An Intermittent Fasting Mimicking Nutrition Bar Extends Physiologic Ketosis in Time Restricted Eating: A Randomized, Controlled, Parallel-Arm Study
Abstract
1. Introduction
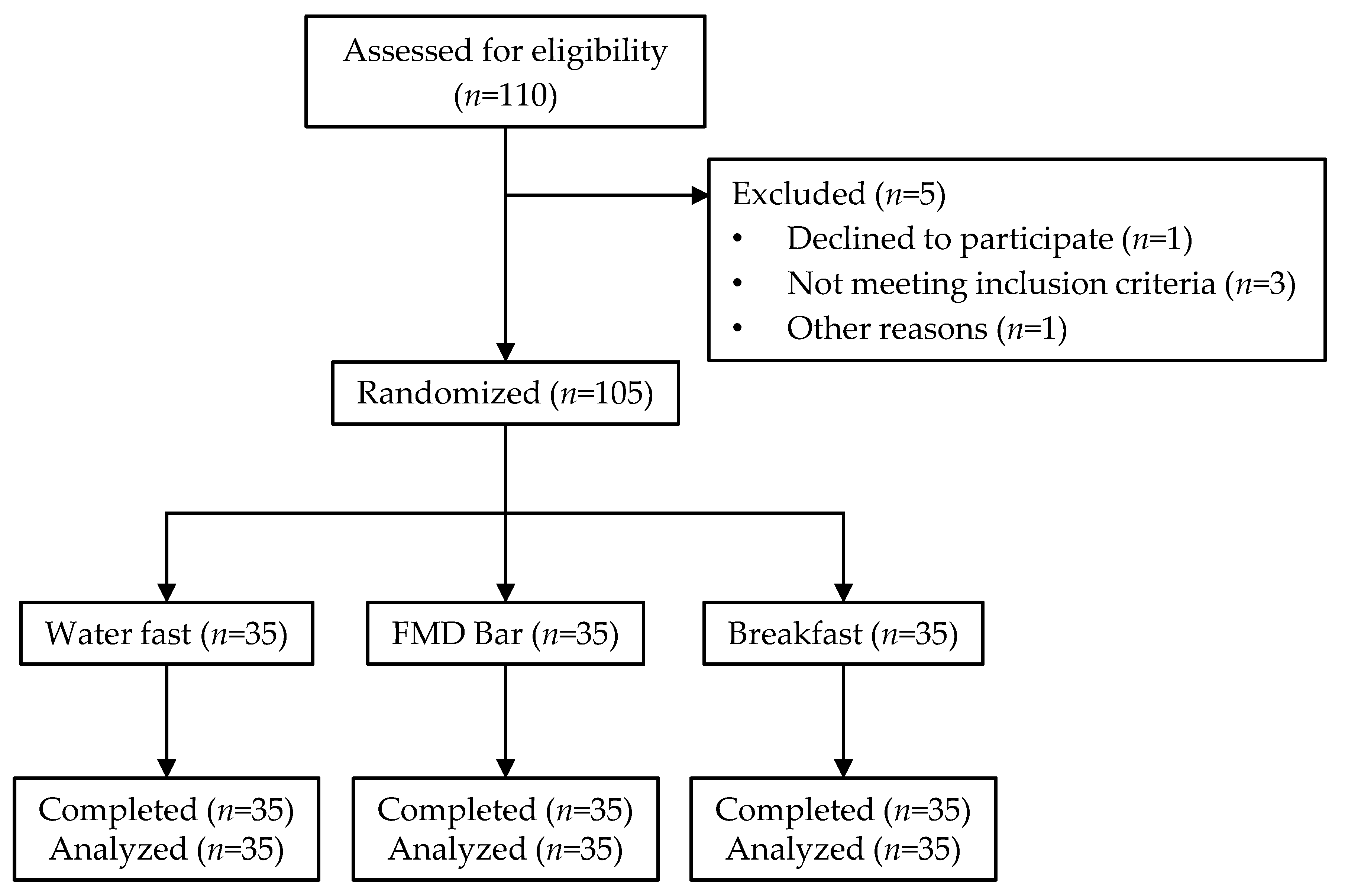
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Blood Glucose and Ketone
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
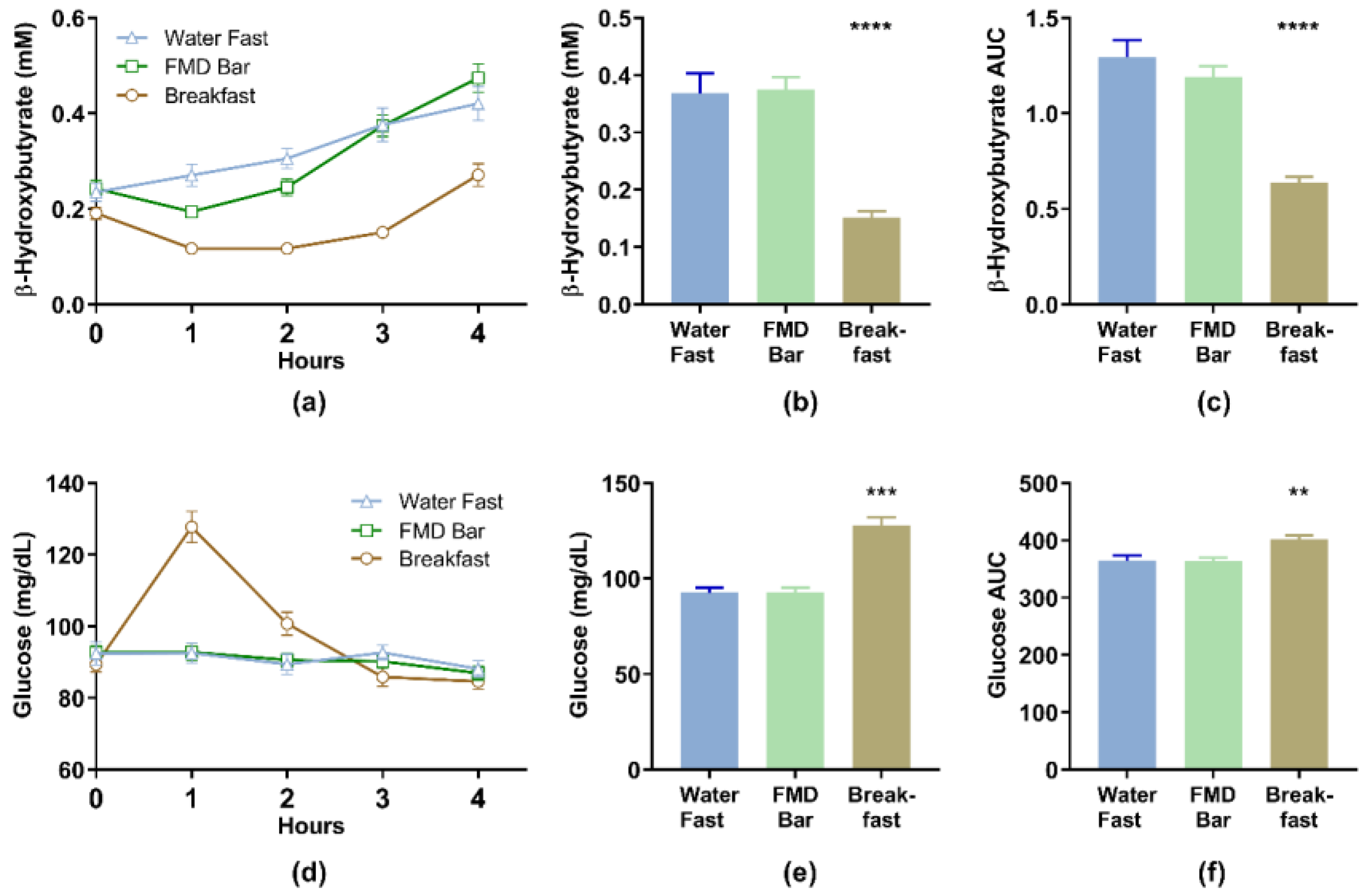
3.2. Blood Beta-Hydroxybutyrate (BHB) and Glucose Levels
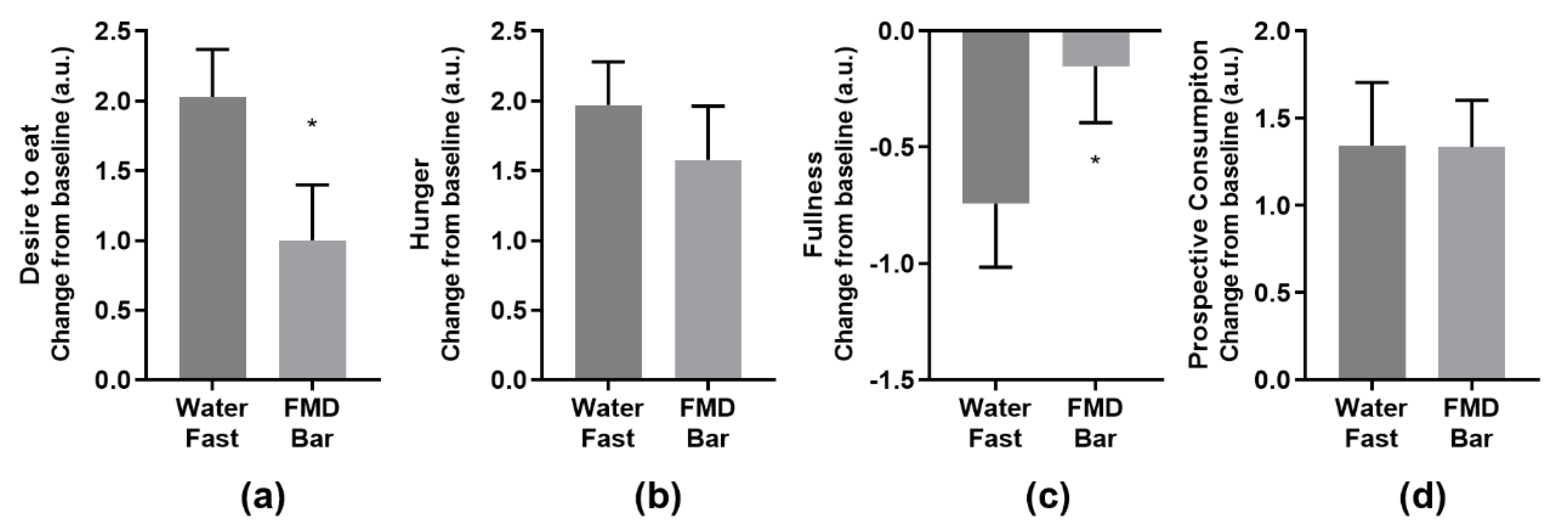
3.3. Subjective Endpoints
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Ravussin, E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003, 78, 361–369. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-Leon, A.M.; Sierra-Perez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef]
- Mattson, M.P.; Duan, W.; Guo, Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: Cellular and molecular mechanisms. J. Neurochem. 2003, 84, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. beta-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal 2016, 28, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metab. Clin. Exp. 2007, 56, 1729–1734. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Varady, K.A.; Hellerstein, M.K. Alternate-day fasting and chronic disease prevention: A review of human and animal trials. Am. J. Clin. Nutr. 2007, 86, 7–13. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- Longo, V.D.; Di Tano, M.; Mattson, M.P.; Guidi, M. Intermittent and periodic fasting, longevity and disease. Nat. Aging 2021, 1, 47–59. [Google Scholar] [CrossRef]
- Stockman, M.C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef]
- Potter, C.; Griggs, R.L.; Brunstrom, J.M.; Rogers, P.J. Breaking the fast: Meal patterns and beliefs about healthy eating style are associated with adherence to intermittent fasting diets. Appetite 2019, 133, 32–39. [Google Scholar] [CrossRef]
