Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns
Abstract
1. Introduction
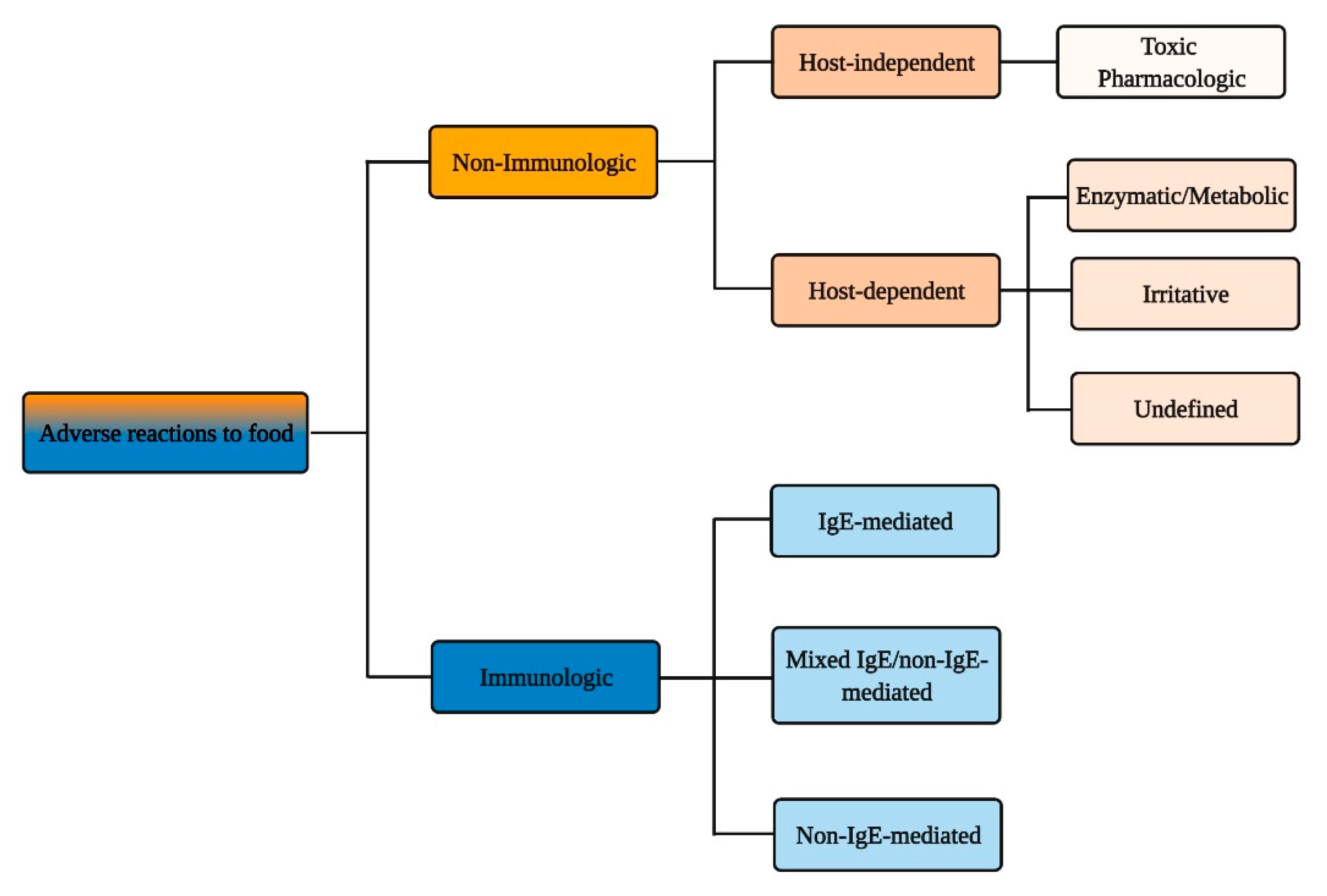
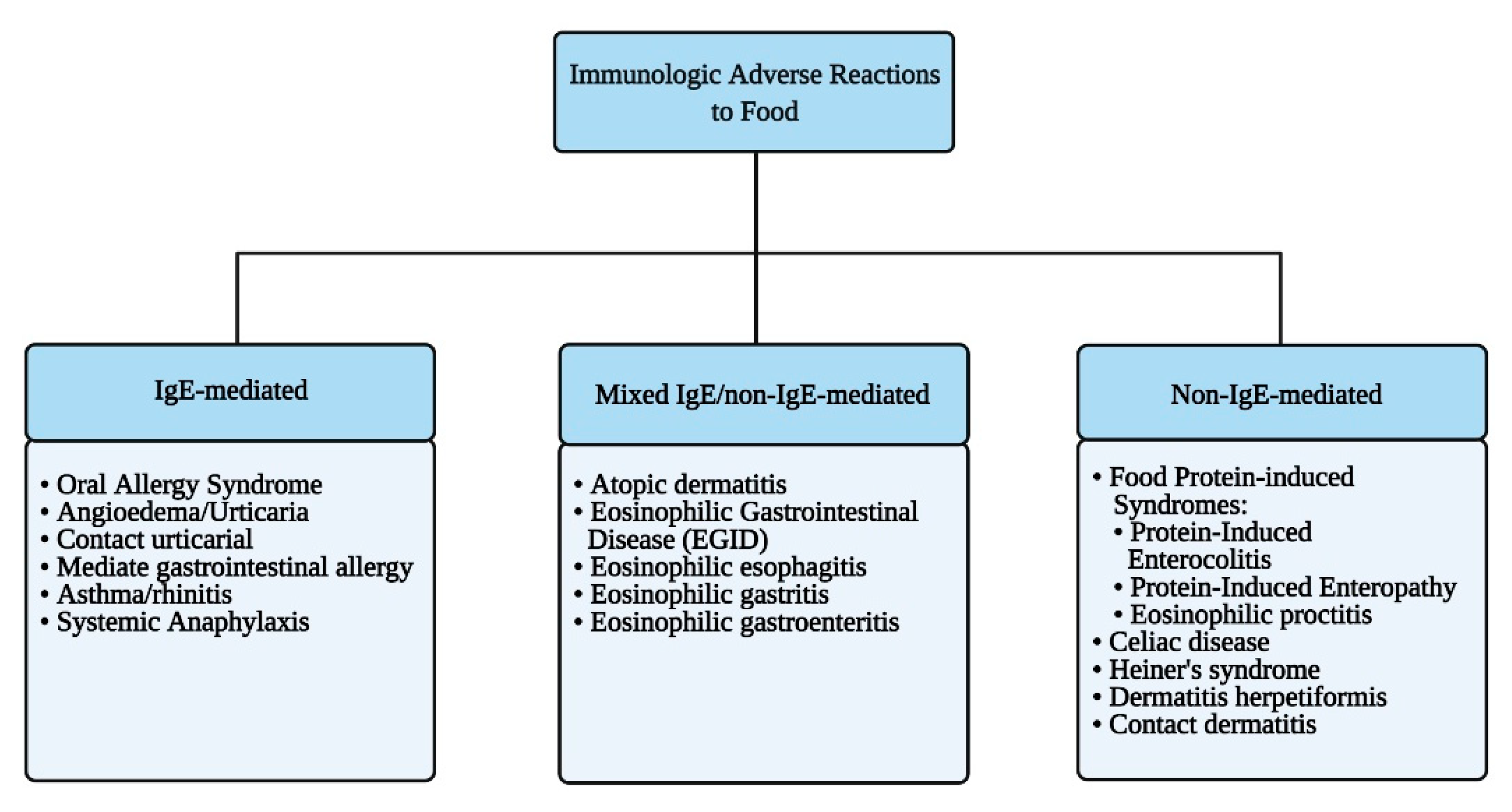
2. Immunologic Adverse Reactions to Food
2.1. IgE-Mediated Food Allergy
2.1.1. Diagnostic and Therapeutic Management of IgE-Mediated Food Allergy
2.1.2. Nutritional Concerns in IgE-Mediated Food Allergy
2.2. Mixed IgE and Non-IgE-Mediated Food Allergy
2.2.1. Diagnostic and Therapeutic Management of Mixed IgE/Non-IgE-Mediated Food Allergy
2.2.2. Nutritional Concerns in Mixed IgE and Non-IgE-Mediated Food Allergy
2.3. Non-IgE-Mediated Food Allergy
2.3.1. Diagnostic and Therapeutic Management of Non-IgE-Mediated Food Protein-Induced Allergy
2.3.2. Nutritional Concerns in Non-IgE-Mediated Food Protein-Induced Allergy
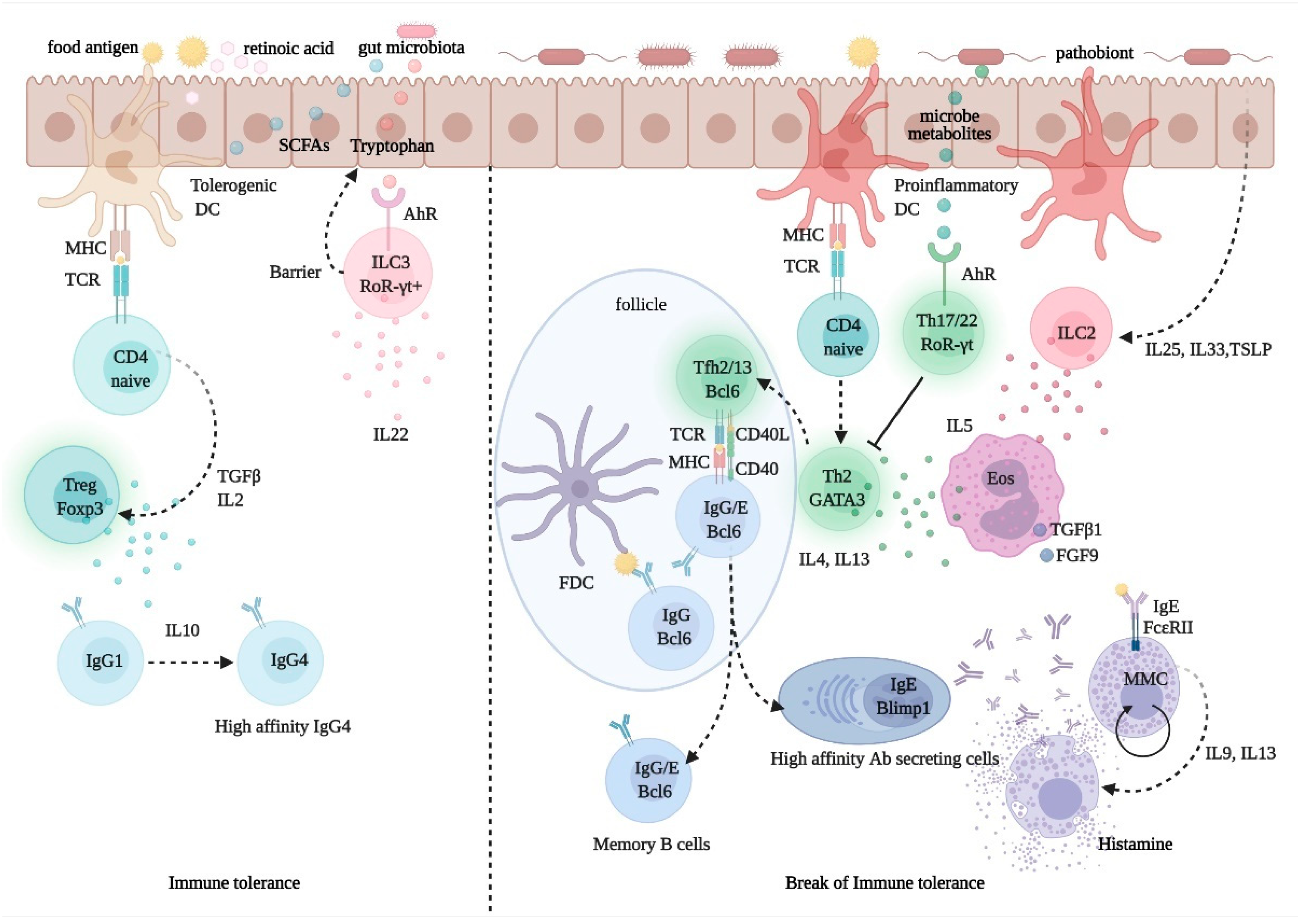
2.4. Pathophysiology of Immunologic Adverse Reactions to Food
3. Non-Immunologic Adverse Reactions to Food
3.1. Host-Independent Non-Immunologic Adverse Reactions to Food
3.1.1. Diagnostic and Therapeutic Management of Host-Independent Reactions to Food
3.1.2. Nutritional Concerns for Host-Independent Reactions to Food
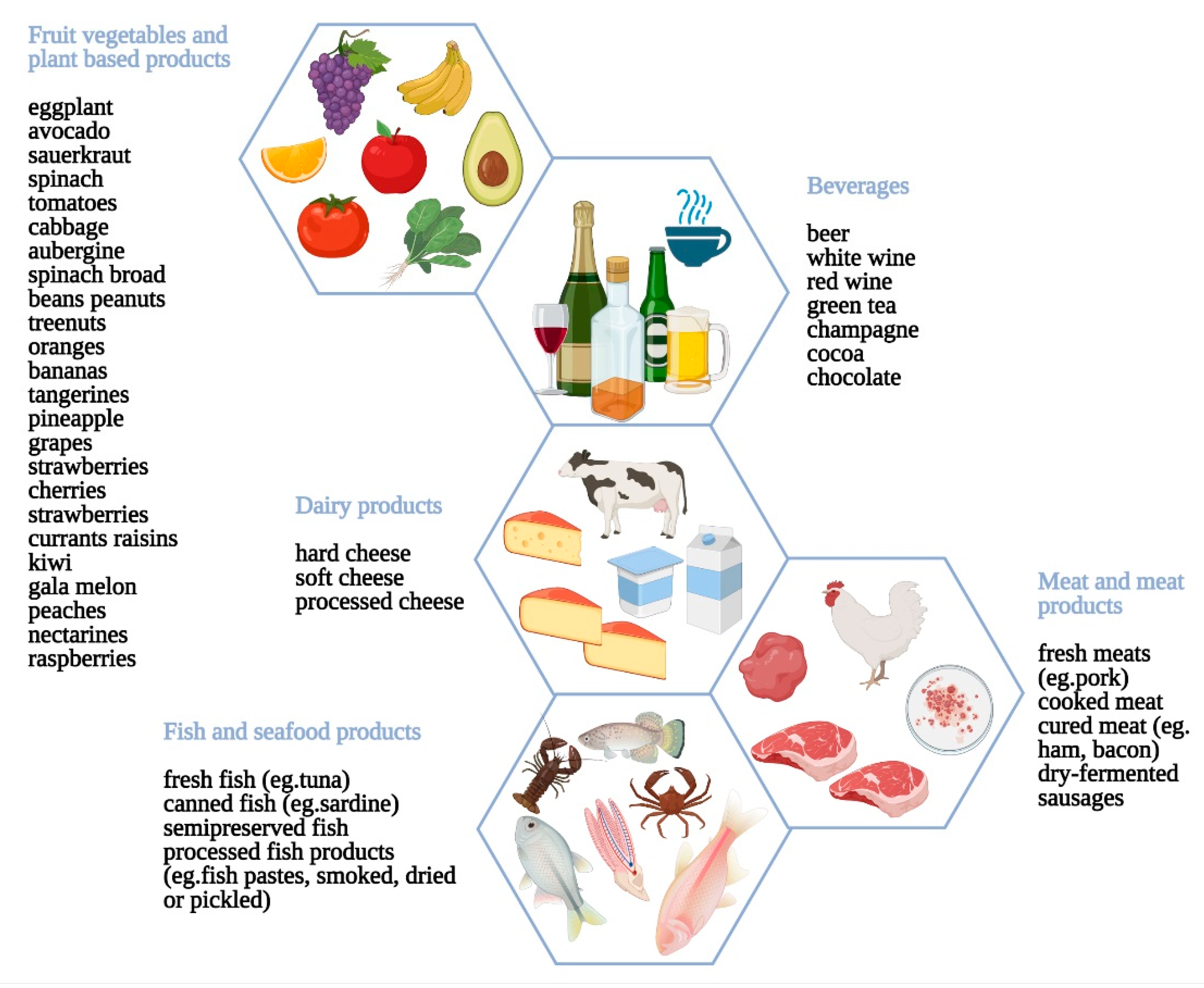
3.2. Host-Dependent Non-Immunologic Adverse Reactions to Food
3.2.1. Diagnostic and Therapeutic Management of Host-Independent Reactions to Food
3.2.2. Nutritional Concerns for Host-Dependent Reactions to Food
3.3. Psychological Correlates of Food Intolerance
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the united states: Summary of the NIAID-sponsored expert panel report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and burden of food allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among us adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Warren, C.M.; Dant, C.; Gupta, R.S.; Nadeau, K.C. Food allergy from infancy through adulthood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1854–1864. [Google Scholar] [CrossRef]
- Santos, A.F. Prevention of food allergy: Can we stop the rise of ige mediated food allergies? Curr. Opin. Allergy Clin. Immunol. 2021, 21, 195–201. [Google Scholar] [CrossRef]
- Wolf, W.A.; Jerath, M.R.; Sperry, S.L.; Shaheen, N.J.; Dellon, E.S. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1272–1279. [Google Scholar] [CrossRef][Green Version]
- Onyimba, F.; Crowe, S.E.; Johnson, S.; Leung, J. Food allergies and intolerances: A clinical approach to the diagnosis and management of adverse reactions to food. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.L.; Adams, H.N.; Gorard, D.A. Review article: The diagnosis and management of food allergy and food intolerances. Aliment. Pharmacol. Ther. 2015, 41, 3–25. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Lang, D.M.; Khan, D.A.; Craig, T.; Dreyfus, D.; Hsieh, F.; Sheikh, J.; Weldon, D.; Zuraw, B.; Bernstein, D.I.; et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J. Allergy Clin. Immunol. 2014, 133, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.P.; Bansil, S.; Uygungil, B. Signs and symptoms of food allergy and food-induced anaphylaxis. Pediatr. Clin. N. Am. 2015, 62, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Wong, W.H.; Chang, C. Clinical spectrum of food allergies: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 46, 225–240. [Google Scholar] [CrossRef]
- Sampson, H.A.; Munoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report--second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. Ann. Emerg. Med. 2006, 47, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A. Update on food allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Waserman, S.; Watson, W. Food allergy. Allergy Asthma Clin. Immunol. 2011, 7, S7. [Google Scholar] [CrossRef]
- Price, A.; Ramachandran, S.; Smith, G.P.; Stevenson, M.L.; Pomeranz, M.K.; Cohen, D.E. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis 2015, 26, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.; Ardusso, L.R.; Bilo, M.B.; El-Gamal, Y.M.; Ledford, D.K.; Ring, J.; Sanchez-Borges, M.; Senna, G.E.; Sheikh, A.; Thong, B.Y.; et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ. J. 2011, 4, 13–37. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Cianferoni, A.; Muraro, A. Food-induced anaphylaxis. Immunol. Allergy Clin. N. Am. 2012, 32, 165–195. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.S.; Chee, R.; Nagendran, V.; Warner, A.; Hayman, G. Dangerous liaison: Sexually transmitted allergic reaction to brazil nuts. J. Investig. Allergol. Clin. Immunol. 2007, 17, 189–191. [Google Scholar]
- Liccardi, G.; Caminati, M.; Senna, G.; Calzetta, L.; Rogliani, P. Anaphylaxis and intimate behaviour. Curr. Opin Allergy Clin. Immunol. 2017, 17, 350–355. [Google Scholar] [CrossRef]
- Lyons, S.A.; Burney, P.G.J.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Clausen, M.; Dubakiene, R.; Fernandez-Perez, C.; Fritsche, P.; Jedrzejczak-Czechowicz, M.; et al. Food allergy in adults: Substantial variation in prevalence and causative foods across europe. J. Allergy Clin. Immunol. Pract. 2019, 7, 1920–1928.e1911. [Google Scholar] [CrossRef]
- Freye, H.B.; Esch, R.E.; Litwin, C.M.; Sorkin, L. Anaphylaxis to the ingestion and inhalation of tenebrio molitor (mealworm) and zophobas morio (superworm). Allergy Asthma Proc. 1996, 17, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Gautreau, M.; Restuccia, M.; Senser, K.; Weisberg, S.N. Familial anaphylaxis after silkworm ingestion. Prehosp. Emerg. Care 2017, 21, 83–85. [Google Scholar] [CrossRef]
- De Pasquale, T.; Buonomo, A.; Illuminati, I.; D’Alo, S.; Pucci, S. Recurrent anaphylaxis: A case of ige-mediated allergy to carmine red (e120). J. Investig. Allergol. Clin. Immunol. 2015, 25, 440–441. [Google Scholar]
