 1.45 | (1.18–1.78) | 6.9 | 1.0 (ref) | 6.9 | 1.00 | (0.79–1.28) |
| n = 67,947 | Adjusted d | 1.26 | (1.01–1.58) | 1.0 (ref) | 0.95 | (0.74–1.21) | |||
| BMI < 25 (303) | Crude | 8.7 | 1.44 | (1.07–1.94) | 5.9 | 1.0 (ref) | 5.5 | 0.94 | (0.69–1.28) |
| n = 48,894 | Adjusted d | 1.43 | (1.05–1.93) | 1.0 (ref) | 0.90 | (0.66–1.22) | |||
| BMI ≥25 (210) | Crude | 11.6 | 1.10 | (0.82–1.49) | 10.3 | 1.0 (ref) | 11.9 | 1.15 | (0.77–1.72) |
| n = 19,053 | Adjusted d | 1.13 | (0.83–1.55) | 1.0 (ref) | 1.10 | (0.73–1.64) | |||
| Cause-specific death, n = 67,947 | p for interaction = 0.21 | ||||||||
| Early stillbirths (112) | Crude | 2.2 | 1.82 | (1.14–2.91) | 1.3 | 1.0 (ref) | 2.3 | 1.87 | (1.19–2.95) |
| Adjusted d | 1.71 | (1.03–2.85) | 1.0 (ref) | 1.72 | (1.09–2.72) | ||||
| Congenital | Crude | 1.6 | 1.10 | (0.67–1.78) | 1.4 | 1.0 (ref) | 0.9 | 0.64 | (0.34–1.23) |
| anomalies (91) | Adjusted d | 0.98 | (0.57–1.69) | 1.0 (ref) | 0.66 | (0.35–1.27) | |||
| Unexplained intra- | Crude | 1.8 | 2.26 | (1.31–3.90) | 0.7 | 1.0 (ref) | 0.3 | 0.42 | (0.15–1.19) |
| uterine death (UID) (59) | Adjusted d | 2.07 | (1.17–3.65) | 1.0 (ref) | 0.39 | (0.14–1.11) | |||
| Placental | Crude | 1.5 | 1.33 | (0.79–2.23) | 1.1 | 1.0 (ref) | 0.8 | 0.72 | (0.36–1.44) |
| dysfunction (77) | Adjusted d | 0.96 | (0.52–1.76) | 1.0 (ref) | 0.64 | (0.32–1.30) | |||
| Umbilical cord | Crude | 0.6 | 1.42 | (0.57–3.55) | 0.3 | 1.0 (ref) | 0.5 | 0.91 | (0.30–2.73) |
| complications (28) | Adjusted d | 1.09 | (0.40–2.94) | 1.0 (ref) | 0.88 | (0.29–2.64) | |||
| Maternal | Crude | 0.3 | 1.40 | (0.45–4.35) | 0.2 | 1.0 (ref) | 0.4 | 2.03 | (0.66–6.26) |
| disease (17) | Adjusted d | 1.39 | (0.46–4.21) | 1.0 (ref) | 2.02 | (0.66–6.17) | |||
| Intrapartum | Crude | 0.7 | 1.05 | (0.49–2.26) | 0.6 | 1.0 (ref) | 0.6 | 0.98 | (0.44–2.19) |
| events (45) | Adjusted d | 0.75 | (0.34–1.66) | 1.0 (ref) | 0.99 | (0.44–2.21) | |||
| Preterm birth (28) | Crude | 0.4 | 1.15 | (0.44–3.00) | 0.5 | 1.0 (ref) | 0.2 | 0.52 | (0.15–1.79) |
| Adjusted d | 1.13 | (0.42–3.03) | 1.0 (ref) | 0.52 | (0.15–1.80) | ||||
| Infections (29) | Crude | 0.7 | 1.82 | (0.82–4.03) | 0.4 | 1.0 (ref) | 0.2 | 0.62 | (0.18–2.17) |
| Adjusted d | 1.60 | (0.63–4.07) | 1.0 (ref) | 0.61 | (0.17–2.15) | ||||
| Other causes e (27) | Crude | 0.3 | 0.88 | (0.27–2.75) | 0.3 | 1.0 (ref) | 0.7 | 2.22 | (0.96–5.12) |
| Adjusted d | 0.95 | (0.31–2.91) | 1.0 (ref) | 2.22 | (0.96–5.13) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohr, E.A.; Wolff, S.; Kirkegaard, H.; Wu, C.; Andersen, A.-M.N.; Olsen, J.; Bech, B.H. Cause-Specific Stillbirth and Neonatal Death According to Prepregnancy Obesity and Early Gestational Weight Gain: A Study in the Danish National Birth Cohort. Nutrients 2021, 13, 1676. https://doi.org/10.3390/nu13051676
Nohr EA, Wolff S, Kirkegaard H, Wu C, Andersen A-MN, Olsen J, Bech BH. Cause-Specific Stillbirth and Neonatal Death According to Prepregnancy Obesity and Early Gestational Weight Gain: A Study in the Danish National Birth Cohort. Nutrients. 2021; 13(5):1676. https://doi.org/10.3390/nu13051676
Chicago/Turabian StyleNohr, Ellen Aagaard, Sanne Wolff, Helene Kirkegaard, Chunsen Wu, Anne-Marie Nybo Andersen, Jørn Olsen, and Bodil Hammer Bech. 2021. "Cause-Specific Stillbirth and Neonatal Death According to Prepregnancy Obesity and Early Gestational Weight Gain: A Study in the Danish National Birth Cohort" Nutrients 13, no. 5: 1676. https://doi.org/10.3390/nu13051676
APA StyleNohr, E. A., Wolff, S., Kirkegaard, H., Wu, C., Andersen, A.-M. N., Olsen, J., & Bech, B. H. (2021). Cause-Specific Stillbirth and Neonatal Death According to Prepregnancy Obesity and Early Gestational Weight Gain: A Study in the Danish National Birth Cohort. Nutrients, 13(5), 1676. https://doi.org/10.3390/nu13051676