- Ballon, A.; Neuenschwander, M.; Schlesinger, S. Breakfast Skipping Is Associated with Increased Risk of Type 2 Diabetes among Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Nutr. 2019, 149, 106–113. [Google Scholar] [CrossRef]
- Carew, A.S.; Mekary, R.A.; Kirkland, S.; Theou, O.; Siddiqi, F.; Urquhart, R.; Blanchard, C.; Parkash, R.; Bennett, M.; Ivey, K.L.; et al. Prospective Study of Skipping Meals to Lose Weight as a Predictor of Incident Type 2 Diabetes With Potential Modification by Cardiometabolic Risk Factors: The Canadian 1995 Nova Scotia Health Survey. Can. J. Diabetes 2020. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Snetselaar, L.G.; Xu, G.; Sun, Y.; Liu, B.; Wallace, R.B.; Bao, W. Association of Skipping Breakfast with Cardiovascular and All-Cause Mortality. J. Am. Coll. Cardiol. 2019, 73, 2025–2032. [Google Scholar] [CrossRef]
- Williams, C.N.; Morse, J.W.; MacDonald, I.A.; Kotoor, R.; Riding, M.D. Increased lithogenicity of bile on fasting in normal subjects. Am. J. Dig. Dis. 1977, 22, 189–194. [Google Scholar] [CrossRef]
- Metzger, A.L.; Adler, R.; Heymsfield, S.; Grundy, S.M. Diurnal variation in biliary lipid composition. Possible role in cholesterol gallstone formation. N. Engl. J. Med. 1973, 288, 333–336. [Google Scholar] [CrossRef]
- Bloch, H.M.; Thornton, J.R.; Heaton, K.W. Effects of fasting on the composition of gallbladder bile. Gut 1980, 21, 1087–1089. [Google Scholar] [CrossRef]
- Sichieri, R.; Everhart, J.E.; Roth, H. A prospective study of hospitalization with gallstone disease among women: Role of dietary factors, fasting period, and dieting. Am. J. Public Health 1991, 81, 880–884. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-Mimicking Diet Promotes Ngn3-Driven beta-Cell Regeneration to Reverse Diabetes. Cell 2017, 168, 775–788.e712. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Wei, M.; Hwang, S.; Morgan, T.E.; Longo, V.D. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp. Gerontol. 2013, 48, 1120–1128. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D.; Vieux, F. Breakfast in the United States: Food and Nutrient Intakes in Relation to Diet Quality in National Health and Examination Survey 2011(-)2014. A Study from the International Breakfast Research Initiative. Nutrients 2018, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ratanapob, N.; VanLeeuwen, J.; McKenna, S.; Wichtel, M.; Rodriguez-Lecompte, J.C.; Menzies, P.; Wichtel, J. Evaluation of the Precision Xtra meter for monitoring blood beta-hydroxybutyrate concentrations in late-gestation ewes. J. Vet. Diagn. Investig. 2019, 31, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.D.; Heuwieser, W.; McArt, J.A.A. Technical note: Comparison of 4 electronic handheld meters for diagnosing hyperketonemia in dairy cows. J. Dairy Sci. 2016, 99, 9136–9142. [Google Scholar] [CrossRef]
- Pineda, A.; Cardoso, F.C. Technical note: Validation of a handheld meter for measuring beta-hydroxybutyrate concentrations in plasma and serum from dairy cows. J. Dairy Sci. 2015, 98, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Agus, M.; Kellogg, M.D. Clinical utility of Abbott Precision Xceed Pro(R) ketone meter in diabetic patients. Pediatr. Diabetes 2011, 12, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Pluddemann, A.; Heneghan, C.; Price, C.P.; Wolstenholme, J.; Thompson, M. Point-of-care blood test for ketones in patients with diabetes: Primary care diagnostic technology update. Br. J. Gen. Pract. 2011, 61, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.; Hendrickx, B.H.; Habets-van der Poel, C.D.; van den Bergh, J.P.; Haagen, A.A.; Bakker, J.A. Accuracy of the Precision(R) point-of-care ketone test examined by liquid chromatography tandem-mass spectrometry (LC-MS/MS) in the same fingerstick sample. Clin. Chem. Lab. Med. 2010, 48, 1781–1784. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Kossoff, E.H.; McGrogan, J.R. Worldwide use of the ketogenic diet. Epilepsia 2005, 46, 280–289. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef]
- Sapir, D.G.; Owen, O.E.; Cheng, J.T.; Ginsberg, R.; Boden, G.; Walker, W.G. The effect of carbohydrates on ammonium and ketoacid excretion during starvation. J. Clin. Investig. 1972, 51, 2093–2102. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Liu, V.; Petocz, P.; Baxter, R.C. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am. J. Clin. Nutr. 2005, 82, 350–354. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocr. 2014, 35, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Hopkins, M.; Beaulieu, K.; Oustric, P.; Blundell, J.E. Issues in Measuring and Interpreting Human Appetite (Satiety/Satiation) and Its Contribution to Obesity. Curr. Obes. Rep. 2019, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
| Ready-to-Eat Dinner 1 | FMD Bar | Breakfast | |
|---|---|---|---|
| Weight (g) | 662 | 40 | 110 |
| Energy (kcal) | 660 | 200 | 435 |
| Protein (g) (% calorie) | 55 (33%) | 4.6 (9%) | 16.9 (16%) |
| Fat (g) (% calorie) | 25 (34%) | 17.3 (77%) | 13.5 (28%) |
| SaFat (g) | 3.5 | 2 | 2 |
| Carbohydrate (g) (% calorie) | 54 (33%) | 7.3 (14%) | 61.4 (56%) |
| Sugar (g) | 13 | 5 | 28 |
| Fiber (g) | 8 | 7 | 5 |
| Characteristics | All (N = 105) | Arm 1 (n = 35) | Arm 2 (n = 35) | Arm 3 (n = 35) | ||||
|---|---|---|---|---|---|---|---|---|
| Water Fast | FMD Bar | Breakfast | ||||||
| Sex, n (%) | 105 | 35 | 35 | 35 | ||||
| Female | 72 | (68.6) | 27 | (77.1) | 23 | (65.7) | 22 | (62.9) |
| Male | 33 | (31.4) | 8 | (22.9) | 12 | (34.3) | 13 | (37.1) |
| Race or ethnic group, n (%) | ||||||||
