Significance of Levocarnitine Treatment in Dialysis Patients
Abstract
1. Introduction
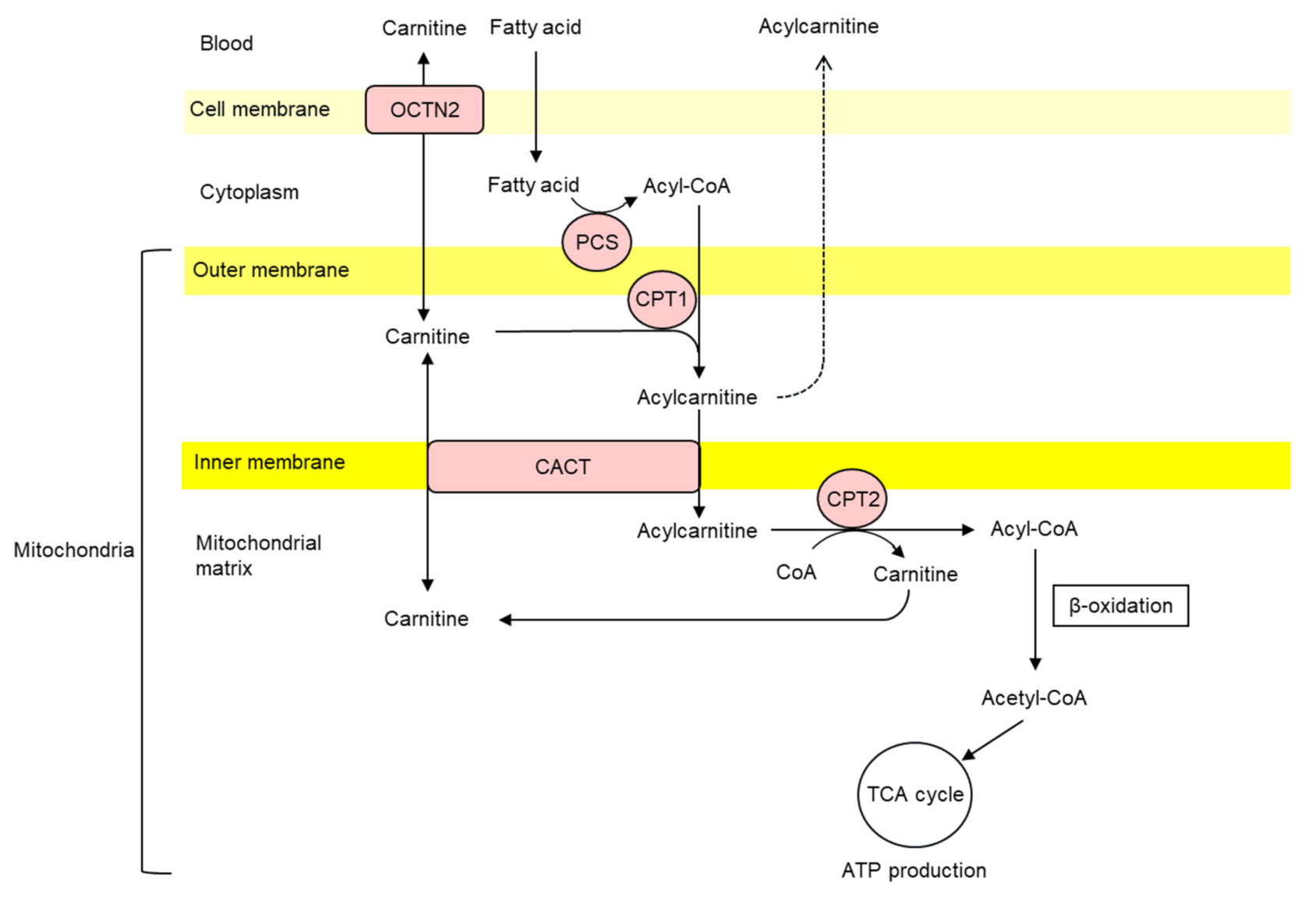
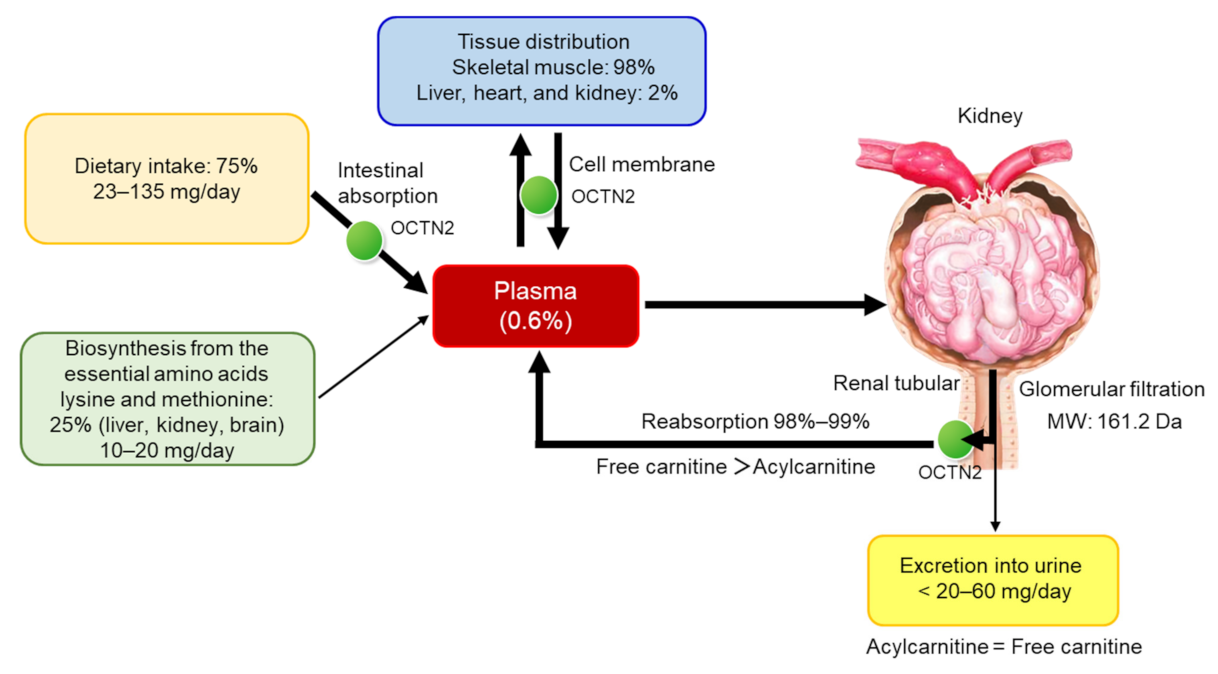
2. Carnitine Homeostasis
3. Carnitine Deficiency in Patients Who Are Undergoing Dialysis Therapy
4. Removal of Carnitine by Dialysis Therapy
5. Carnitine Supplementation in Dialysis Patients
6. Anemia
7. Cardiac Function
8. Muscle Symptoms and Quality of Life
9. Plasma Lipid Profiles and Inflammation-Related Parameters
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golper, T.A.; Ahmad, S. L-carnitine administration to hemodialysis patients: Has it times come? Semin. Dial. 1992, 5, 94–98. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Koziol, B.J.; Shapiro, J.I.; Brass, E.P. Carnitine metabolism during exercise in patients on chronic hemodialysis. Kidney Int. 1992, 41, 1613–1619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guarnieri, G.; Situlin, R.; Biolo, G. Carnitine metabolism in uremia. Am. J. Kidney Dis. 2001, 38, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Faull, R.; Fornasini, G.; Lemanowicz, E.F.; Longo, A.; Pace, S.; Nation, R.L. Pharmacokinetics of L-carnitine in patients with end-stage renal disease undergoing long-term hemodialysis. Clin. Pharmacol. Ther. 2000, 68, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Borum, P.R. Carnitine. Annu. Rev. Nutr. 1983, 3, 233–259. [Google Scholar] [CrossRef]
- Moorthy, A.V.; Rosenblum, M.; Rajaram, R.; Shug, A.L. A comparison of plasma and muscle carnitine levels in patients on peritoneal or hemodialysis for chronic renal failure. Am. J. Nephrol. 1983, 3, 205–208. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Chenard, C.A. Metabolic fate of dietary carnitine in human adults: Identification and quantification of urinary and faecal metabolism. J. Nutr. 1991, 121, 539–546. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Belke, D.D.; Gamble, J.; Itoi, T.; Schönekess, B.O. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochem. Biophys. Acta 1994, 1213, 263–276. [Google Scholar] [CrossRef]