- de Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; Allergy, E.F.; Anaphylaxis Guidelines, G. Prevalence of common food allergies in europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Antonicelli, L.; Arena, A.; Bommarito, L.; Caruso, B.; Colombo, G.; Crivellaro, M.; De Carli, M.; Della Torre, E.; Della Torre, F.; et al. Causes of food-induced anaphylaxis in italian adults: A multi-centre study. Int. Arch. Allergy Immunol. 2009, 150, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Fernandez-Rivas, M.; Caringi, M.; Amato, S.; Mistrello, G.; Asero, R. Allergy to peanut lipid transfer protein (ltp): Frequency and cross-reactivity between peanut and peach ltp. Eur. Ann. Allergy Clin. Immunol. 2009, 41, 106–111. [Google Scholar]
- Asero, R.; Mistrello, G.; Amato, S.; Roncarolo, D.; Martinelli, A.; Zaccarini, M. Peach fuzz contains large amounts of lipid transfer protein: Is this the cause of the high prevalence of sensitization to ltp in mediterranean countries? Eur. Ann. Allergy Clin. Immunol. 2006, 38, 118–121. [Google Scholar] [PubMed]
- Asero, R.; Mistrello, G.; Roncarolo, D.; Amato, S. Relationship between peach lipid transfer protein specific ige levels and hypersensitivity to non-rosaceae vegetable foods in patients allergic to lipid transfer protein. Ann. Allergy Asthma Immunol. 2004, 92, 268–272. [Google Scholar] [CrossRef]
- Wilson, J.M.; Schuyler, A.J.; Workman, L.; Gupta, M.; James, H.R.; Posthumus, J.; McGowan, E.C.; Commins, S.P.; Platts-Mills, T.A.E. Investigation into the alpha-gal syndrome: Characteristics of 261 children and adults reporting red meat allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 2348–2358.e2344. [Google Scholar] [CrossRef] [PubMed]
- Beaudouin, E.; Renaudin, J.M.; Morisset, M.; Codreanu, F.; Kanny, G.; Moneret-Vautrin, D.A. Food-dependent exercise-induced anaphylaxis--update and current data. Eur. Ann. Allergy Clin. Immunol. 2006, 38, 45–51. [Google Scholar]
- Povesi Dascola, C.; Caffarelli, C. Exercise-induced anaphylaxis: A clinical view. Ital. J. Pediatr. 2012, 38, 43. [Google Scholar] [CrossRef]
- Castells, M.C.; Horan, R.F.; Sheffer, A.L. Exercise-Induced anaphylaxis. Curr. Allergy Asthma Rep. 2003, 3, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. Eaaci food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Ocmant, A.; Mulier, S.; Hanssens, L.; Goldman, M.; Casimir, G.; Mascart, F.; Schandene, L. Basophil activation tests for the diagnosis of food allergy in children. Clin. Exp. Allergy 2009, 39, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Douiri, A.; Becares, N.; Wu, S.Y.; Stephens, A.; Radulovic, S.; Chan, S.M.; Fox, A.T.; Du Toit, G.; Turcanu, V.; et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014, 134, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Vivinus-Nebot, M.; Bourrier, T.; Saggio, B.; Albertini, M.; Bernard, A. Benefit of the basophil activation test in deciding when to reintroduce cow’s milk in allergic children. Allergy 2011, 66, 92–100. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Leung, N.; Wang, L.X.; Lisann, L.; Sicherer, S.H.; Scurlock, A.M.; Pesek, R.; Perry, T.T.; Jones, S.M.; et al. Correlations between basophil activation, allergen-specific ige with outcome and severity of oral food challenges. Ann. Allergy Asthma Immunol. 2015, 114, 319–326. [Google Scholar] [CrossRef]
- Santos, A.F.; Du Toit, G.; Douiri, A.; Radulovic, S.; Stephens, A.; Turcanu, V.; Lack, G. Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J. Allergy Clin. Immunol. 2015, 135, 179–186. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Rosa, J.S.; Mukai, K.; Hamilton, R.; Smith, B.M.; Gupta, R.; Galli, S.J.; Desai, M.; et al. Development of a tool predicting severity of allergic reaction during peanut challenge. Ann. Allergy Asthma Immunol. 2018, 121, 69–76.e62. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; van Wijk, R.G.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American academy of allergy, asthma & immunology-european academy of allergy and clinical immunology practall consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar]
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.; Hoest, A.; Lack, G.; et al. Eaaci food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014, 69, 590–601. [Google Scholar] [CrossRef]
- Lack, G. Clinical practice. Food allergy. N. Engl. J. Med. 2008, 359, 1252–1260. [Google Scholar] [CrossRef]
- Nelson, H.S.; Lahr, J.; Rule, R.; Bock, A.; Leung, D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J. Allergy Clin. Immunol. 1997, 99, 744–751. [Google Scholar] [CrossRef]
- Oppenheimer, J.J.; Nelson, H.S.; Bock, S.A.; Christensen, F.; Leung, D.Y. Treatment of peanut allergy with rush immunotherapy. J. Allergy Clin. Immunol. 1992, 90, 256–262. [Google Scholar] [CrossRef]
- PALISADE Group of Clinical Investigators; Vickery, B.P.; Vereda, A.; Casale, T.B.; Beyer, K.; du Toit, G.; Hourihane, J.O.; Jones, S.M.; Shreffler, W.G.; Marcantonio, A.; et al. Ar101 oral immunotherapy for peanut allergy. N. Engl. J. Med. 2018, 379, 1991–2001. [Google Scholar]
- Cianferoni, A.; Hanna, E.; Lewis, M.; Alfaro, M.K.; Corrigan, K.; Buonanno, J.; Datta, R.; Brown-Whitehorn, T.; Spergel, J. Safety review of year 1 oral immunotherapy clinic: Multifood immunotherapy in real-world setting. J. Allergy Clin. Immunol. 2021, 147, AB245. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Greenhawt, M.; Sussman, G.; Begin, P.; Nowak-Wegrzyn, A.; Petroni, D.; Beyer, K.; Brown-Whitehorn, T.; Hebert, J.; Hourihane, J.O.; et al. Effect of epicutaneous immunotherapy vs. placebo on reaction to peanut protein ingestion among children with peanut allergy: The pepites randomized clinical trial. JAMA 2019, 321, 946–955. [Google Scholar] [CrossRef]
- Kulis, M.; Smeekens, J.; Kim, E.; Zarnitsyn, V.; Patel, S. Peanut protein-loaded microneedle patches are immunogenic and distinct from subcutaneous delivery. J. Allergy Clin. Immunol. 2021, 147, AB237. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Wood, R.A.; Stablein, D.; Burks, A.W.; Liu, A.H.; Jones, S.M.; Fleischer, D.M.; Leung, D.Y.; Grishin, A.; Mayer, L.; et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (consortium of food allergy research) of food allergy. J. Allergy Clin. Immunol. 2010, 125, 1077–1083.e1078. [Google Scholar] [CrossRef] [PubMed]