| White | 82 | (78.1) | 25 | (71.4) | 30 | (85.7) | 27 | (77.1) |
| Black | 6 | (5.7) | 2 | (5.7) | 1 | (2.9) | 3 | (8.6) |
| Hispanic | 12 | (11.4) | 7 | (20) | 3 | (8.6) | 2 | (5.7) |
| Asian | 5 | (4.8) | 1 | (2.9) | 1 | (2.9) | 3 | (8.6) |
| Age (years) | 46.1 | ±11.6 | 46.0 | ±11.5 | 46.4 | ±10.9 | 45.9 | ±12.5 |
| Weight (kg) | 71.9 | ±12.8 | 67.7 | ±11.1 | 73.0 | ±12.3 | 75.0 | ±13.9 |
| BMI | 25.0 | ±3.5 | 23.6 | ±2.9 | 25.4 | ±3.4 | 25.9 | ±3.8 |
| β-Hydroxybutyrate (mM) | Glucose (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | p 1 | p 2 | Mean | SD | 95% CI | p 1 | p 2 | |
| Baseline | ||||||||||
| Water Fast | 0.25 | 0.15 | 0.20–0.30 | 92.4 | 18.9 | 85.9–98.9 | ||||
| FMD Bar | 0.24 | 0.10 | 0.21–0.28 | 0.616 | 92.7 | 10.8 | 89.1–96.4 | - | ||
| Breakfast | 0.19 | 0.07 | 0.17–0.22 | 0.254 | 0.032 | 89.3 | 12.4 | 85.1–93.6 | - | - |
| Hour 1 | ||||||||||
| Water Fast | 0.27 | 0.14 | 0.22–0.31 | 92.5 | 16.5 | 86.9–98.2 | ||||
| FMD Bar | 0.19 | 0.06 | 0.17–0.22 | 0.005 | 92.8 | 14.6 | 87.8–97.9 | 0.998 | ||
| Breakfast | 0.12 | 0.05 | 0.10–0.13 | <0.001 | 0.002 | 127.8 | 25.8 | 118.9–136.6 | <0.001 | <0.001 |
| Hour 2 | ||||||||||
| Water Fast | 0.30 | 0.13 | 0.26–0.34 | 89.43 | 17 | 83.5–95.3 | ||||
| FMD Bar | 0.25 | 0.10 | 0.21–0.28 | 0.061 | 90.63 | 11 | 86.9–94.4 | - | ||
| Breakfast | 0.12 | 0.05 | 0.10–0.13 | <0.001 | <0.001 | 100.7 | 19 | 94.3–107.2 | - | - |
| Hour 3 | ||||||||||
| Water Fast | 0.37 | 0.21 | 0.30–0.44 | 92.7 | 12.3 | 88.5–97.0 | ||||
| FMD Bar | 0.37 | 0.13 | 0.33–0.42 | 0.986 | 90.2 | 10.8 | 86.5–93.9 | - | ||
| Breakfast | 0.15 | 0.07 | 0.13–0.18 | <0.001 | <0.001 | 85.9 | 15.7 | 80.5–91.3 | - | - |
| Hour 4 | ||||||||||
| Water Fast | 0.41 | 0.21 | 0.34–0.49 | 88.1 | 13.7 | 83.4–92.8 | ||||
| FMD Bar | 0.47 | 0.17 | 0.41–0.53 | 0.331 | 86.9 | 11.4 | 83.0–90.9 | 0.917 | ||