- Marzo, A.; Martelli, E.A.; Urso, R.; Rocchetti, M.; Rizza, V.; Kelly, J.G. Metabolism and disposition of intravenously administered acetyl-L-carnitine in healthy volunteers. Eur. J. Clin. Pharmacol. 1989, 37, 59–63. [Google Scholar]
- Matera, M.; Bellinghieri, G.; Costantino, G.; Santoro, D.; Calvani, M.; Savica, V. History of L-Carnitine: Implications for renal disease. J. Ren. Nutr. 2003, 13, 2–14. [Google Scholar] [CrossRef]
- Tamai, I.; Ohashi, R.; Nezu, J.; Yabuuchi, H.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Molecular and functional identification of sodium-dependent high affinity human carnitine trasporter OTCN2. J. Biol. Chem. 1998, 273, 20378–20382. [Google Scholar] [CrossRef]
- Tamai, I.; China, K.; Sai, Y.; Kobayashi, D.; Nezu, J.; Kawahara, E.; Tsuji, A. Na (+)-coupled transporter of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellar localization in kidney. Biochim. Biophys. Acta 2001, 1512, 273–284. [Google Scholar]
- Koizumi, A.; Nozaki, J.; Ohura, T.; Kayo, T.; Wada, Y.; Nezu, J.; Ohashi, R.; Tamai, I.; Shoji, Y.; Takada, G.; et al. Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum. Mol. Genet. 1999, 8, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.V.; Murthy, M.S.R. Carnitine-acylcarnitine translocase deficiency: Implications in human pathology. Biochim. Biophys. Acta 1994, 1226, 269–276. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system: From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef]
