Evaluation of Serum Selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Selenium Analysis
2.3. Statistical Analysis
3. Results
3.1. Serum Selenium Levels of the General Population from Western Romania
3.2. Serum Selenium Levels of a Young Population from Western Romania
4. Discussion
4.1. Serum Selenium Levels of the General Population from Western Romania
4.2. Serum Selenium Levels of a Young Population from Western Romania
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliasgharpour, M.; Farzami, M. Trace Elements in Human Nutrition: A Review. Inter. J. Med. Invest. 2013, 2, 115–128. [Google Scholar]
- Prashanth, L.; Kattapagari, K.; Chitturi, R.; Baddam, V.; Prasad, L. A Review on Role of Essential Trace Elements in Health and Disease. J. Dr. Ntr. Univ. Health Sci. 2015, 4, 75–85. [Google Scholar] [CrossRef]
- Wada, O. What are Trace Elements? Their Deficiency and Excess States. Jpn. Med. Assoc. J. 2004, 47, 351–358. [Google Scholar]
- Al-Fartusie, F.; Mohssan, S. Essential Trace Elements and Their Vital Roles in Human Body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Croft, L.; Lu, J.; Holmgren, A.; Khanna, K. From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Skalnaya, M.; Skalny, A. Essential Trace Elements in Human Health: A Physician’s View; Publishing House of Tomsk State University: Tomsk, Russia, 2018. [Google Scholar]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species-A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of Selenium and Selenoprotein for Brain Function: From Antioxidant Protection to Neuronal Signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.J.; Han, H.; Zhou, G.B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.J.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Pal, A. Role of Copper and Selenium in Reproductive Biology: A Brief Update. Biochem Pharm. 2015, 4, 5. [Google Scholar]
- Bleau, G.; Lemarbre, J.; Faucher, G.; Roberts, K.D.; Chapdelaine, A. Semen Selenium and Human Fertility. Fertil. Steril. 1984, 42, 890–894. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Dhundasi, S. Recent Advances in Heavy Metals Induced Effect on Male Reproductive FunctioŽ A Retrospective. J. Med. Sci. 2009, 2, 37–42. [Google Scholar]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Wu, S.; Motley, A.K.; Stevenson, T.D.; Winfrey, V.P.; Capecchi, M.R.; Atkins, J.F.; Burk, R.F. Production of Selenoprotein P (Sepp1) by Hepatocytes is Central to Selenium Homeostasis. J. Biol. Chem. 2012, 287, 40414–40424. [Google Scholar] [CrossRef]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of Selenoprotein P and other Plasma Selenium Biomarkers for the Assessment of the Selenium Nutritional Requirement: A Placebo-Controlled, Double-Blind Study of Selenomethionine Supplementation in Selenium-Deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Zhou, X.; Smith, A.M.; Failla, M.L.; Hill, K.E.; Yu, Z. Estrogen Status Alters Tissue Distribution and Metabolism of Selenium in Female Rats. J. Nutr. Biochem. 2012, 23, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, T.M.T.; Halls, D.J. Measurement of Selenium in Clinical Specimens. Ann. Clin. Biochem. 1999, 36, 301–315. [Google Scholar] [CrossRef]
- Safaralizadeh, R.; Kardar, G.A.; Pourpak, Z.; Moin, M.; Zare, A.; Teimourian, S. Serum Concentration of Selenium in Healthy Individuals Living in Tehran. Nutr. J. 2005, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, S. Clinical Biochemistry Reference Ranges Handbook; National Health Service: Leeds, UK, 2018. [Google Scholar]
- Arnaud, J.; Bertrais, S.; Roussel, A.M.; Arnault, N.; Ruffieux, D.; Favier, A.; Berthelin, S.; Estaquio, C.; Galan, P.; Czernichow, S.; et al. Serum Selenium Determinants in French Adults: The SU.VI.M.AX study. Br. J. Nutr. 2006, 95, 313–320. [Google Scholar] [CrossRef]
- Millán Adame, E.; Florea, D.; Sáez Pérez, L.; Molina López, J.; López-González, B.; Pérez de la Cruz, A.; Planells del Pozo, E. Deficient Selenium Status of a Healthy Adult Spanish Population. Nutr. Hosp. 2012, 27, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.A.; Cristea, C.; Calomfirescu, C.; Matei, E.; Ursu, C.; Rădulescu, S.; Georgescu, D. Raportul Naţional al Stării de Sănătate a Populaţiei României din 2017. 2018. Available online: https://insp.gov.ro/sites/cnepss/wp-content/uploads/2014/11/SSPR-2017-1.pdf (accessed on 20 March 2020).
- Cucu, M.A.; Cristea, C.; Matei, E.; Galan, A.; Ursu, C.; Dima, C.; Georgescu, D. Raportul Naţional al Stării de Sănătate a Populaţiei României din 2019. 2020. Available online: https://insp.gov.ro/sites/cnepss/wp-content/uploads/2020/12/Raport-Starea-de-Sanatate-2019.pdf (accessed on 25 January 2021).