- Novembre, E.; Leo, G.; Cianferoni, A.; Bernardini, R.; Pucci, N.; Vierucci, A. Severe hypoproteinemia in infant with ad. Allergy 2003, 58, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Serranti, D.; Barni, S.; Pucci, N.; Rossi, M.E.; de Martino, M.; Novembre, E. A kwashiorkor case due to the use of an exclusive rice milk diet to treat atopic dermatitis. Nutr. J. 2015, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, B.; von Berg, A.; Bollrath, C.; Berdel, D.; Schauer, U.; Rieger, C.; Haschke-Becher, E.; Wahn, U. Safety and efficacy of a new extensively hydrolyzed formula for infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2008, 19, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Chini, L.; Paone, F.; Moschese, V.; Knafelz, D.; Panel, P.; Emanuele, E.; Palermo, D.; Di Fazio, A.; Rossi, P. Clinical comparison of different replacement milk formulas in children with allergies to cow’s milk proteins. 24-month follow-up study. Minerva Pediatr. 1996, 48, 71–77. [Google Scholar] [PubMed]
- Muraro, M.A.; Giampietro, P.G.; Galli, E. Soy formulas and nonbovine milk. Ann. Allergy Asthma Immunol. 2002, 89, 97–101. [Google Scholar] [CrossRef]
- Halken, S.; Host, A.; Hansen, L.G.; Osterballe, O. Safety of a new, ultrafiltrated whey hydrolysate formula in children with cow milk allergy: A clinical investigation. Pediatr. Allergy Immunol. 1993, 4, 53–59. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of lactobacillus gg on tolerance acquisition in infants with cow’s milk allergy: A randomized trial. J. Allergy Clin. Immunol. 2012, 129, 580–582. [Google Scholar] [CrossRef]
- Klemola, T.; Vanto, T.; Juntunen-Backman, K.; Kalimo, K.; Korpela, R.; Varjonen, E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: A prospective, randomized study with a follow-up to the age of 2 years. J. Pediatr. 2002, 140, 219–224. [Google Scholar] [CrossRef]
- Reche, M.; Pascual, C.; Fiandor, A.; Polanco, I.; Rivero-Urgell, M.; Chifre, R.; Johnston, S.; Martin-Esteban, M. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2010, 21, 577–585. [Google Scholar] [CrossRef]
- Terracciano, L.; Bouygue, G.R.; Sarratud, T.; Veglia, F.; Martelli, A.; Fiocchi, A. Impact of dietary regimen on the duration of cow’s milk allergy: A random allocation study. Clin. Exp. Allergy 2010, 40, 637–642. [Google Scholar] [CrossRef]
- Fiocchi, A.; Brozek, J.; Schunemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World allergy organization (wao) diagnosis and rationale for action against cow’s milk allergy (dracma) guidelines. Pediatr. Allergy Immunol. 2010, 21, 1–125. [Google Scholar] [CrossRef]
- Fox, A.T.; Du Toit, G.; Lang, A.; Lack, G. Food allergy as a risk factor for nutritional rickets. Pediatr. Allergy Immunol. 2004, 15, 566–569. [Google Scholar] [CrossRef]
- Salcedo, G.; Sanchez-Monge, R.; Diaz-Perales, A.; Garcia-Casado, G.; Barber, D. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin. Exp. Allergy 2004, 34, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Mills, C. Nonspecific lipid-transfer proteins in plant foods and pollens: An important allergen class. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 275–279. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.M.; Aceves, S.S.; Dellon, E.S.; Gupta, S.K.; Spergel, J.M.; Furuta, G.T.; Rothenberg, M.E. Pathophysiology of eosinophilic esophagitis. Gastroenterology 2018, 154, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Morotti, R.A.; Konstantinou, G.N.; Yershov, O.; Chehade, M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: A historical cohort. Allergy 2012, 67, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M.; Aceves, S.S.; Spergel, J.M.; Rothenberg, M.E. The management of eosinophilic esophagitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 332–340. [Google Scholar] [CrossRef]
- Dellon, E.S.; Hirano, I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018, 154, 319–332.e313. [Google Scholar] [CrossRef]
- Clayton, F.; Fang, J.C.; Gleich, G.J.; Lucendo, A.J.; Olalla, J.M.; Vinson, L.A.; Lowichik, A.; Chen, X.; Emerson, L.; Cox, K.; et al. Eosinophilic esophagitis in adults is associated with igg4 and not mediated by ige. Gastroenterology 2014, 147, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on atopic dermatitis: Diagnosis, severity assessment, and treatment selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.; Tofte, S.J.; Hanifin, J.M. Does food allergy cause atopic dermatitis? Food challenge testing to dissociate eczematous from immediate reactions. Dermatol. Ther. 2006, 19, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Spergel, J.M.; Ruchelli, E.; Verma, R.; Mascarenhas, M.; Semeao, E.; Flick, J.; Kelly, J.; Brown-Whitehorn, T.; Mamula, P.; et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin. Gastroenterol. Hepatol. 2005, 3, 1198–1206. [Google Scholar] [CrossRef]
- Kelly, K.J. Eosinophilic gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 2000, 30, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.E.; Spergel, J.M.; Ruchelli, E.; Liacouras, C.A. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am. J. Gastroenterol. 2003, 98, 777–782. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Eosinophilic gastrointestinal disorders (egid). J. Allergy Clin. Immunol. 2004, 113, 11–28. [Google Scholar] [CrossRef]
- Sampson, H.A.; Sicherer, S.H.; Birnbaum, A.H. Aga technical review on the evaluation of food allergy in gastrointestinal disorders. American gastroenterological association. Gastroenterology 2001, 120, 1026–1040. [Google Scholar] [CrossRef]
- Uppal, V.; Kreiger, P.; Kutsch, E. Eosinophilic gastroenteritis and colitis: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 175–188. [Google Scholar] [CrossRef]
- Cotton, C.C.; Eluri, S.; Wolf, W.A.; Dellon, E.S. Six-Food elimination diet and topical steroids are effective for eosinophilic esophagitis: A meta-regression. Dig. Dis. Sci. 2017, 62, 2408–2420. [Google Scholar] [CrossRef]