- Zammit, V.A. Carnitine acyltransferase: Functional significance of subcellular distribution and membrane topology. Prog. Lipid Res. 1999, 38, 199–244. [Google Scholar] [CrossRef]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Zhou, Y.T.; Levi, M.; Unger, R.H. Fatty acid-induced beta cell apoptosis: A link between obesity and diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 2498–2502. [Google Scholar] [CrossRef]
- Winter, S.C.; Zorn, E.M.; Vance, W.H. Carnitine deficiency. Lancet 1990, 335, 981–982. [Google Scholar] [CrossRef]
- Suzuki, Y.K.; Tokuyama, K.; Kinoshita, M. Urinary profile of L-Carnitine and its derivatives in starved normal persons and ACTH injected patients with myopathy. J. Nutr. Sci. Vitaminol. 1983, 29, 303–312. [Google Scholar] [CrossRef]
- Kanda, E.; Kato, A.; Masakane, I.; Kanno, Y. A new nutritional risk index for predicting mortality in hemodialysis patients: Nationwide cohort study. PLoS ONE 2019, 14, e0214524. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kalantar-Zadeh, K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat. Rev. Nephrol 2015, 11, 302–313. [Google Scholar] [CrossRef]
- Tein, I. Carnitine transport: Pathophysiology and metabolism of known molecular defects. J. Inherit. Metab. Dis. 2003, 26, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Hoppel, C. Genetic disorders of carnitine metabolism and their nutritional management. Annu. Rev. Nutr. 1998, 18, 179–206. [Google Scholar] [CrossRef]
- Evans, A.M. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. 2003, 42 (Suppl. 4), S13–S26. [Google Scholar] [CrossRef]
- Evans, A.M.; Fornaini, G. Pharmacokinetics of L-carnitine. Clin. Pharm. 2003, 42, 941–967. [Google Scholar] [CrossRef]
- Japan Pediatric Society. Available online: http://www.jpeds.or.jp/modules/guidelines/index.php?content_id=2 (accessed on 21 September 2018).