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female Age-Related Fertility Decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Baird, D.T.; Collins, J.; Egozcue, J.; Evers, L.H.; Gianaroli, L.; Leridon, H.; Sunde, A.; Templeton, A.; Van Steirteghem, A.; Cohen, J.; et al. Fertility and Ageing. Hum. Reprod. Update 2005, 11, 261–276. [Google Scholar] [CrossRef]
- Harris, I.D.; Fronczak, C.; Roth, L.; Meacham, R.B. Fertility and the Aging Male. Rev. Urol. 2011, 13, e184–e190. [Google Scholar] [PubMed]
- Hassan, M.A.M.; Killick, S.R. Effect of Male Age on Fertility: Evidence for the Decline in Male Fertility with Increasing Age. Fertil. Steril. 2003, 79, 1520–1527. [Google Scholar] [CrossRef]
- National Institute of Statistics-Romania. Tendinte sociale. 2019, 17. Available online: https://insse.ro/cms/sites/default/files/field/publicatii/tendinte_sociale.pdf (accessed on 2 April 2021).
- National Institute of Statistics-Romania. Populaţia După Domiciliu* la 1 Ianuarie 2020 a Ajuns la 22 175 mii Persoane. 2020. Available online: https://insse.ro/cms/sites/default/files/com_presa/com_pdf/popdom1ian2020r.pdf (accessed on 2 April 2021).
- Thomson, C.D. SELENIUM|Physiology. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5117–5124. ISBN 9780122270550. [Google Scholar]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium Speciation from Food Source to Metabolites: A Critical Review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Noisel, N.; Carrier, G.; Bouchard, M. Study of Selenium Intake and Disposition in Various Matrices Based on Mathematical Algorithms Derived from Pooled Biomonitoring Data. Int. J. Hyg. Environ. Health 2014, 217. [Google Scholar] [CrossRef]
- Burri, J.; Haldimann, M.; Dudler, V. Selenium Status of the Swiss Population: Assessment and Change Over a Decade. J. Trace Elem. Med. Biol. 2008, 22, 112–119. [Google Scholar] [CrossRef]
- Rocourt, C.R.; Cheng, W.H. Selenium Supranutrition: Are the Potential Benefits of Chemoprevention Outweighed by the Promotion of Diabetes and Insulin Resistance? Nutrients 2013, 5, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Hollenbach, B.; Laurberg, P.; Carlé, A.; Hög, A.; Jørgensen, T.; Vejbjerg, P.; Ovesen, L.; Schomburg, L. Serum Selenium and Selenoprotein P Status in Adult Danes—8-year followup. J. Trace Elem. Med. Biol. 2009, 23, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; López-Jurado, M.; Aranda, P.; Llopis, J. Plasma Levels of Copper, Manganese and Selenium in an Adult Population in Southern Spain: Influence of Age, Obesity and Lifestyle Factors. Sci. Total Environ. 