Vitamin B6 Status among Vegetarians: Findings from a Population-Based Survey
Abstract
1. Introduction
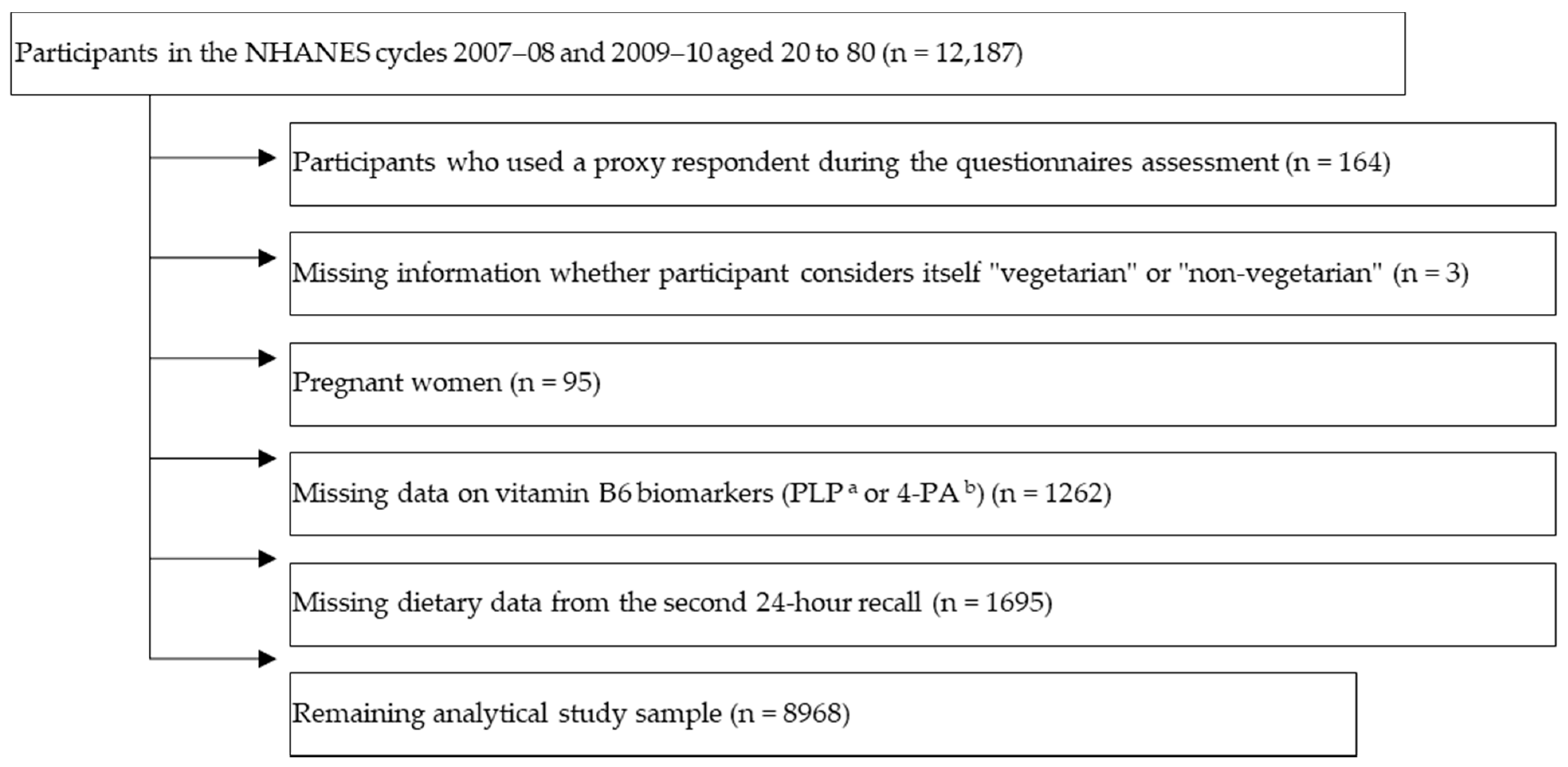
2. Materials and Methods
2.1. Study Population
2.2. Nutritional Assessment
2.3. Covariates
2.4. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Vitamin B6 Levels across the Groups
3.3. Predictors of Vitamin B6 Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Unweighted Participants Counts (Survey-Weighted Frequencies) | ||||
|---|---|---|---|---|
| Meat-Eaters 8765 (97.7) | Flexitarians 112 (1.3) | Pescatarians 35(0.4) | Vegetarians 56 (0.6) | |
| Dietary intake, median (IQR) | ||||
| Meat (oz. eq.) | 1.2 (2.5) | 0.2 (1.2) | 0.0 (0.0) | 0.0 (0.0) |
| Seafood (oz. eq.) | 0.0 (0.3) | 0.0 (0.2) | 0.4 (0.9) | 0.0 (0.0) |
| Poultry (oz. eq.) | 1.0 (2.4) | 0.7 (1.7) | 0.0 (0.0) | 0.0 (0.0) |
| Fruit (cup eq.) | 0.7 (1.4) | 0.9 (1.2) | 1.0 (1.3) | 1.1 (1.8) |
| Vegetables and legumes a (cup eq.) | 0.5 (0.5) | 0.6 (0.6) | 0.7 (0.6) | 0.7 (0.9) |
| Dairy (cup eq.) | 1.4 (1.4) | 1.0 (1.2) | 1.2 (1.7) | 1.3 (1.7) |
| Eggs (oz. eq.) | 0.2 (0.8) | 0.2 (0.7) | 0.1 (0.7) | 0.1 (0.2) |
| Dietary fiber (g/d) | 15.2 (10.1) | 15.8 (14.0) | 22.8 (14.7) | 22.4 (18.2) |
| Unweighted Participants Counts (Survey-Weighted Frequencies) | ||||
|---|---|---|---|---|
| Meat-Eaters | Flexitarians | Pescatarians | Vegetarians | |
| Serum parameters, mean b (SE) | ||||
| C-reactive protein(mg/dL) | 0.2 (0.004) | 0.1 (0.01) | 0.1 (0.02) | 0.1 (0.02) |
| Cotinine (ng/mL) | 0.3 (0.03) | 0.1 (0.04) | 0.1 (0.02) | 0.2 (0.1) |
| Glycosylated hemoglobin (%) | 5.6 (0.01) | 5.7 (0.07) | 5.3 (0.04) | 5.4 (0.045) |
| Albumin (g/dL) | 4.3 (0.007) | 4.3 (0.03) | 4.2 (0.1) | 4.3 (0.046) |
| Creatinine (mg/dL) | 0.9 (0.005) | 0.8 (0.02) | 0.7 (0.02) | 0.8 (0.02) |
| Alkaline phosphotase (U/L) | 64.1 (0.3) | 64.1 (2.9) | 62.3 (4.6) | 60.3 (2.4) |
| Meat-Eaters | Flexitarians | Pescatarians | Vegetarians | p b | |