- Evans, A.M.; Faull, R.J.; Nation, R.L.; Prasad, S.; Elias, T.; Reuter, S.E.; Fornasini, G. Impact of hemodialysis on endogenous plasma and muscle carnitine levels in patients with end-stage renal disease. Kidney Int. 2004, 66, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, L.G.; Palmieri, G.; Mauriello, A.; Vacha, G.M.; D’Iddio, S.; Giorcelli, G.; Corsi, M. Morphometric evidence of the trophic effect of L-carnitine on human skeletal muscle. Nephron 1990, 55, 16–23. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Higuchi, T.; Akiya, Y.; Horikami, T.; Tei, R.; Furukawa, T.; Takashima, H.; Tomita, H.; Abe, M. Prevalence of carnitine deficiency and decreased carnitine levels in patients on hemodialysis. Blood Purif. 2019, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sirolli, V.; Rossi, C.; Di Castelnuovo, A.; Felaco, P.; Amoroso, L.; Zucchelli, M.; Ciavardelli, D.; Di Ilio, C.; Sacchetta, P.; Bernardini, S.; et al. Toward personalized hemodialysis by low molecular weight aminocontaining compounds: Future perspective of patient metabolic fingerprint. Blood Transfus. 2012, 10, 78–88. [Google Scholar]
- Reuter, S.E.; Evans, A.M.; Faull, R.J.; Chace, D.H.; Fornaini, G. Impact of haemodialysis on individual endogenous plasma acylcarnitine concentrations in end-stage renal disease. Ann. Clin. Biochem. 2005, 42, 387–393. [Google Scholar] [CrossRef]
- Marzo, A.; Arrigoni Martelli, E.; Mancinelli, A.; Cardace, G.; Corbelletta, C.; Bassani, E.; Solbiati, M. Protein binding of L-carnitine family components. Eur. J. Drug Metab. Pharmacokinet. 1991, 3, 364–368. [Google Scholar]
- Debska-Slizien, A.; Kawecka, A.; Wojnarowski, K.; Prajs, J.; Malgorzewicz, S.; Kunicka, D.; Zdrojewski, Z.; Walysiak, S.; Lipinski, J.; Rutkowski, B. Correlation between plasma carnitine, muscle carnitine and glycogen levels in maintenance hemodialysis patients. Int. J. Artif. Organs 2000, 23, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Kamei, D.; Tsuchiya, K.; Mineshima, M.; Nitta, K. Association between 4-year all-cause mortality and carnitine profile in maintenance hemodialysis patients. PLoS ONE 2018, 13, e0201591. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Watanabe, Y.; Masakane, I.; Wada, A.; Shoji, T.; Hasegawa, T.; Nakamoto, H.; Yamagata, K.; Kazama, J.J.; Fujii, N.; et al. Overview of regular dialysis treatment in Japan (as of 31 December 2011). Ther. Aphel. Dial. 2013, 17, 567–611. [Google Scholar] [CrossRef]
- Masakane, I.; Kikuchi, K.; Kawanishi, H. Evidence for the clinical advantages of predilution on-line hemodiafiltration. Contrib. Nephrol. 2017, 189, 17–23. [Google Scholar]
- Penne, E.L.; van der Weerd, N.C.; Blankestijn, P.J.; van den Dorpel, M.A.; Grooteman, M.P.; Nubé, M.J.; Ter Wee, P.M.; Lévesque, R.; Bots, M.L.; CONTRAST investigators. Role of residual kidney function and convective volume on change in beta2-microglobulin levels in hemodiafiltration patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 80–86. [Google Scholar] [CrossRef]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macià, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Constantin-Teodosiu, D.; Kirby, D.P.; Short, A.H.; Burden, R.P.; Morgan, A.G.; Greenha, P.L. Free and esterified carnitine in continuous ambulatory peritoneal dialysis patients. Kidney Int. 1996, 49, 158–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sotirakopoulos, N.; Athanasiou, G.; Tsitsios, T.; Mavromatidis, K. The influence of L-carnitine supplementation on hematocrit and hemoglobin levels in patients with end stage renal failure on CAPD. Ren. Fail. 2002, 24, 505–510. [Google Scholar] [CrossRef]
- Grzegorzewska, A.E.; Mariak, I.; Dobrowolska-Zachwieja, A. Continuous ambulatory peritoneal dialysis (CAPD) adequacy influences serum free carnitine level. Int. Urol. Nephrol. 1999, 31, 533–540. [Google Scholar] [CrossRef]