2010, 408, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, J.; Li, H. Selenium, Aging and Aging-Related Diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- González-Estecha, M.; Palazón-Bru, I.; Bodas-Pinedo, A.; Trasobares, E.; Palazón-Bru, A.; Fuentes, M.; Cuadrado-Cenzual, M.Á.; Calvo-Manuel, E. Relationship Between Serum Selenium, Sociodemographic Variables, other Trace Elements and Lipid Profile in an Adult Spanish Population. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (Gms) 2017, 43, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Post, C.; Aaseth, J. Relatively High Mortality Risk in Elderly Swedish Subjects with Low Selenium Status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef]
- Robberecht, H.; De Bruyne, T.; Davioud-Charvet, E.; Mackrill, J.; Hermans, N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Des. 2019, 25, 1694–1706. [Google Scholar] [CrossRef]
- Kim, Y.J.; Galindev, O.; Sei, J.H.; Bae, S.M.; Im, H.; Wen, L.; Seo, Y.R.; Ahn, W.S. Serum Selenium Level in Healthy Koreans. Biol. Trace Elem. Res. 2009, 131, 103–109. [Google Scholar] [CrossRef]
- Bocca, B.; Madeddu, R.; Asara, Y.; Tolu, P.; Marchal, J.A.; Forte, G. Assessment of Reference Ranges for Blood Cu, Mn, Se and Zn in a Selected Italian Population. J. Trace Elem. Med. Biol. 2011, 25, 19–26. [Google Scholar] [CrossRef]
- Thomson, C.D. Assessment of Requirements for Selenium and adequacy of Selenium Status: A Review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef]
- Chen, C.J.; Lai, J.S.; Wu, C.C.; Lin, T.S. Serum Selenium in Adult Taiwanese. Sci. Total. Environ. 2006, 367, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Alis, R.; Santos-Lozano, A.