- Steinbach, E.C.; Hernandez, M.; Dellon, E.S. Eosinophilic esophagitis and the eosinophilic gastrointestinal diseases: Approach to diagnosis and management. J. Allergy Clin. Immunol. Pract. 2018, 6, 1483–1495. [Google Scholar] [CrossRef]
- Gonsalves, N. Eosinophilic gastrointestinal disorders. Clin. Rev. Allergy Immunol. 2019, 57, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2020, 158, 111–122.e110. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, P.A.; Sicherer, S.H.; Borkowski, T.A.; Cohen, B.A.; Sampson, H.A. Prevalence of ige-mediated food allergy among children with atopic dermatitis. Pediatrics 1998, 101, E8. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Caubet, J.C.; Boguniewicz, M.; Eigenmann, P.A. Evaluation of food allergy in patients with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Cho, S.I.; Ahn, I.S.; Lee, H.B.; Kim, H.O.; Park, C.W.; Lee, C.H. Treatment of atopic dermatitis with a low-histamine diet. Ann. Dermatol. 2011, 23, S91–S95. [Google Scholar] [CrossRef]
- Mehta, H.; Groetch, M.; Wang, J. Growth and nutritional concerns in children with food allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Bussmann, C.; Zuber, M.; Vannini, S.; Simon, H.U.; Schoepfer, A. Eosinophilic esophagitis: Analysis of food impaction and perforation in 251 adolescent and adult patients. Clin. Gastroenterol. Hepatol. 2008, 6, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Klinnert, M.D. Psychological impact of eosinophilic esophagitis on children and families. Immunol. Allergy Clin. N. Am. 2009, 29, 99–107. [Google Scholar] [CrossRef]
- Spergel, J.M.; Brown-Whitehorn, T.F.; Beausoleil, J.L.; Franciosi, J.; Shuker, M.; Verma, R.; Liacouras, C.A. 14 years of eosinophilic esophagitis: Clinical features and prognosis. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 30–36. [Google Scholar] [CrossRef]
- Wang, R.; Hirano, I.; Doerfler, B.; Zalewski, A.; Gonsalves, N.; Taft, T. Assessing adherence and barriers to long-term elimination diet therapy in adults with eosinophilic esophagitis. Dig. Dis. Sci. 2018, 63, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e822. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Sampson, H.A.; Wood, R.A.; Sicherer, S.H. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics 2003, 111, 829–835. [Google Scholar] [CrossRef]
- Caubet, J.C.; Ford, L.S.; Sickles, L.; Jarvinen, K.M.; Sicherer, S.H.; Sampson, H.A.; Nowak-Wegrzyn, A. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J. Allergy Clin. Immunol. 2014, 134, 382–389. [Google Scholar] [CrossRef]
- Diaz, J.J.; Espin, B.; Segarra, O.; Dominguez-Ortega, G.; Blasco-Alonso, J.; Cano, B.; Rayo, A.; Moreno, A.; Gastrointestinal Allergy Working Group of the Spanish Society of Pediatric Gastroenterology, Hepatology and Nutrition (SEGHNP). Food protein-induced enterocolitis syndrome: Data from a multicenter retrospective study in spain. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sopo, S.M.; Giorgio, V.; Dello Iacono, I.; Novembre, E.; Mori, F.; Onesimo, R. A multicentre retrospective study of 66 italian children with food protein-induced enterocolitis syndrome: Different management for different phenotypes. Clin. Exp. Allergy 2012, 42, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Nowak-Wegrzyn, A.; Vadas, P. Fpies in adults. Ann. Allergy Asthma Immunol. 2018, 121, 736–738. [Google Scholar] [CrossRef]
- Fernandes, B.N.; Boyle, R.J.; Gore, C.; Simpson, A.; Custovic, A. Food protein-induced enterocolitis syndrome can occur in adults. J. Allergy Clin. Immunol. 2012, 130, 1199–1200. [Google Scholar] [CrossRef]
- Tan, J.A.; Smith, W.B. Non-Ige-Mediated gastrointestinal food hypersensitivity syndrome in adults. J. Allergy Clin. Immunol. Pract. 2014, 2, 355–357.e351. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Delgado, P.; Caparros, E.; Moreno, M.V.; Cueva, B.; Fernandez, J. Food protein-induced enterocolitis-like syndrome in a population of adolescents and adults caused by seafood. J. Allergy Clin. Immunol. Pract. 2019, 7, 670–672. [Google Scholar] [CrossRef]
- Ichimura, S.; Kakita, H.; Asai, S.; Mori, M.; Takeshita, S.; Ueda, H.; Muto, T.; Kondo, T.; Yamada, Y. A rare case of fetal onset, food protein-induced enterocolitis syndrome. Neonatology 2019, 116, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.R.; Rieu, P.; Semmekrot, B.A.; Van Krieken, J.H.; Tolboom, J.J.; Draaisma, J.M. Allergic colitis presenting within the first hours of premature life. Acta Paediatr. 2005, 94, 1514–1515. [Google Scholar] [CrossRef]
- Lozinsky, A.C.; Morais, M.B. Eosinophilic colitis in infants. J. Pediatr. 2014, 90, 16–21. [Google Scholar] [CrossRef]
- Labrosse, R.; Graham, F.; Caubet, J.C. Non-Ige-Mediated gastrointestinal food allergies in children: An update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Goldberg, M.R.; Rajuan, N.; Cohen, A.; Leshno, M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: A large-scale, prospective population-based study. J. Allergy Clin. Immunol. 2011, 127, 647–653.e3. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Nowak-Wegrzyn, A. Manifestations, diagnosis, and management of food protein-induced enterocolitis syndrome. Pediatr. Ann. 2013, 42, 135–140. [Google Scholar] [CrossRef]
- Zingone, F.; Iovino, P.; Bucci, C.; Ciacci, C. Coeliac disease: No difference in milk and dairy products consumption in comparison with controls. BMJ Nutr. Prev. Health 2019, 2, 39–42. [Google Scholar] [CrossRef]
- Ciacci, C.; Iovino, P.; Amoruso, D.; Siniscalchi, M.; Tortora, R.; Di Gilio, A.; Fusco, M.; Mazzacca, G. Grown-Up coeliac children: The effects of only a few years on a gluten-free diet in childhood. Aliment. Pharmacol. Ther. 2005, 21, 421–429. [Google Scholar] [CrossRef]
- D’Amato, G.; Akdis, C.A. Global warming, climate change, air pollution and allergies. Allergy 2020, 75, 2158–2160. [Google Scholar] [CrossRef] [PubMed]