- Shimizu, S.; Takashima, H.; Tei, R.; Furukawa, T.; Okamura, M.; Kitai, M.; Nagura, C.; Maruyama, T.; Higuchi, T.; Abe, M. Prevalence of carnitine deficiency and decreased carnitine levels in patients on peritoneal dialysis. Nutrients 2019, 11, 2645. [Google Scholar] [CrossRef]
- Schreiber, B. Levocarnitine and dialysis: A review. Nutr. Clin. Pract. 2005, 20, 218–243. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin. Ther. 1995, 17, 176–185. [Google Scholar] [CrossRef]
- Golper, T.A.; Wolfson, M.; Ahmad, S.; Hirschberg, R.; Kurtin, P.; Katz, L.A.; Nicora, R.; Ashbrook, D.; Kopple, J.D. Multicenter trial of L-carnitine in maintenance hemodialysis patients. I. Carnitine concentrations and lipid effects. Kidney Int. 1990, 38, 904–911. [Google Scholar] [CrossRef]
- Ahmad, S.; Robertson, H.T.; Golper, T.A.; Wolfson, M.; Kurtin, P.; Katz, L.A.; Hirschberg, R.; Nicora, R.; Ashbrook, D.W.; Kopple, J.D. Multicenter trial of L-carnitine in maintenance hemodialysis patients. II. Clinical and biochemical effects. Kidney Int. 1990, 38, 912–918. [Google Scholar] [CrossRef]
- Fagher, B.; Cederblad, G.; Eriksson, M.; Monti, M.; Moritz, U.; Nilsson-Ehle, P.; Thysell, H. L-carnitine and haemodialysis: Double blind study on muscle function and metabolism and peripheral nerve function. Scand. J. Clin. Lab. Investig. 1985, 45, 169–178. [Google Scholar] [CrossRef]
- Sahajwalla, C.G.; Helton, E.D.; Purich, E.D.; Hoppel, C.L.; Cabana, B.E. Comparison of L-carnitine pharmacokinetics with and without baseline correction following administration of single 20-mg/kg intravenous dose. J. Pharm. Sci. 1995, 84, 634–639. [Google Scholar] [CrossRef]
- Segre, G.; Bianchi, E.; Corsi, M.; D’Iddio, S.; Ghirardi, O.; Maccari, F. Plasma and urine pharmacokinetics of free and of short-chain carnitine after administration of carnitine in man. Arzneimittelforschung 1988, 38, 1830–1834. [Google Scholar]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Lang, D.H.; Yeung, C.K.; Peter, R.M.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.M.; Rettie, A.E. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Al-Waiz, M.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 1987, 17, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C.; Zhang, A.Q.; Noblet, J.M.; Gillespie, S.; Jones, N.; Smith, R.L. Metabolic disposition of [14C]-trimethylamine N-oxide in rat: Variation with dose and route of administration. Xenobiotica 1997, 27, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiotadependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef]
- Eknoyan, G.; Latos, D.L.; Lindberg, J. Practice recommendations for the use of L-carnitine in dialysis-related carnitine disorder. National Kidney Foundation Carnitine Consensus Conference. Am. J. Kidney Dis. 2003, 41, 868–876. [Google Scholar] [CrossRef]
- Parfrey, P.S.; Foley, R.N.; Wittreich, B.H.; Sullivan, D.J.; Zagari, M.J.; Frei, D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J. Am. Soc. Nephrol. 2005, 16, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Johnson, D.W.; Tonelli, M.; Garg, A.X.; Pellegrini, F.; Ravani, P.; Jardine, M.; Perkovic, V.; et al. Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann. Intern. Med. 2010, 153, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thamer, M.; Stefanik, K.; Kaufman, J.; Cotter, D.J. Epoetin requirements predict mortality in hemodialysis patients. Am. J. Kidney Dis. 2004, 44, 866–876. [Google Scholar] [CrossRef]
- Gunnell, J.; Yeun, J.Y.; Depner, T.A.; Kaysen, G.A. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am. J. Kidney Dis. 1999, 33, 63–72. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I.; Saito, K.; Nangaku, M.; Hattori, M.; Suzuki, T.; Morita, S.; Ashida, A.; et al. 2015 Japanese Society for Dialysis Therapy: Guidelines for Renal Anemia in Chronic Kidney Disease. Ren. Replace. Ther. 2017, 3, 36. [Google Scholar] [CrossRef]