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Fiuza-Luces, C.; Garatachea, N.; Lucia, A.; Emanuele, E. Trace Elements Levels in Centenarian ’Dodgers’. J. Trace Elem. Med. Biol. 2016, 35, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, B.; Cabral Pinto, M.; Nogueira, J.; Pinto, P.; Almeida, A.; Pinto, E.; Marinho-Reis, P.; Diniz, L.; Moreira, P.I.; Simões, M.R.; et al. Associations between Trace Elements and Cognitive Decline: An Exploratory 5-Year Follow-Up Study of an Elderly Cohort. Int. J. Environ. Res. Public Health 2020, 17, 6051. [Google Scholar] [CrossRef] [PubMed]
- Retondario, A.; Fernandes, R.; Rockenbach, G.; Alves, M.A.; Bricarello, L.P.; Trindade, E.; Vasconcelos, F.A.G. Selenium Intake and Metabolic Syndrome: A Systematic Review. Clin. Nutr. 2019, 38, 603–614. [Google Scholar] [CrossRef]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.M.; Guallar, E. Serum Selenium Concentrations and Diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ. Health Perspect. 2009, 117, 1409–1413. [Google Scholar] [CrossRef]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Kuo, C.S.; Lee, L.T.; Huang, K.C. High Serum Selenium Levels are Associated with Increased Risk for Diabetes Mellitus Independent of Central Obesity and Insulin Resistance. Bmj Open Diabetes Res. Amp. Care 2016, 4, e000253. [Google Scholar] [CrossRef]
- Fontenelle, L.; Feitosa, M.; Morais, J.; Severo, J.; Freitas, T.; Beserra, J.; Henriques, G.; Marreiro, D. The Role of Selenium in Insulin Resistance. Braz. J. Pharm. Sci. 2018, 54, s2175–s97902018000100139. [Google Scholar] [CrossRef]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Chiang, C.H.; Yao, C.A.; Huang, K.C. Gender Differences with Dose-Response Relationship between Serum Selenium Levels and Metabolic Syndrome-A Case-Control Study. Nutrients 2019, 11, 477. [Google Scholar] [CrossRef]
- Sahebari, M.; Rezaieyazdi, Z.; Khodashahi, M. Selenium and Autoimmune Diseases: A Review Article. Curr. Rheumatol. Rev. 2019, 15, 123–134. [Google Scholar] [CrossRef]
- Federige, M.A.F.; Romaldini, J.H.; Miklos, A.B.P.P.; Koike, M.K.; Takei, K.; Portes, E.d.S. Serum Selenium and Selenoprotein-P Levels in Autoimmune Thyroid Diseases Patients in a Select Center: A Transversal Study. Arch. Endocrinol. Metab. 2017, 61, 600–607. [Google Scholar] [CrossRef]
- Alijani, E.; Abbasi, N. The Effect of Selenium on Hashimoto’s Thyroiditis; Systemic Review and Metaanalysis. J. Clin. Toxicol. 2020, 10, 440. [Google Scholar] [CrossRef]