- Satitsuksanoa, P.; Jansen, K.; Głobińska, A.; van de Veen, W.; Akdis, M. Regulatory immune mechanisms in tolerance to food allergy. Front. Immunol. 2018, 9, 2939. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.D.; Wünschmann, S.; Pomés, A. Proteases as th2 adjuvants. Curr. Allergy Asthma Rep. 2007, 7, 363–367. [Google Scholar] [CrossRef]
- Tordesillas, L.; Gómez-Casado, C.; Garrido-Arandia, M.; Murua-García, A.; Palacín, A.; Varela, J.; Konieczna, P.; Cuesta-Herranz, J.; Akdis, C.A.; O’Mahony, L.; et al. Transport of pru p 3 across gastrointestinal epithelium—An essential step towards the induction of food allergy? Clin. Exp. Allergy 2013, 43, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Cevhertas, L.; Nadeau, K.; Akdis, M.; Akdis, C.A. Environmental factors in epithelial barrier dysfunction. J. Allergy Clin. Immunol. 2020, 145, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.X.; Brough, H. The role of environmental exposure to peanut in the development of clinical allergy to peanut. Clin. Exp. Allergy 2017, 47, 1232–1238. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Berdyshev, E.; Goleva, E. Cutaneous barrier dysfunction in allergic diseases. J. Allergy Clin. Immunol. 2020, 145, 1485–1497. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Salzano, F.A.; Marino, L.; Salzano, G.; Botta, R.M.; Cascone, G.; D’Agostino Fiorenza, U.; Selleri, C.; Casolaro, V. Microbiota composition and the integration of exogenous and endogenous signals in reactive nasal inflammation. J. Immunol. Res. 2018, 2018, 2724951. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Crestani, E.; Chatila, T.A. Dietary and microbial determinants in food allergy. Immunity 2020, 53, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Simonyte Sjödin, K.; Vidman, L.; Rydén, P.; West, C.E. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Skevaki, C. Early life microbial exposures and allergy risks: Opportunities for prevention. Nat. Rev. Immunol. 2020, 21, 177–191. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.M.; Zakrzewska, M.; Romaszko, E.; Wroblewska, B. Gestational dysfunction-driven diets and probiotic supplementation correlate with the profile of allergen-specific antibodies in the serum of allergy sufferers. Nutrients 2020, 12, 2381. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Makela, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Charbonnier, L.M.; Noval Rivas, M.; Georgiev, P.; Li, N.; Gerber, G.; Bry, L.; Chatila, T.A. Myd88 adaptor-dependent microbial sensing by regulatory t cells promotes mucosal tolerance and enforces commensalism. Immunity 2015, 43, 289–303. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary fiber and bacterial scfa enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as bioactive human milk protective component against food allergy. Allergy 2020. [Google Scholar] [CrossRef]
- Vonk, M.M.; Blokhuis, B.R.J.; Diks, M.A.P.; Wagenaar, L.; Smit, J.J.; Pieters, R.H.H.; Garssen, J.; Knippels, L.M.J.; van Esch, B. Butyrate enhances desensitization induced by oral immunotherapy in cow’s milk allergic mice. Mediat. Inflamm. 2019, 2019, 9062537. [Google Scholar] [CrossRef] [PubMed]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 practall document of the American Academy of Allergy, asthma & immunology and the european academy of allergy and clinical immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [PubMed]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and preventive effects in allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. The microbiota regulates type 2 immunity through rorγt+ t cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-Dependent crosstalk between macrophages and ilc3 promotes intestinal homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef]
- Neeland, M.R.; Koplin, J.J.; Dang, T.D.; Dharmage, S.C.; Tang, M.L.; Prescott, S.L.; Saffery, R.; Martino, D.J.; Allen, K.J. Early life innate immune signatures of persistent food allergy. J. Allergy Clin. Immunol. 2018, 142, 857–864.e853. [Google Scholar] [CrossRef]
- Morita, H.; Moro, K.; Koyasu, S. Innate lymphoid cells in allergic and nonallergic inflammation. J. Allergy Clin. Immunol. 2016, 138, 1253–1264. [Google Scholar] [CrossRef]
- Christianson, C.A.; Goplen, N.P.; Zafar, I.; Irvin, C.; Good, J.T., Jr.; Rollins, D.R.; Gorentla, B.; Liu, W.; Gorska, M.M.; Chu, H.; et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and il-33. J. Allergy Clin. Immunol. 2015, 136, 59–68.e14. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.Y.; Iijima, K.; Bartemes, K.; Kita, H. Group 2 innate lymphoid cells promote an early antibody response to a respiratory antigen in mice. J. Immunol. 2016, 197, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, I.; Matha, L.; Steer, C.A.; Ghaedi, M.; Poon, G.F.; Takei, F. Allergen-Experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity 2016, 45, 198–208. [Google Scholar] [CrossRef]
- Lee, J.B.; Chen, C.Y.; Liu, B.; Mugge, L.; Angkasekwinai, P.; Facchinetti, V.; Dong, C.; Liu, Y.J.; Rothenberg, M.E.; Hogan, S.P.; et al. Il-25 and cd4+ th2 cells enhance type 2 innate lymphoid cell-derived il-13 production, which promotes ige-mediated experimental food allergy. J. Allergy Clin. Immunol. 2016, 137, 1216–1225.e1215. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a t follicular helper cell subset that drives anaphylactic ige. Science 2019, 365, 6456. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T follicular helper cell biology: A decade of discovery and diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Keen, J.C.; Cianferoni, A.; Florio, G.; Guo, J.; Chen, R.; Roman, J.; Wills-Karp, M.; Casolaro, V.; Georas, S.N. Characterization of a novel pma-inducible pathway of interleukin-13 gene expression in t cells. Immunology 2006, 117, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.E.; Reinhardt, R.L.; Bando, J.K.; Sullivan, B.M.; Ho, I.C.; Locksley, R.M. Divergent expression patterns of il-4 and il-13 define unique functions in allergic immunity. Nat. Immunol. 2011, 13, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, V.; Fang, X.; Tancowny, B.; Fan, J.; Wu, F.; Srikantan, S.; Asaki, S.Y.; De Fanis, U.; Huang, S.K.; Gorospe, M.; et al. Posttranscriptional regulation of il-13 in t cells: Role of the rna-binding protein hur. J. Allergy Clin. Immunol. 2008, 121, 853–859.e854. [Google Scholar] [CrossRef]