|---|---|---|---|---|---|
| Model 1 | |||||
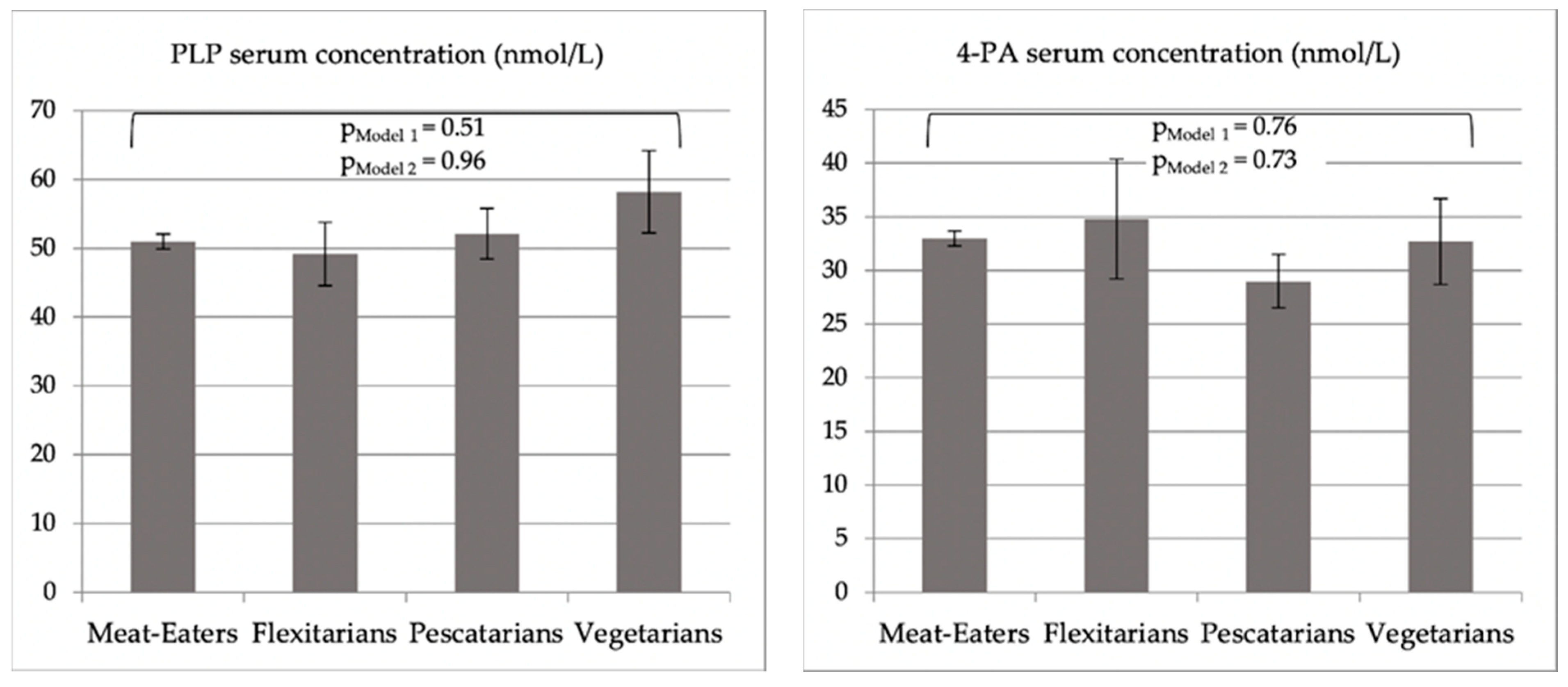
| PLP c, mean ± SE | 51.1 ± 1.0 | 50.2 ± 1.1 | 54.7 ± 1.1 | 59.6 ± 1.2 | 0.51 |
| 4-PA d, mean ± SE | 34.7 ± 1.0 | 34.4 ± 1.2 | 35.0 ± 1.2 | 41.2 ± 1.2 | 0.76 |
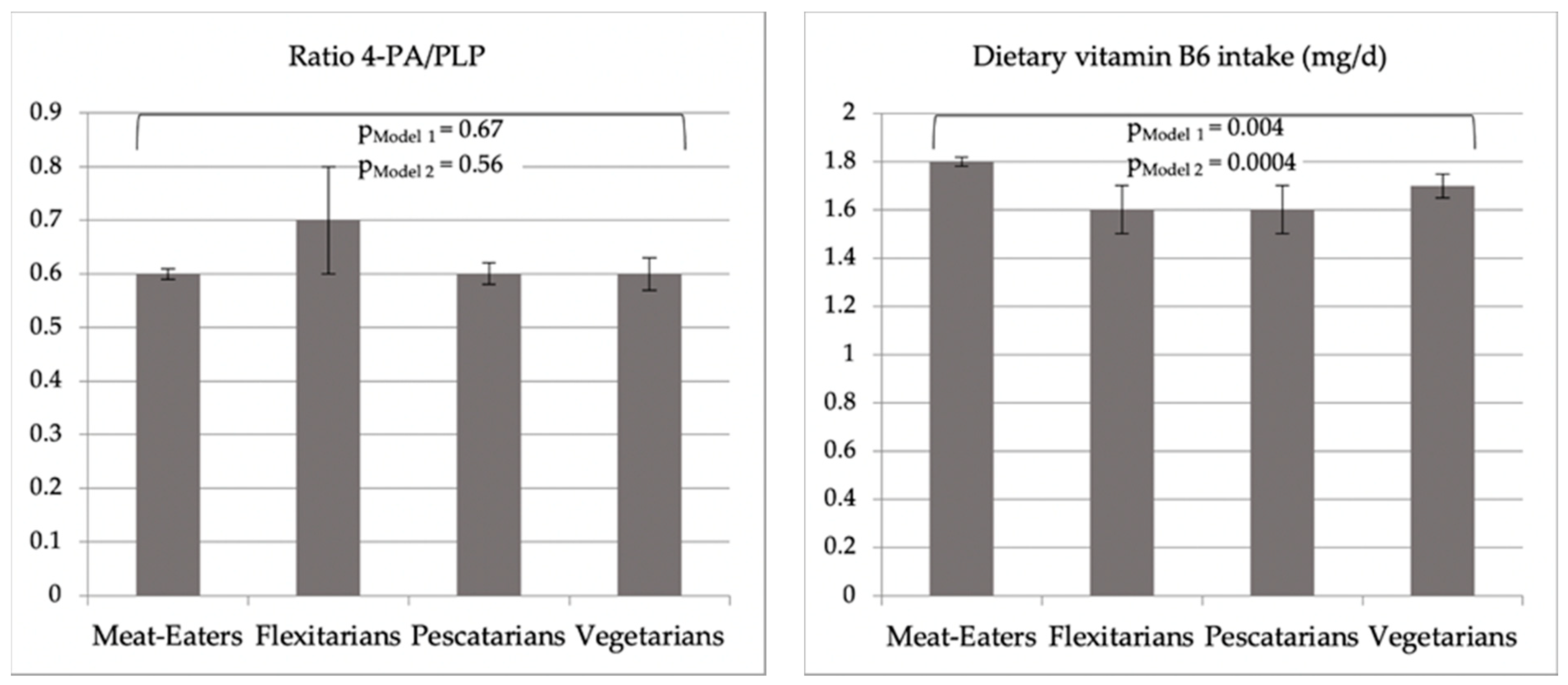
| Ratio, 4-PA/PLP, mean ± SE | 2.6 ± 1.0 | 3.4 ± 1.3 | 2.4 ± 1.1 | 2.6 ± 1.0 | 0.67 |
| Model 2 | |||||
| PLP, mean ± SE | 70.1 ± 1.2 | 69.2 ± 1.2 | 64.5 ± 1.3 | 69.4 ± 1.2 | 0.96 |
| 4-PA, mean ± SE | 76.4 ± 1.4 | 82.5 ± 1.3 | 67.1 ± 1.4 | 81.0 ± 1.4 | 0.73 |
| Ratio, 4-PA/PLP, mean ± SE | 5.9 ± 1.8 | 8.0 ± 1.9 | 6.1 ± 1.8 | 6.4 ± 1.8 | 0.56 |
| Unweighted Participants Counts (Survey-Weighted Frequencies) | ||||
|---|---|---|---|---|
| Meat-Eaters 8765 (97.7) | Flexitarians 112 (1.3) | Pescatarians 35 (0.4) | Vegetarians 56 (0.6) | |
| Categories, n (%) | ||||
| Adequate (PLP > 30 nmol/L) | 5987 (68.3) | 83 (74.1%) | 28 (80.0%) | 42 (75.0%) |
| Moderately deficient (PLP 20–30 nmol/L) | 1487 (17.0) | 16 (14.3%) | 5 (14.3%) | 5 (8.9%) |
| Deficient (PLP < 20 nmol/L) | 1291 (14.7) | 13 (11.6%) | 2 (5.7%) | 9 (16.1%) |
References
- Battaglia Richi, E.; Baumer, B.; Conrad, B.; Darioli, R.; Schmid, A.; Keller, U. Health Risks Associated with Meat Consumption: A Review of Epidemiological Studies. Int. J. Vitam. Nutr. Res. 2015, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef]
- Segovia-Siapco, G.; Sabaté, J. Health and sustainability outcomes of vegetarian dietary patterns: A revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur. J. Clin. Nutr. 2019, 72, 60–70. [Google Scholar] [CrossRef]
- Karavasiloglou, N.; Selinger, E.; Gojda, J.; Rohrmann, S.; Kühn, T. Differences in Bone Mineral Density between Adult Vegetarians and Nonvegetarians Become Marginal when Accounting for Differences in Anthropometric Factors. J. Nutr. 2020, 150, 1266–1271. [Google Scholar] [CrossRef]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 728–734. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- Reynolds, R.D. Bioavailability of vitamin B-6 from plant foods. Am. J. Clin. Nutr. 1988, 48, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Roth-Maier, D.A.; Kettler, S.I.; Kirchgessner, M. Availability of vitamin B6 from different food sources. Int. J. Food Sci. Nutr. 2002, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chang, S.J.; Chiu, Y.T.; Chang, H.H.; Cheng, C.H. The status of plasma homocysteine and related B-vitamins in healthy young vegetarians and nonvegetarians. Eur. J. Nutr. 2003, 42, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Schüpbach, R.; Wegmüller, R.; Berguerand, C.; Bui, M.; Herter-Aeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur. J. Nutr. 2017, 56, 283–293. [Google Scholar] [CrossRef]
- Hung, C.J.; Huang, P.C.; Lu, S.C.; Li, Y.H.; Huang, H.B.; Lin, B.F.; Chang, S.J.; Chou, H.F. Plasma homocysteine levels in Taiwanese vegetarians are higher than those of omnivores. J. Nutr. 2002, 132, 152–158. [Google Scholar] [CrossRef]
- Majchrzak, D.; Singer, I.; Männer, M.; Rust, P.; Genser, D.; Wagner, K.H.; Elmadfa, I. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann. Nutr. Metab. 2006, 50, 485–491. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, Ø.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef]
- Derbyshire, E.J. Flexitarian Diets and Health: A Review of the Evidence-Based Literature. Front. Nutr. 2016, 3, 55. [Google Scholar] [CrossRef]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Available online: https://www.cdc.gov/nchs/data/series/sr_02/sr02_160.pdf (accessed on 3 January 2021).