- Bednarczuk, T.; Schomburg, L. Challenges and Perspectives of Selenium Supplementation in Graves’ Disease and Orbitopathy. Horm. (Athens) 2020, 19, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Colucci, R.; Dragoni, F.; Moretti, S. Oxidative Stress and Immune System in Vitiligo and Thyroid Diseases. Oxid. Med. Cell Longev 2015, 2015, 631927. [Google Scholar] [CrossRef]
- Fan, K.-C.; Yang, T.-H.; Huang, Y.-C. Vitiligo and Thyroid Disease: A Systematic Review and Meta-Analysis. Eur. J. Dermatol. 2018, 28, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.; Al-Tawil, N.; Abid, F. Association of Boron, Copper, Germanium, Magnesium, Selenium and Zinc with Incidence of Rheumatoid Arthritis. Am. J. Intern. Med. 2015, 3, 132–140. [Google Scholar] [CrossRef][Green Version]
- Sahebari, M.; Abrishami-Moghaddam, M.; Moezzi, A.; Ghayour-Mobarhan, M.; Mirfeizi, Z.; Esmaily, H.; Ferns, G. Association Between Serum Trace Element Concentrations and the Disease Activity of Systemic Lupus Erythematosus. Lupus 2014, 23, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum Selenium Levels and All-Cause, Cancer, and Cardiovascular Mortality Among US Adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Huzarski, T.; Jakubowska, A.; Gronwald, J.; Cybulski, C.; Oszurek, O.; Dębniak, T.; Jaworska-Bieniek, K.; Lener, M.; Białkowska, K.; et al. Serum Selenium Level and Cancer Risk: A Nested Case-Control Study. Hered. Cancer Clin. Pr. 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef]
- Wąsowicz, W.; Zachara, B.A. Selenium Concentrations in the Blood and Urine of a Healthy Polish Sub-Population. J. Clin. Chem. Clin. Biochem. 1987, 25, 409–412. [Google Scholar] [CrossRef]
- Shortt, C.T.; Duthie, G.G.; Robertson, J.D.; Morrice, P.C.; Nicol, F.; Arthur, J.R. Selenium Status of a Group of Scottish Adults. Eur. J. Clin. Nutr. 1997, 51, 400–404. [Google Scholar] [CrossRef][Green Version]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in Food and the Human Body: A Review. Sci. Total. Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Nomikos, T.; Panagiotakos, D.; Pergantis, S.; Fragopoulou, E.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary Habits of Greek Adults and Serum Total Selenium Concentration: The ATTICA study. Eur. J. Nutr. 2010, 49, 465–472. [Google Scholar] [CrossRef]