- Xiong, H.; Dolpady, J.; Wabl, M.; Curotto de Lafaille, M.A.; Lafaille, J.J. Sequential class switching is required for the generation of high affinity ige antibodies. J. Exp. Med. 2012, 209, 353–364. [Google Scholar] [CrossRef]
- Takhar, P.; Corrigan, C.J.; Smurthwaite, L.; O’Connor, B.J.; Durham, S.R.; Lee, T.H.; Gould, H.J. Class switch recombination to ige in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Vicario, M.; Blanchard, C.; Stringer, K.F.; Collins, M.H.; Mingler, M.K.; Ahrens, A.; Putnam, P.E.; Abonia, J.P.; Santos, J.; Rothenberg, M.E. Local b cells and ige production in the oesophageal mucosa in eosinophilic oesophagitis. Gut 2010, 59, 12–20. [Google Scholar] [CrossRef]
- Croote, D.; Darmanis, S.; Nadeau, K.C.; Quake, S.R. High-Affinity allergen-specific human antibodies cloned from single ige b cell transcriptomes. Science 2018, 362, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Aranda, C.J.; Curotto de Lafaille, M.A. The secret life of ige-producing cells. Immunity 2019, 50, 285–287. [Google Scholar] [CrossRef] [PubMed]
- He, J.S.; Subramaniam, S.; Narang, V.; Srinivasan, K.; Saunders, S.P.; Carbajo, D.; Wen-Shan, T.; Hidayah Hamadee, N.; Lum, J.; Lee, A.; et al. Igg1 memory b cells keep the memory of ige responses. Nat. Commun. 2017, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; Sokolowska, M.; Morita, H.; Rückert, B.; Kast, J.I.; Wawrzyniak, M.; Sangasapaviliya, A.; Pradubpongsa, P.; Fuengthong, R.; Thantiworasit, P.; et al. Der p 1-specific regulatory t-cell response during house dust mite allergen immunotherapy. Allergy 2019, 74, 976–985. [Google Scholar] [CrossRef]
- Foong, R.X.; Santos, A.F. Biomarkers of diagnosis and resolution of food allergy. Pediatr. Allergy Immunol. 2020, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Kamemura, N.; Nagao, M.; Irahara, M.; Kagami, S.; Fujisawa, T.; Kido, H. Differential response in allergen-specific ige, iggs, and iga levels for predicting outcome of oral immunotherapy. Pediatr. Allergy Immunol. 2016, 27, 276–282. [Google Scholar] [CrossRef]
- Wright, B.L.; Kulis, M.; Orgel, K.A.; Burks, A.W.; Dawson, P.; Henning, A.K.; Jones, S.M.; Wood, R.A.; Sicherer, S.H.; Lindblad, R.W.; et al. Component-Resolved analysis of iga, ige, and igg4 during egg oit identifies markers associated with sustained unresponsiveness. Allergy 2016, 71, 1552–1560. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Rogosch, T.; Kerzel, S.; Dey, F.; Wagner, J.J.; Zhang, Z.; Maier, R.F.; Zemlin, M. Igg4 and ige transcripts in childhood allergic asthma reflect divergent antigen-driven selection. J. Immunol. 2014, 193, 5801–5808. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kim, H.S.; Kim, D.K.; Nam, S.T.; Kim, H.W.; Park, Y.H.; Lee, D.; Lee, M.B.; Lee, J.H.; Kim, B.; et al. Mesenteric il-10-producing cd5+ regulatory b cells suppress cow’s milk casein-induced allergic responses in mice. Sci. Rep. 2016, 6, 19685. [Google Scholar] [CrossRef]
- van de Veen, W.; Stanic, B.; Wirz, O.F.; Jansen, K.; Globinska, A.; Akdis, M. Role of regulatory B cells in immune tolerance to allergens and beyond. J. Allergy Clin. Immunol. 2016, 138, 654–665. [Google Scholar] [CrossRef]
- van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Rückert, B.; Akdis, C.A.; Akdis, M. Igg4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Runge, T.M.; Dellon, E.S. Do we know what causes eosinophilic esophagitis? A mechanistic update. Curr. Gastroenterol. Rep. 2015, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Brandt, E.B.; Rothenberg, M.E. Il-5 promotes eosinophil trafficking to the esophagus. J. Immunol. 2002, 168, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Aceves, S.S. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol. Clin. N. Am. 2014, 43, 297–316. [Google Scholar] [CrossRef]
- Morita, H.; Nomura, I.; Orihara, K.; Yoshida, K.; Akasawa, A.; Tachimoto, H.; Ohtsuka, Y.; Namai, Y.; Futamura, M.; Shoda, T.; et al. Antigen-Specific t-cell responses in patients with non-IgE-mediated gastrointestinal food allergy are predominantly skewed to T(h)2. J. Allergy Clin. Immunol. 2013, 131, 590–592.e6. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Bencharitiwong, R.; Grishin, A.; Caubet, J.C.; Bardina, L.; Sicherer, S.H.; Sampson, H.A.; Nowak-Wegrzyn, A. The role of casein-specific iga and tgf-beta in children with food protein-induced enterocolitis syndrome to milk. Pediatr. Allergy Immunol. 2014, 25, 651–656. [Google Scholar] [CrossRef]
- Khoshoo, V.; Bhan, M.K.; Kumar, R.; Arora, N.K.; Stintzing, G. Is cow’s milk protein sensitive enteropathy a cell mediated immunological phenomenon? Acta Paediatr. Scand. 1991, 80, 1092–1093. [Google Scholar] [CrossRef]
- Odze, R.D.; Bines, J.; Leichtner, A.M.; Goldman, H.; Antonioli, D.A. Allergic proctocolitis in infants: A prospective clinicopathologic biopsy study. Hum. Pathol. 1993, 24, 668–674. [Google Scholar] [CrossRef]
- Maglio, M.; Troncone, R. Intestinal anti-tissue transglutaminase2 autoantibodies: Pathogenic and clinical implications for celiac disease. Front. Nutr. 2020, 7, 73. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Di Sabatino, A.; Corazza, G.R. The immune recognition of gluten in coeliac disease. Clin. Exp. Immunol. 2005, 140, 408–416. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; White, M.; Lockett, C.; Xu, H.; Shin, A. High prevalence of food intolerances among us internet users. Public Health Nutr. 2021, 24, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Genetics Home Reference. Available online: https://ghr.nlm.nih.gov/condition/hereditary-fructose-intolerance#definition (accessed on 16 December 2020).