- National Health and Nutrition Examination Survey (NHANES): Laboratory Procedures Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/manuals/manual_lab.pdf (accessed on 3 January 2021).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS); National Health and Nutrition Examination Survey Data; Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. NHANES 2007–2008. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2007 (accessed on 3 January 2021).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS); National Health and Nutrition Examination Survey Data; Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. NHANES 2009–2010. Available online: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2009 (accessed on 3 January 2021).
- National Health and Nutrition Examination Survey (NHANES): Ethics Review Board (ERB) Approval. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed on 6 January 2021).
- National Health and Nutrition Examination Survey (NHANES): MEC In-Person Dietary Interviewers Procedures Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dietarymec.pdf (accessed on 3 January 2021).
- Laboratory Procedure Manual: Vitamin B6 (pyridoxal 5’-phosphate; 4-pyridoxic acid). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/Vit_B6_F_MET_VITAMIN_B6.pdf (accessed on 3 January 2021).
- Ulvik, A.; Midttun, Ø.; Pedersen, E.R.; Eussen, S.J.; Nygård, O.; Ueland, P.M. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am. J. Clin. Nutr. 2014, 100, 250–255. [Google Scholar] [CrossRef]
- Vásquez, E.; Batsis, J.A.; Germain, C.M.; Shaw, B.A. Impact of obesity and physical activity on functional outcomes in the elderly: Data from NHANES 2005-2010. J. Aging Health 2014, 26, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Mercer, A.; Hassanali, J.; Carmack, C.; Doss, D.; Murillo, R. Gender Differences in the Association Between Alcohol Use and Sedentary Behavior Among Adults. Am. J. Health Promot. 2018, 32, 1576–1581. [Google Scholar] [CrossRef]
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; pp. 150, 170–176. [Google Scholar]