- Belhadj, M.; Kazi Tani, L.S.; Dennouni Medjati, N.; Harek, Y.; Dali Sahi, M.; Sun, Q.; Heller, R.; Behar, A.; Charlet, L.; Schomburg, L. Se Status Prediction by Food Intake as Compared to Circulating Biomarkers in a West Algerian Population. Nutrients 2020, 12, 3599. [Google Scholar] [CrossRef]
- Pagalea, A.; Uta, D.S.V. Romanian Consumer Lifestyle and Attitude towards Bio Products Purchase. Procedia—Soc. Behav. Sci. 2012, 62, 1308–1312. [Google Scholar] [CrossRef]
- Voinea, L.; Vranceanu, D.; Filip, A.; Popescu, D.; Negrea, M.; Dina, R. Research on Food Behavior in Romania from the Perspective of Supporting Healthy Eating Habits. Sustainability 2019, 11, 5255. [Google Scholar] [CrossRef]
- Kim, K.; Wactawski-Wende, J.; Michels, K.A.; Schliep, K.C.; Plowden, T.C.; Chaljub, E.N.; Mumford, S.L. Dietary Minerals, Reproductive Hormone Levels and Sporadic Anovulation: Associations in Healthy Women with Regular Menstrual Cycles. Br. J. Nutr. 2018, 120, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Smith, A.M. Plasma Selenium and Plasma and Erythrocyte Glutathione Peroxidase Activity Increase with Estrogen During the Menstrual Cycle. J. Am. Coll Nutr. 2003, 22, 43–51. [Google Scholar] [CrossRef]
- Lucky, H.S.; Sajal, G.; Yesul, K.; Ashok, A. Female Infertility and Antioxidants. Curr. Women’s Health Rev. 2010, 6, 84–95. [Google Scholar] [CrossRef]
- Bizerea, T.O.; Dezsi, S.G.; Marginean, O.; Stroescu, R.; Rogobete, A.; Bizerea-Spiridon, O.; Ilie, C. The Link Between Selenium, Oxidative Stress and Pregnancy Induced Hypertensive Disorders. Clin. Lab. 2018, 64, 1593–1610. [Google Scholar] [CrossRef] [PubMed]
- Mamon, M.A.C.; Menodiado, C.M.M.; Siasu, G.L.; Ramos, G.B. Selenium Supplementation within the Periconception Period: Influence on Maternal Liver and Renal Histoarchitecture. Asian Pac. J. Reprod. 2016, 5, 295–300. [Google Scholar] [CrossRef]
- Zadrozna, M.; Gawlik, M.; Nowak, B.; Marcinek, A.; Mrowiec, H.; Walas, S.; Wietecha-Posłuszny, R.; Zagrodzki, P. Antioxidants Activities and Concentration of Selenium, Zinc and Copper in Preterm and IUGR Human Placentas. J. Trace Elem. Med. Biol. 2009, 23, 144–148. [Google Scholar] [CrossRef]