- Panel, N.-S.E.; Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; et al. Guidelines for the diagnosis and management of food allergy in the united states: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar]
- Werfel, T. Skin manifestations in food allergy. Allergy 2001, 56, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, F.; Mangini, F.; Berardi, M.; Sterpeta Loffredo, M.; Chinellato, I.; Dellino, A.; Cristofori, F.; Di Domenico, F.; Mastrototaro, M.F.; Cappiello, A.; et al. Intolerance to food additives: An update. Minerva Pediatr. 2008, 60, 1401–1409. [Google Scholar]
- Kang, M.G.; Song, W.J.; Park, H.K.; Lim, K.H.; Kim, S.J.; Lee, S.Y.; Kim, S.H.; Cho, S.H.; Min, K.U.; Chang, Y.S. Basophil activation test with food additives in chronic urticaria patients. Clin. Nutr. Res. 2014, 3, 9–16. [Google Scholar] [CrossRef]
- Halpern, G.M.; Scott, J.R. Non-Ige antibody mediated mechanisms in food allergy. Ann. Allergy 1987, 58, 14–27. [Google Scholar] [PubMed]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schafer, C.; et al. German guideline for the management of adverse reactions to ingested histamine: Guideline of the german society for allergology and clinical immunology (DGAKI), the german society for pediatric allergology and environmental medicine (GPA), the german association of allergologists (AEDA), and the swiss society for allergology and immunology (SGAI). Allergo J. Int. 2017, 26, 72–79. [Google Scholar]
- Schnedl, W.J.; Lackner, S.; Enko, D.; Schenk, M.; Holasek, S.J.; Mangge, H. Evaluation of symptoms and symptom combinations in histamine intolerance. Intest. Res. 2019, 17, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Comas-Baste, O.; Sanchez-Perez, S.; Veciana-Nogues, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine intolerance: The current state of the art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Williams, M.; Reeves, L.; Meyer, R.; Venter, C. Sensitivity to food additives, vaso-active amines and salicylates: A review of the evidence. Clin. Transl. Allergy 2015, 5, 34. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L. Adverse reactions to the sulphite additives. Gastroenterol. Hepatol. Bed Bench 2012, 5, 16–23. [Google Scholar]
- Andreozzi, L.; Giannetti, A.; Cipriani, F.; Caffarelli, C.; Mastrorilli, C.; Ricci, G. Hypersensitivity reactions to food and drug additives: Problem or myth? Acta Biomed. 2019, 90, 80–90. [Google Scholar]
- Bover-Cid, S.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Processing contaminants: Biogenic amines. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 381–391. [Google Scholar]
- San Mauro Martin, I.; Brachero, S.; Garicano Vilar, E. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016, 44, 475–483. [Google Scholar] [CrossRef]
- Kettner, L.; Seitl, I.; Fischer, L. Evaluation of porcine diamine oxidase for the conversion of histamine in food-relevant amounts. J. Food Sci. 2020, 85, 843–852. [Google Scholar] [CrossRef]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30, 10–17. [Google Scholar]
- Malakar, S. Bioactive food chemicals and gastrointestinal symptoms: A focus of salicylates. J. Gastroenterol. Hepatol. 2017, 32, 73–77. [Google Scholar] [CrossRef]
- Enattah, N.; Pekkarinen, T.; Valimaki, M.J.; Loyttyniemi, E.; Jarvela, I. Genetically defined adult-type hypolactasia and self-reported lactose intolerance as risk factors of osteoporosis in finnish postmenopausal women. Eur. J. Clin. Nutr. 2005, 59, 1105–1111. [Google Scholar] [CrossRef]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose intolerance in adults: Biological mechanism and dietary management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef]
- Montalto, M.; Nucera, G.; Santoro, L.; Curigliano, V.; Vastola, M.; Covino, M.; Cuoco, L.; Manna, R.; Gasbarrini, A.; Gasbarrini, G. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: A crossover double-blind placebo-controlled study. Eur. J. Clin. Nutr. 2005, 59, 489–493. [Google Scholar] [CrossRef]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef]
- Iovino, P.; Tremolaterra, F.; Consalvo, D.; Sabbatini, F.; Mazzacca, G.; Ciacci, C. Perception of electrocutaneous stimuli in irritable bowel syndrome. Am. J. Gastroenterol. 2006, 101, 596–603. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Savaiano, D.A. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am. J. Clin. Nutr. 1996, 64, 232–236. [Google Scholar] [CrossRef]
- Zingone, F.; Bucci, C.; Iovino, P.; Ciacci, C. Consumption of milk and dairy products: Facts and figures. Nutrition 2017, 33, 322–325. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Cong, Y.; Chu, H.; Fried, M.; Dai, N.; Fox, M. Bloating and distention in irritable bowel syndrome: The role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am. J. Gastroenterol. 2013, 108, 1516–1525. [Google Scholar] [CrossRef]
- Iovino, P.; Bucci, C.; Tremolaterra, F.; Santonicola, A.; Chiarioni, G. Bloating and functional gastro-intestinal disorders: Where are we and where are we going? World J. Gastroenterol. 2014, 20, 14407–14419. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in fodmaps reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e65. [Google Scholar] [CrossRef]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 2017, 152, 124–133.e122. [Google Scholar] [CrossRef]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential effects of fodmaps (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by mri. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.; Iovino, P.; Laxative Inadequate Relief Survey (LIRS) Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol. Motil. 2016, 28, 581–591. [Google Scholar] [CrossRef]
- Chen, B.R.; Du, L.J.; He, H.Q.; Kim, J.J.; Zhao, Y.; Zhang, Y.W.; Luo, L.; Dai, N. Fructo-Oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress-induced irritable bowel syndrome mouse model. World J. Gastroenterol. 2017, 23, 8321–8333. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. Fodmaps alter symptoms and the metabolome of patients with ibs: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Valeur, J.; Roseth, A.G.; Knudsen, T.; Malmstrom, G.H.; Fiennes, J.T.; Midtvedt, T.; Berstad, A. Fecal fermentation in irritable bowel syndrome: Influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion 2016, 94, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabro, A.; Troncone, R.; Corazza, G.R.; Study Group for Non-Celiac Gluten Sensitivity. An italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabro, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-celiac gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Carroccio, A. Suspected nonceliac gluten sensitivity confirmed in few patients after gluten challenge in double-blind, placebo-controlled trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of non-celiac gluten sensitivity (ncgs): The salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierod, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 2018, 154, 529–539.e522. [Google Scholar] [CrossRef]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (ncgs) and wheat-sensitive irritable bowel syndrome (ibs): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Bucci, C.; Zingone, F.; Russo, I.; Morra, I.; Tortora, R.; Pogna, N.; Scalia, G.; Iovino, P.; Ciacci, C. Gliadin does not induce mucosal inflammation or basophil activation in patients with nonceliac gluten sensitivity. Clin. Gastroenterol. Hepatol. 2013, 11, 1294–1299.e1291. [Google Scholar] [CrossRef]
- Mumolo, M.G.; Rettura, F.; Melissari, S.; Costa, F.; Ricchiuti, A.; Ceccarelli, L.; de Bortoli, N.; Marchi, S.; Bellini, M. Is gluten the only culprit for non-celiac gluten/wheat sensitivity? Nutrients 2020, 12, 3785. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac wheat sensitivity: An immune-mediated condition with systemic manifestations. Gastroenterol. Clin. N. Am. 2019, 48, 165–182. [Google Scholar] [CrossRef]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: Effective management strategies for lactose intolerance. Ann. Intern. Med. 2010, 152, 797–803. [Google Scholar] [CrossRef]
- Savaiano, D.A.; Ritter, A.J.; Klaenhammer, T.R.; James, G.M.; Longcore, A.T.; Chandler, J.R.; Walker, W.A.; Foyt, H.L. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (rp-g28): A randomized, double-blind clinical trial. Nutr. J. 2013, 12, 160. [Google Scholar] [CrossRef]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive enzyme supplementation in gastrointestinal diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Ritter, A.J.; Savaiano, D.; Monteagudo-Mera, A.; Anderson, C.; Magness, S.T.; Klaenhammer, T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA 2017, 114, E367–E375. [Google Scholar] [CrossRef]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Algera, J.; Colomier, E.; Simren, M. The dietary management of patients with irritable bowel syndrome: A narrative review of the existing and emerging evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef] [PubMed]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome-etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Hookway, C.; Buckner, S.; Crosland, P.; Longson, D. Irritable bowel syndrome in adults in primary care: Summary of updated nice guidance. BMJ 2015, 350, h701. [Google Scholar] [CrossRef]
- Barrett, J.S. How to institute the low-fodmap diet. J. Gastroenterol. Hepatol. 2017, 32, 8–10. [Google Scholar] [CrossRef]