- Coburn, S.P.; Reynolds, R.D.; Mahuren, J.D.; Schaltenbrand, W.E.; Wang, Y.; Ericson, K.L.; Whyte, M.P.; Zubovic, Y.M.; Ziegler, P.J.; Costill, D.L.; et al. Elevated plasma 4-pyridoxic acid in renal insufficiency. Am. J. Clin. Nutr. 2002, 75, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, W.; Górska-Warsewicz, H.; Kulykovets, O. Meat, Meat Products and Seafood as Sources of Energy and Nutrients in the Average Polish Diet. Nutrients 2018, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
| Unweighted Participants Counts (Survey-Weighted Frequencies) | ||||
|---|---|---|---|---|
| Meat-Eaters 8765 (97.7) | Flexitarians 112 (1.3) | Pescatarians 35 (0.4) | Vegetarians 56 (0.6) | |
| Age, mean a (SE) | 44.7 (0.4) | 47.4 (2.5) | 38.8 (1.8) | 37.1 (1.2) |
| Sex, n (%) | ||||
| Female | 4519 (51.6) | 69 (61.6) | 23 (65.7) | 35 (62.5) |
| Male | 4246 (48.4) | 43 (38.4) | 12 (34.3) | 21 (37.5) |
| Educational attainment, n (%) | ||||
| College or higher | 4229 (48.2) | 53 (47.3) | 23 (65.7) | 49 (87.5) |
| High school or lower | 4536 (51.8) | 59 (52.7) | 12 (34.3) | 7 (12.5) |
| Ethnicity, n (%) | ||||
| Mexican American | 1494 (17.0) | 21 (18.8) | 2 (5.7) | 5 (8.9) |
| Non-Hispanic Black | 1610 (18.4) | 17 (15.2) | 5 (14.3) | 2 (3.6) |
| Non-Hispanic White | 4427 (50.5) | 40 (35.7) | 21 (60.0) | 29 (51.8) |
| Other | 1234 (14.1) | 34 (30.4) | 7 (20.0) | 20 (35.7) |
| Body mass index (kg/m2), mean (SE) | 28.1 (0.1) | 26.1 (0.4) | 25.4 (0.7) | 24.9 (0.5) |
| Smoking status, n (%) | ||||
| Current smoker | 1884 (21.5) | 9 (8.0) | 3 (8.6) | 6 (10.7) |
| Former smoker | 2254 (25.7) | 29 (25.9) | 11 (31.4) | 11 (19.6) |
| Non-smoker | 4627 (52.8) | 74 (66.1) | 21 (60.0) | 39 (69.6) |
| Alcohol consumption, n (%) | ||||
| Binge or Heavy drinker | 2837 (32.4) | 24 (21.4) | 14 (40.0) | 15 (26.8) |
| Moderate drinker | 2686 (30.6) | 24 (21.4) | 9 (25.7) | 18 (32.1) |
| Non drinker | 1654 (18.9) | 26 (23.2) | 5 (14.3) | 6 (10.7) |
| Unknown/missing | 1588 (18.1) | 38 (33.9) | 7 (20.0) | 17 (30.4) |
| Physical activity, n (%) | ||||