- Ba, Z. Selenium in Pregnant Women: Mini Review. J. Nutr. Food Sci. 2016. [Google Scholar] [CrossRef]
- McArdle, H.J.; Ashworth, C.J. Micronutrients in Fetal Growth and Development. Br. Med. Bull. 1999, 55, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, G.; Akbaba, E.; Sahin, C.; Kara, M. The Relationship Between Gestational Diabetes Mellitus and Selenoprotein-P Plasma 1 (SEPP1) Gene Polymorphisms. Gynecol. Endocrinol. 2018, 34, 849–852. [Google Scholar] [CrossRef]
- Hofstee, P.; Cuffe, J.S.M.; Perkins, A.V. Analysis of Selenoprotein Expression in Response to Dietary Selenium Deficiency During Pregnancy Indicates Tissue Specific Differential Expression in Mothers and Sex Specific Changes in the Fetus and Offspring. Int. J. Mol. Sci. 2020, 21, 2210. [Google Scholar] [CrossRef]
- Wu, H.; Jia, X.; Zhao, H.; Huang, Y.; Liu, C.; Huang, Z.; Li, S.; Wang, J. Identification of SEPP1 Polymorphisms is not a Genetic Risk Factor for Preeclampsia in Chinese Han Women: A Clinical Trial and Experimental Study. Medicine 2017, 96, e7249. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; MacPherson, A.; Yates, R.W.; Hussain, B.; Dixon, J. The Effect of Oral Selenium Supplementation on Human Sperm Motility. Br. J. Urol. 1998, 82, 76–80. [Google Scholar] [CrossRef]
- Oguntibeju, O.; Esterhuyse, J.S.; Truter, E. Selenium: Its Potential Role in Male Infertility. Pak. J. Med. Sci. 2009, 25, 332–337. [Google Scholar]
- Oluboyo, A.O.; Adijeh, R.U.; Onyenekwe, C.C.; Oluboyo, B.O.; Mbaeri, T.C.; Odiegwu, C.N.; Chukwuma, G.O.; Onwuasoanya, U.F. Relationship Between Serum Levels of Testosterone, Zinc and Selenium in Infertile Males Attending Fertility Clinic in Nnewi, South East Nigeria. Afr. J. Med. Med. Sci. 2012, 41, 51–54. [Google Scholar] [PubMed]
- Villaverde, A.I.S.B.; Fioratti, E.G.; Ramos, R.S.; Neves, R.C.F.; Ferreira, J.C.P.; Cardoso, G.S.; Padilha, P.M.; Lopes, M.D. Blood and Seminal Plasma Concentrations of Selenium, Zinc and Testosterone and their Relationship to Sperm Quality and Testicular Biometry in Domestic Cats. Anim. Reprod. Sci. 2014, 150, 50–55. [Google Scholar] [CrossRef]
- Rezaeian, Z.; Yazdekhasti, H.; Nasri, S.; Rajabi, Z.; Fallahi, P.; Amidi, F. Effect of Selenium on Human Sperm Parameters after Freezing and Thawing Procedures. Asian Pac. J. Reprod. 2016, 5, 462–466. [Google Scholar] [CrossRef]