- Tuck, C.; Barrett, J. Re-Challenging fodmaps: The low fodmap diet phase two. J. Gastroenterol. Hepatol. 2017, 32, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The low fodmap diet in the management of irritable bowel syndrome: An evidence-based review of fodmap restriction, reintroduction and personalisation in clinical practice. J. Hum. Nutr. Diet. 2018, 31, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Body, J.J.; Bruyere, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; et al. Effects of dairy products consumption on health: Benefits and beliefs--a commentary from the belgian bone club and the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their fodmap content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low fodmap diet: Evidence, doubts, and hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Mumolo, M.G.; Bronzini, F.; Pancetti, A.; Bertani, L.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Marchi, S.; et al. Low fermentable oligo- di- and mono-saccharides and polyols (fodmaps) or gluten free diet: What is best for irritable bowel syndrome? Nutrients 2020, 12, 3368. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; de Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A low-fodmap diet for irritable bowel syndrome: Some answers to the doubts from a long-term follow-up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Trott, N.; Aziz, I.; Rej, A.; Surendran Sanders, D. How patients with ibs use low fodmap dietary information provided by general practitioners and gastroenterologists: A qualitative study. Nutrients 2019, 11, 1313. [Google Scholar] [CrossRef]
- Zingone, F.; Bartalini, C.; Siniscalchi, M.; Ruotolo, M.; Bucci, C.; Morra, I.; Iovino, P.; Ciacci, C. Alterations in diets of patients with nonceliac gluten sensitivity compared with healthy individuals. Clin. Gastroenterol. Hepatol. 2017, 15, 63–68.e2. [Google Scholar] [CrossRef][Green Version]
- Lillestol, K.; Berstad, A.; Lind, R.; Florvaag, E.; Arslan Lied, G.; Tangen, T. Anxiety and depression in patients with self-reported food hypersensitivity. Gen. Hosp. Psychiatry 2010, 32, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Knibb, R.C.; Armstrong, A.; Booth, D.A.; Platts, R.G.; Booth, I.W.; MacDonald, A. Psychological characteristics of people with perceived food intolerance in a community sample. J. Psychosom. Res. 1999, 47, 545–554. [Google Scholar] [CrossRef]
- De Petrillo, A.; Hughes, L.D.; McGuinness, S.; Roberts, D.; Godfrey, E. A systematic review of psychological, clinical and psychosocial correlates of perceived food intolerance. J. Psychosom. Res. 2021, 141, 110344. [Google Scholar] [CrossRef]
- Stockhorst, U.; Enck, P.; Klosterhalfen, S. Role of classical conditioning in learning gastrointestinal symptoms. World J. Gastroenterol. 2007, 13, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Halpert, A.; Godena, E. Irritable bowel syndrome patients’ perspectives on their relationships with healthcare providers. Scand. J. Gastroenterol. 2011, 46, 823–830. [Google Scholar] [CrossRef]
- Hulme, K.; Chilcot, J.; Smith, M.A. Doctor-patient relationship and quality of life in irritable bowel syndrome: An exploratory study of the potential mediating role of illness perceptions and acceptance. Psychol. Health Med. 2018, 23, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Siniscalchi, M.; Capone, P.; Gallotta, S.; Ciacci, C.; Iovino, P. Prevalence of functional dyspepsia and its subgroups in patients with eating disorders. World J. Gastroenterol. 2012, 18, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Gagliardi, M.; Asparago, G.; Carpinelli, L.; Angrisani, L.; Iovino, P. Anhedonia and functional dyspepsia in obese patients: Relationship with binge eating behaviour. World J. Gastroenterol. 2020, 26, 2632–2644. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Gagliardi, M.; Guarino, M.P.L.; Siniscalchi, M.; Ciacci, C.; Iovino, P. Eating disorders and gastrointestinal diseases. Nutrients 2019, 11, 3038. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Boyman, O.; Caramori, G.; Cari, L.; Fan Chung, K.; Diamant, Z.; et al. Immune modulation via t regulatory cell enhancement: Disease-modifying therapies for autoimmunity and their potential for chronic allergic and inflammatory diseases-an EAACI position paper of the task force on immunopharmacology (TIPCO). Allergy 2021, 76, 90–113. [Google Scholar] [CrossRef] [PubMed]

| Cutaneous: Urticaria-angioedema/pruritus | By food ingestion or contact (an estimated 20% of acute urticarial cases are food allergy-related) [8,9]; severity of IgE-mediated food allergy can be determined by the percentage of the involved skin [10] |
| Respiratory: Rhinoconjunctivitis/asthma | Rarely isolated; commonly associated with other organ/apparatus involvement; can be triggered by allergen ingestion or by inhalation via aerosols (as in baker’s asthma) |
| Neurological | Dizziness or weakness, change in the mental status, unconsciousness (generally associated with anaphylaxis) |
| Cardiovascular | Tachycardia, hypotension, cardiovascular collapse (generally associated with anaphylaxis) |
| Food | U.S. | EU | |
|---|---|---|---|
| Self-Reported | Self-Reported | Challenge-Confirmed | |
| Cow’s milk | 1.9 (1.8–2.1) | 6.0 (5.7–6.4) | 0.6 (0.5–0.8) |
| Wheat | 0.8 (0.7–0.9) | 3.6 (3.0–4.2) | 0.2 (0.2–0.3) |
| Egg | 0.8 (0.7–0.9) | 2.5 (2.3–2.7) | 0.1 (0.01–0.2) |
| Tree nuts | 1.2 (1.1–1.3) | 2.2 (1.8–2.5) | 0.3 (0.1–0.4) |
| Peanut | 1.8 (1.7–1.9) | 1.3 (1.2–1.5) | 0.2 (0.2–0.3) |
| Fish | 0.9 (0.8–1.0) | 1.3 (0.9–1.7) | 0.5 (0.08–0.8) |
| Shellfish | 2.9 (2.7–3.1) | 1.3 (0.9–1.7) | 0.1 (0.02–0.2) |
| Soy | 0.6 (0.5–0.7) | 0.4 (0.3–0.6) | 0.1 (0.06–0.3) |
| In Vivo Tests | |
|---|---|
| Elimination diet | This involves an eating plan that omits a food or group of foods believed to cause an adverse reaction. By removing certain foods for a period of time and then reintroducing them during a “challenge” period, it allows the identification of which foods are causing symptoms. The elimination of 6 foods, i.e., eggs, soy, cow’s milk, wheat, seafood, and peanut/tree nuts, can be therapeutic and diagnostic in EoE. |
| Oral food challenge (OFC) | OFC is the gold standard for diagnosis of food allergy. It consists of administering the suspect food at established doses and observing the clinical response in a protected clinical setting. |
| Skin prick test (SPT) | Commercial extracts of allergen are inoculated subcutaneously to detect the presence of sIgE bound to mast cells. |
| Skin Prick by Prick (PbP) | PbP is similar to the SPT but is performed using fresh, cooked or raw food. |
| Atopy Patch Test (APT) | The suspect food is applied directly on the skin using special supports and removed after 48–72 h to study non-IgE (cell-mediated) or mixed IgE/cell-mediated responses. |
| In vitro Tests | |
| Total serum IgE (tIgE) | The total concentration of IgE in the blood is measured; this is useful for assessing the presence of an allergic background but does not identify specific triggers. |
| Radioallergoimmunosorbent (RAST) detection of allergen-specific IgE (sIgEs) | Fluorescent enzyme-labeled antibody assay measures absolute sIgE levels. Values may correlate with the likelihood of clinical reaction for specific foods. |
| Component Resolved Diagnosis (CRD) | CRD is similar to RAST, but it utilizes purified native or recombinant allergens to detect sIgE antibodies against individual allergenic molecules. |
| Basophil Activation Test (BAT) | BAT measures by flow cytometry the expression of activation markers on the surface of basophils following the cross-linking of IgE bound to the high-affinity IgE receptor (FcεRI) by allergen or anti-IgE. |
| Allergen | Deficiency | Substitute |
|---|---|---|
| Cow’s Milk | Calcium, vitamin D, protein, phosphorus, magnesium, potassium, vitamin B12, zinc | Almond milk, oat milk, coconut milk, rice milk, cashew milk, hems milk, macadamia milk |
| Wheat | Fiber, folate, vitamin B12, selenium, manganese, phosphorus, copper | Rice, quinoa, millet, amaranth, buckwheat, sorghum, teff |
| Egg | Retinol (vitamin A), riboflavin, thiamin, vitamin B6, vitamin B12, biotin, folate, pantothenic acid, potassium, magnesium, phosphorus, iron, selenium, zinc, iodine | Tofu, mashed banana, yogurt, buttermilk, chia seeds |
| Tree Nuts | Protein, fat, MUFA, PUFA, linoleic acid, carbohydrates, fiber, calcium, iron, magnesium, phosphorus, potassium, sodium, selenium, zinc, copper, vitamin C, thiamin, riboflavin, niacin, pantothenic acid, vitamin B6, folate, vitamin B12, vitamin A, β-carotene, lycopene, lutein, zeaxanthin, vitamin E | Pumpkin seeds, sunflower seeds, chickpeas, sesame seeds, olives, avocado |
| Peanut | Protein, fat, fiber, magnesium, folate, vitamin E, copper, arginine | Sunflower seeds, sesame seeds, flax seeds, tree nuts (almonds, cashews, walnuts) |
| Fish | Omega-fatty acids, proteins, iron, zinc, copper, vitamin B12, vitamin D | Walnuts, flaxseed oil, soy oil, canola oil, egg, sesame butter, leafy green vegetables (spinach, spirulina) |
| Shellfish | Omega-fatty acids, proteins, irons, zinc, copper, vitamin B12 | Coldwater fish (salmon, tuna, mackerel, sardines), egg, nuts, seeds |
| Soy | Protein, fat, fiber, vitamin C, vitamin K, thiamine, riboflavin, folate, iron, magnesium, phosphorus, potassium, zinc, manganese, copper, vitamin E, niacin, vitamin B6, pantothenic acid | Fresh vegetables, plant proteins, grains |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. https://doi.org/10.3390/nu13051638
Gargano D, Appanna R, Santonicola A, De Bartolomeis F, Stellato C, Cianferoni A, Casolaro V, Iovino P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients. 2021; 13(5):1638. https://doi.org/10.3390/nu13051638
Chicago/Turabian StyleGargano, Domenico, Ramapraba Appanna, Antonella Santonicola, Fabio De Bartolomeis, Cristiana Stellato, Antonella Cianferoni, Vincenzo Casolaro, and Paola Iovino. 2021. "Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns" Nutrients 13, no. 5: 1638. https://doi.org/10.3390/nu13051638
APA StyleGargano, D., Appanna, R., Santonicola, A., De Bartolomeis, F., Stellato, C., Cianferoni, A., Casolaro, V., & Iovino, P. (2021). Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients, 13(5), 1638. https://doi.org/10.3390/nu13051638