| Moderate or vigorous | 3969 (45.3) | 43 (38.4) | 25 (71.4) | 35 (62.5) |
| None | 4796 (54.7) | 69 (61.6) | 10 (28.6) | 21 (37.5) |
| Vitamin B6 vitamers, mean (SE) | ||||
| PLP b (nmol/L) | 51.0 (1.1) | 49.2 (4.6) | 52.1 (3.7) | 58.2 (6.0) |
| 4-PA c (nmol/L) | 33.0 (0.7) | 34.8 (5.6) | 29.0 (2.5) | 32.7 (4.0) |
| Ratio, 4-PA/PLP | 0.6 (0.01) | 0.7 (0.1) | 0.6 (0.02) | 0.6 (0.03) |
| Dietary vitamin B6 Supplement Use d, n (%) | ||||
| No | 5975 (68.2) | 79 (70.5) | 22 (62.9) | 31 (55.4) |
| Yes | 2790 (31.8) | 33 (29.5) | 13 (37.1) | 25 (44.6) |
| Dietary vitamin B6 Intake (mg/d), mean (SE) | 1.8 (0.02) | 1.6 (0.1) | 1.6 (0.1) | 1.7 (0.05) |
| Total e vitamin B6 Intake (mg/d), mean (SE) | 2.8 (0.1) | 2.5 (0.3) | 3.3 (0.9) | 2.9 (0.3) |
| Pyridoxal-5′-Phosphate | 4-Pyridoxic Acid | Ratio, 4-PA/PLP | ||||
|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | |
| Diet Type | <0.1 | 0.96 | <0.1 | 0.73 | <0.1 | 0.56 |
| Age | <0.1 | 0.16 | 2.1 | <0.0001 | 0.1 | 0.003 |
| Sex | 0.1 | 0.44 | 0.8 | 0.06 | 1.6 | 0.003 |
| Fasting duration | 0.3 | 0.0013 | 1.2 | <0.0001 | 0.3 | <0.0001 |
| Vitamin B6 supplement use | 18.1 | <0.0001 | 18.5 | <0.0001 | 0.7 | <0.0001 |
| Dietary vitamin B6 intake | 5.4 | <0.0001 | 4.2 | <0.0001 | <0.1 | 0.91 |
| Body mass index | 0.7 | <0.0001 | 0.3 | 0.0008 | 0.1 | 0.04 |
| Educational attainment | <0.1 | 0.055 | <0.1 | 0.30 | <0.1 | 0.38 |
| Ethnicity | 0.2 | <0.0001 | 1.5 | <0.0001 | 0.2 | 0.002 |
| Smoking status | 0.1 | 0.14 | <0.1 | 0.38 | 0.1 | 0.07 |
| Alcohol consumption | 0.1 | 0.06 | 0.1 | 0.31 | <0.1 | 0.29 |
| Physical activity | 0.2 | 0.01 | <0.1 | 0.24 | 0.1 | 0.02 |
| Prevalent diabetes mellitus | 0.2 | 0.001 | 0.1 | 0.32 | 0.3 | 0.02 |
| History of cancer | 0.1 | 0.11 | <0.1 | 0.92 | <0.1 | 0.33 |
| History cardiovascular diseases | <0.1 | 0.23 | <0.1 | 0.10 | 0.1 | 0.02 |
| Prevalent liver disease | 0.1 | 0.08 | <0.1 | 0.34 | <0.1 | 0.02 |
| Oral contraceptive use | 0.1 | 0.03 | 0.1 | 0.35 | <0.1 | 0.10 |