- Shi, L.; Song, R.; Yao, X.; Ren, Y. Effects of Selenium on the Proliferation, Apoptosis and Testosterone Production of Sheep Leydig Cells in Vitro. Theriogenology 2017, 93, 24–32. [Google Scholar] [CrossRef] [PubMed]
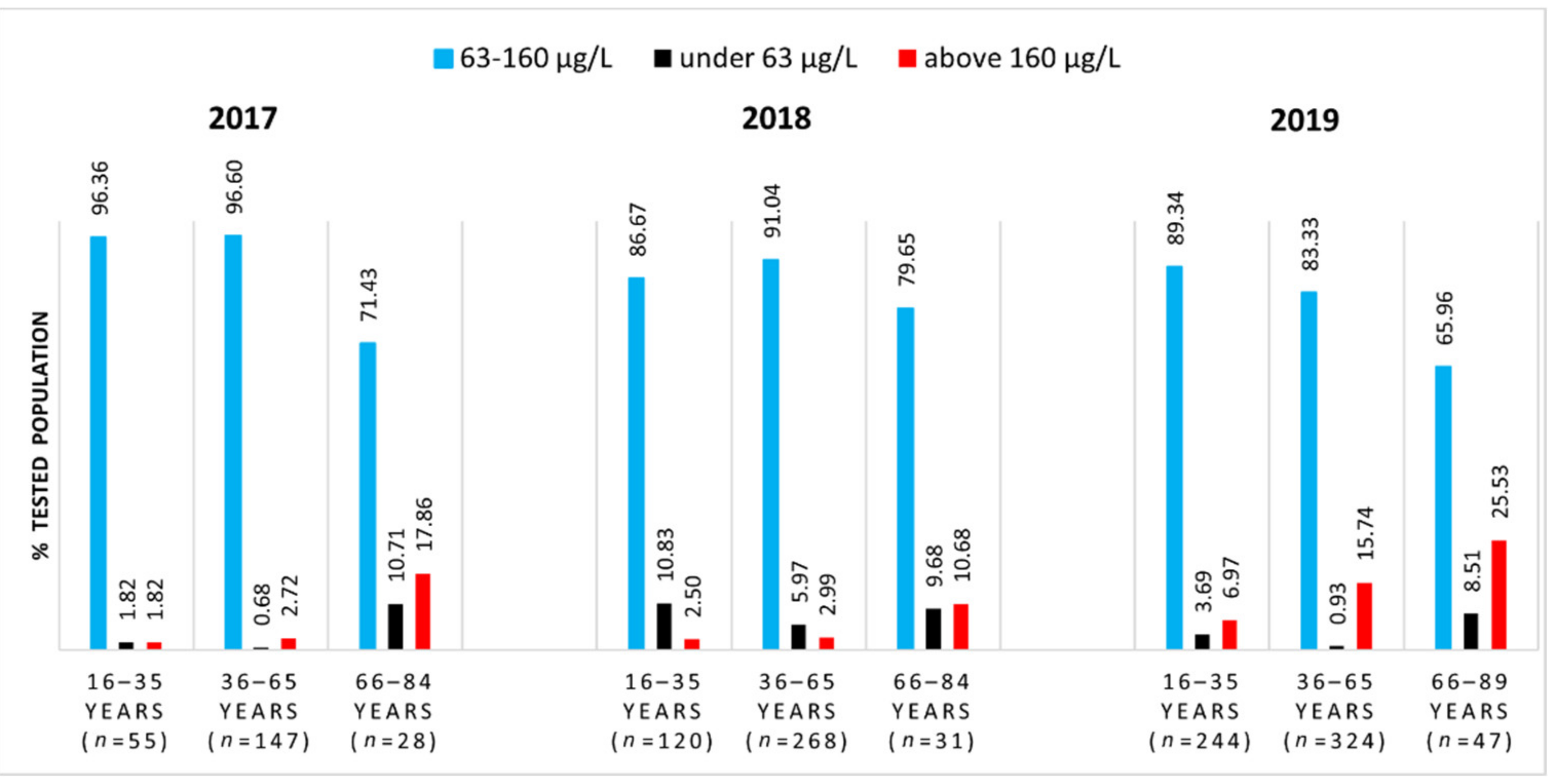

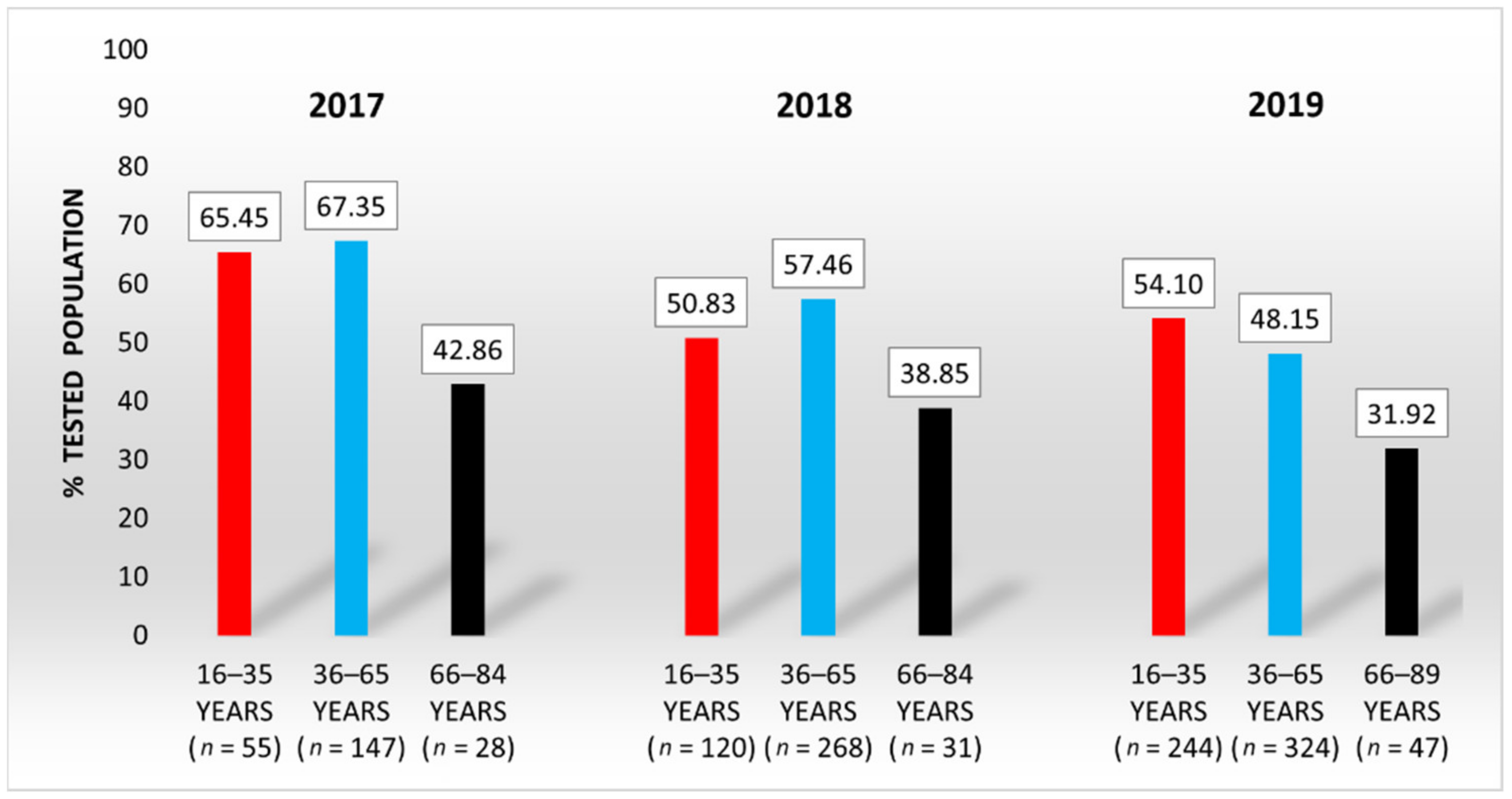
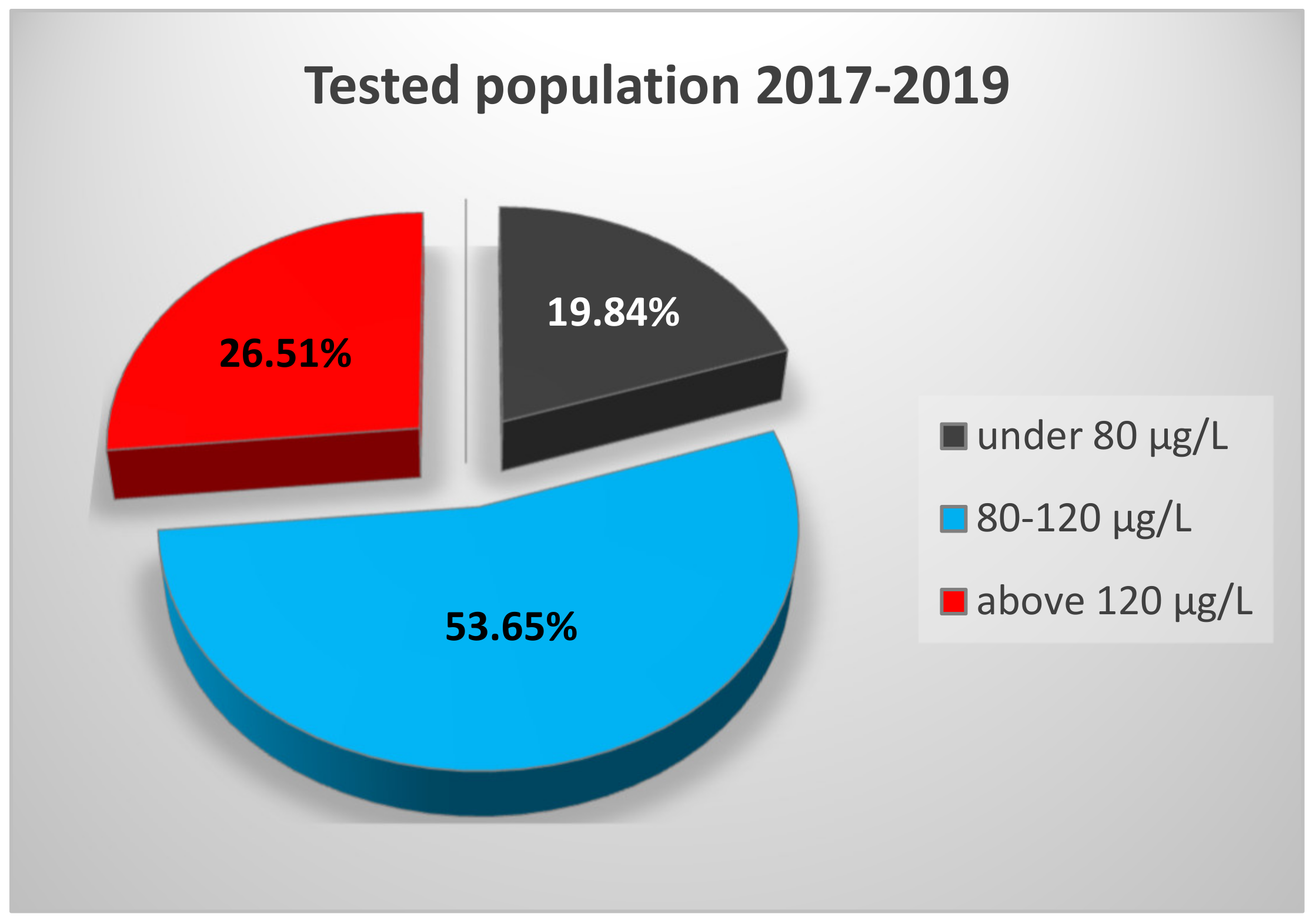
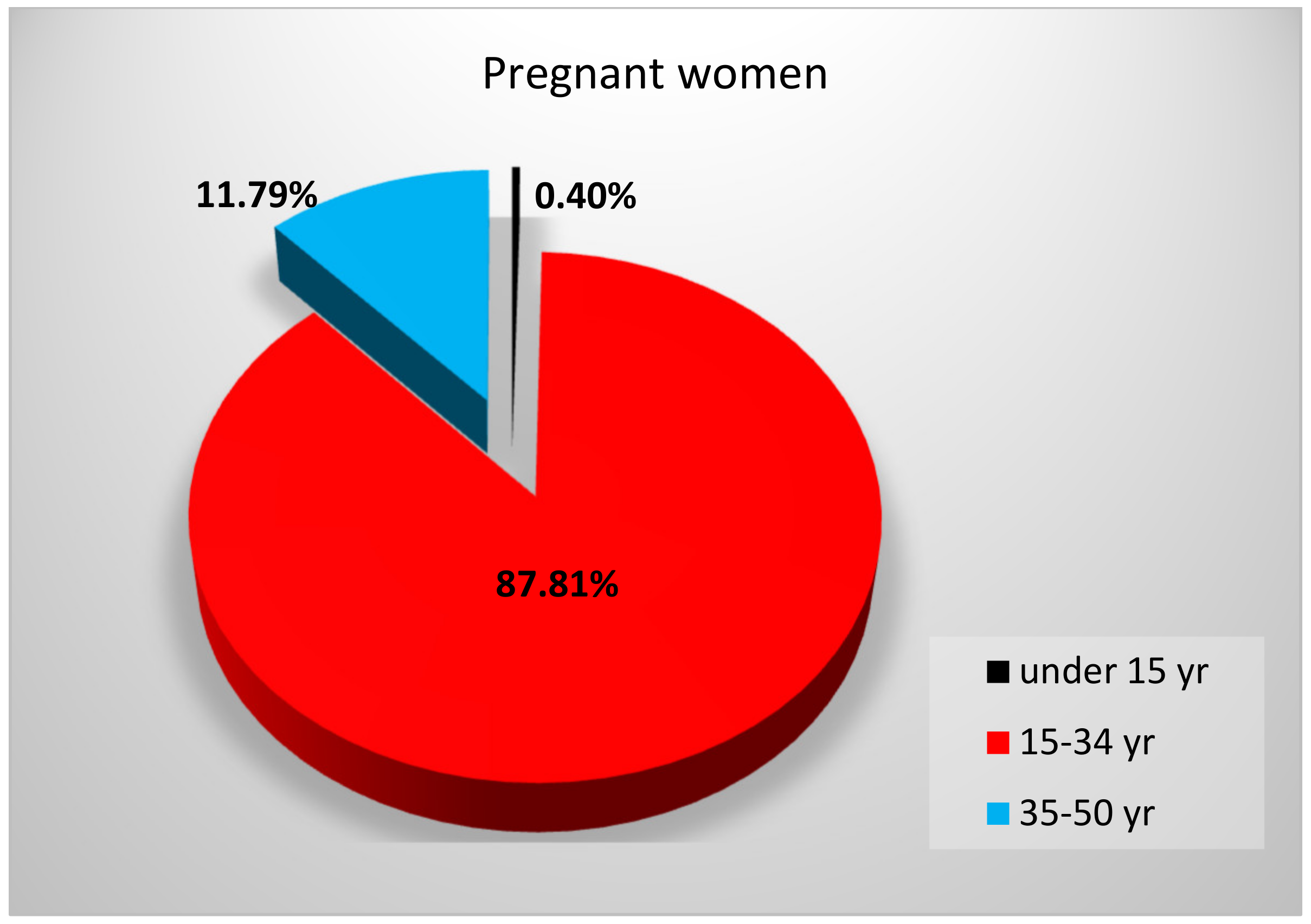


| Year | Age Range (yr) | Number of Subjects | Median SeS 1 (μg/L) | SeS Range (μg/L) |
|---|---|---|---|---|
| 2017 | 16–35 | 55 | 96.11 ± 15.00 | 51.09–165.59 |
| 36–65 | 147 | 97.27 ± 16.08 | 58.54–219.66 | |
| ≥66 | 28 | 98.66 ± 25.77 | 52.32–184.60 | |
| 2018 | 16–35 | 120 | 89.00 ± 17.49 | 46.63–176.46 |
| 36–65 | 268 | 92.35 ± 18.70 | 42.69–222.93 | |
| ≥66 | 31 | 99.56 ± 24.85 | 37.64–205.37 | |
| 2019 | 16–35 | 244 | 98.45 ± 24.20 | 23.80–259.00 |
| 36–65 | 324 | 115.00 ± 27.18 | 51.25–299.00 | |
| ≥66 | 47 | 129.00 ± 33.50 | 48.33–281.00 |
| Gender | Number of Subjects | Total Number of Subjects | Median SeS 1 (μg/L) | SeS Range (μg/L) | ||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | ||||
| Female | 42 | 91 | 216 | 349 | 94.15 ± 22.22 | 23.80–259.00 |
| Male | 13 | 29 | 28 | 70 | 106.05 ± 17.52 | 60.05–180.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizerea-Moga, T.O.; Pitulice, L.; Bizerea-Spiridon, O.; Moga, T.V. Evaluation of Serum Selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania. Nutrients 2021, 13, 1497. https://doi.org/10.3390/nu13051497
Bizerea-Moga TO, Pitulice L, Bizerea-Spiridon O, Moga TV. Evaluation of Serum Selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania. Nutrients. 2021; 13(5):1497. https://doi.org/10.3390/nu13051497
Chicago/Turabian StyleBizerea-Moga, Teofana Otilia, Laura Pitulice, Otilia Bizerea-Spiridon, and Tudor Voicu Moga. 2021. "Evaluation of Serum Selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania" Nutrients 13, no. 5: 1497. https://doi.org/10.3390/nu13051497
APA StyleBizerea-Moga, T. O., Pitulice, L., Bizerea-Spiridon, O., & Moga, T. V. (2021). Evaluation of Serum Selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania. Nutrients, 13(5), 1497. https://doi.org/10.3390/nu13051497