| Breakfast | 0.27 | 0.14 | 0.22–0.32 | 0.003 | <0.001 | 84.6 | 12.8 | 80.2–89.0 | 0.481 | 0.726 |
| AUC0–4h | ||||||||||
| Water Fast | 1.27 | 0.53 | 1.09–1.45 | 365.0 | 50.0 | 347.8–382.1 | ||||
| FMD Bar | 1.17 | 0.34 | 1.06–1.29 | 0.485 | 363.5 | 37.3 | 350.7–376.3 | 0.989 | ||
| Breakfast | 0.62 | 0.18 | 0.56–0.68 | <0.001 | <0.001 | 401.4 | 42.3 | 386.9–415.9 | 0.002 | 0.001 |
| t | df | p | Mean Difference | 95% CI | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| BHB (hour 3) | 0.137 | 58.2 | 0.891 | 0.006 | −0.089 | 0.078 |
| Glucose (AUC0–4) | −0.140 | 68.0 | 0.889 | −1.471 | −19.55 | 22.50 |
| BHB(AUC0–4) | −0.889 | 68.0 | 0.377 | −0.094 | −0.118 | 0.307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.W.; Wei, M.; Caputo, S.; Wilson, M.L.; Antoun, J.; Hsu, W.C. An Intermittent Fasting Mimicking Nutrition Bar Extends Physiologic Ketosis in Time Restricted Eating: A Randomized, Controlled, Parallel-Arm Study. Nutrients 2021, 13, 1523. https://doi.org/10.3390/nu13051523
Huang AW, Wei M, Caputo S, Wilson ML, Antoun J, Hsu WC. An Intermittent Fasting Mimicking Nutrition Bar Extends Physiologic Ketosis in Time Restricted Eating: A Randomized, Controlled, Parallel-Arm Study. Nutrients. 2021; 13(5):1523. https://doi.org/10.3390/nu13051523
Chicago/Turabian StyleHuang, Angie W., Min Wei, Sara Caputo, Melissa L. Wilson, Joseph Antoun, and William C. Hsu. 2021. "An Intermittent Fasting Mimicking Nutrition Bar Extends Physiologic Ketosis in Time Restricted Eating: A Randomized, Controlled, Parallel-Arm Study" Nutrients 13, no. 5: 1523. https://doi.org/10.3390/nu13051523
APA StyleHuang, A. W., Wei, M., Caputo, S., Wilson, M. L., Antoun, J., & Hsu, W. C. (2021). An Intermittent Fasting Mimicking Nutrition Bar Extends Physiologic Ketosis in Time Restricted Eating: A Randomized, Controlled, Parallel-Arm Study. Nutrients, 13(5), 1523. https://doi.org/10.3390/nu13051523