| Menopausal status | 0.8 | <0.0001 | 0.1 | 0.04 | 0.1 | 0.24 |
| Prescription drug use | ||||||
| Theophylline | 0.1 | 0.0015 | <0.1 | 0.15 | 0.4 | 0.02 |
| L-Dopamin | <0.1 | 0.30 | <0.1 | 0.08 | <0.1 | 0.18 |
| NSAID | <0.1 | 0.25 | <0.1 | 0.64 | <0.1 | 0.43 |
| COX2 Inhibitors | 0.1 | 0.006 | 0.1 | 0.01 | <0.1 | 0.31 |
| Isoniazid | 0.1 | 0.0001 | 0.1 | <0.0001 | <0.1 | 0.93 |
| Hydralazine | <0.1 | 0.27 | <0.1 | 0.71 | <0.1 | 0.32 |
| Serum parameters | ||||||
| C-reactive protein | 0.2 | 0.0096 | <0.1 | 0.22 | 0.1 | <0.0001 |
| Cotinine | 0.7 | <0.0001 | 0.5 | <0.0001 | <0.1 | 0.25 |
| Glycohemoglobin | 0.1 | 0.02 | 0.1 | 0.007 | 0.2 | 0.002 |
| Albumin | 3.4 | <0.0001 | 0.1 | 0.10 | 1.0 | <0.0001 |
| Creatinine | 0.3 | 0.0002 | 5.0 | <0.0001 | 8.2 | <0.0001 |
| Alkaline phosphatase | 4.0 | <0.0001 | 0.1 | 0.04 | 0.8 | <0.0001 |
| Model R2 | 40.98 | 39.33 | 16.50 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schorgg, P.; Bärnighausen, T.; Rohrmann, S.; Cassidy, A.; Karavasiloglou, N.; Kühn, T. Vitamin B6 Status among Vegetarians: Findings from a Population-Based Survey. Nutrients 2021, 13, 1627. https://doi.org/10.3390/nu13051627
Schorgg P, Bärnighausen T, Rohrmann S, Cassidy A, Karavasiloglou N, Kühn T. Vitamin B6 Status among Vegetarians: Findings from a Population-Based Survey. Nutrients. 2021; 13(5):1627. https://doi.org/10.3390/nu13051627
Chicago/Turabian StyleSchorgg, Paula, Till Bärnighausen, Sabine Rohrmann, Aedin Cassidy, Nena Karavasiloglou, and Tilman Kühn. 2021. "Vitamin B6 Status among Vegetarians: Findings from a Population-Based Survey" Nutrients 13, no. 5: 1627. https://doi.org/10.3390/nu13051627
APA StyleSchorgg, P., Bärnighausen, T., Rohrmann, S., Cassidy, A., Karavasiloglou, N., & Kühn, T. (2021). Vitamin B6 Status among Vegetarians: Findings from a Population-Based Survey. Nutrients, 13(5), 1627. https://doi.org/10.3390/nu13051627