- Kooistra, M.P.; Struyvenberg, A.; Vanes, A. The response to recombinant human erythropoietin in patients with the anemia of end-stage renal disease is correlated with serum carnitine levels. Nephron 1991, 57, 127–128. [Google Scholar] [CrossRef]
- Matsumura, M.; Hatakeyama, S.; Koni, I.; Mabuchi, H.; Muramoto, H. Correlation between serum carnitine levels and erythrocyte osmotic fragility in hemodialysis patients. Nephron 1996, 72, 574–578. [Google Scholar] [CrossRef]
- Steiber, A.L.; Weatherspoon, L.J.; Spry, L.; Davis, A.T. Serum carnitine concentrations correlated to clinical outcome parameters in chronic hemodialysis patients. Clin. Nutr. 2004, 23, 27–34. [Google Scholar] [CrossRef]
- Kletzmayr, J.; Mayer, G.; Legenstein, E.; Heinz-Peer, G.; Leitha, T.; Hörl, W.H.; Kovarik, J. Anemia and carnitine supplementation in hemodialyzed patients. Kidney Int. Suppl. 1999, 55, S93–S106. [Google Scholar] [CrossRef]
- Caruso, U.; Leone, L.; Cravotto, E.; Nava, D. Effects of L-carnitine on anemia in aged hemodialysis patients treated with recombinant human erythropoietin: A pilot study. Dial. Transplant. 1998, 27, 498–506. [Google Scholar]
- Sotirakopoulos, N.; Athanasiou, G.; Tsitsios, T.; Stambolidou, M.; Missirlis, Y.; Mavromatidis, K. Effect of L-carnitine supplementation on red blood cells deformability in hemodialysis patients. Ren. Fail. 2000, 22, 73–80. [Google Scholar]
- de los Reyes, B.; Perez-García, R.; Liras, A.; Arenas, J. Reduced carnitine palmitoyl transferase activity and altered acyl-trafficking in red blood cells from hemodialysis patients. Biochim. Biophys. Acta 1996, 1315, 37–39. [Google Scholar] [CrossRef]
- Arduini, A.; Rossi, M.; Mancinelli, G.; Belfiglio, M.; Scurti, R.; Radatti, G.; Shohet, S.B. Effect of L-carnitine and acetyl-L-carnitine on the human erythrocyte membrane stability and deformability. Life Sci. 1990, 47, 2395–2400. [Google Scholar] [CrossRef]
- Hörl, W.H. Is there a role for adjuvant therapy in patients being treated with epoetin? Nephrol. Dial. Transplant. 1999, 14, 50–60. [Google Scholar] [CrossRef][Green Version]
- Hurot, J.M.; Cucherat, M.; Haugh, M.; Fouque, D. Effects of L-carnitine supplementation in maintenance hemodialysis patients: A systematic review. J. Am. Soc. Nephrol. 2002, 13, 708–714. [Google Scholar]
- Zhu, Y.; Xue, C.; Ou, J.; Xie, Z.; Deng, J. Effect of L-carnitine supplementation on renal anemia in patients on hemodialysis: A meta-analysis. Int. Urol. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Bonomini, M.; Clutterbuck, E.J.; Laffan, M.A.; Pusey, C.D. Effect of L-carnitine administration on erythrocyte survival in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 2671–2672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, Y.; Amano, I.; Hirose, S.; Tsuruta, Y.; Hara, S.; Murata, M.; Imai, T. Effects of L-carnitine supplementation on renal anemia in poor responders to erythropoietin. Blood Purif. 2001, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P.; Adler, S.; Sietsema, K.E.; Hiatt, W.R.; Orlando, A.M.; Amato, A. Intravenous L-carnitine increases plasma carnitine, reduces fatigue, and may preserve exercise capacity in hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 1018–1028. [Google Scholar] [CrossRef]
- Savica, V.; Santoro, D.; Mazzaglia, G.; Ciolino, F.; Monardo, P.; Calvani, M.; Bellinghieri, G.; Kopple, J.D. L-carnitine infusions may suppress serum C-reactive protein and improve nutritional status in maintenance hemodialysis patients. J. Ren. Nutr. 2005, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.X.; Wu, E.L. Effect of levocarnitine/iron saccharate combination on renal anaemia and oxidative stress in patients undergoing haemodialysis. Trop. J. Pharm. Res. 2016, 15, 2269–2274. [Google Scholar] [CrossRef][Green Version]
- Mitwalli, A.H.; Al-Wakeel, J.S.; Alam, A.; Tarif, N.; Abu-Aisha, H.; Rashed, M.; Al Nahed, N. L-carnitine supplementation in hemodialysis patients. Saudi J. Kidney Dis. Transplant. 2005, 16, 17–22. [Google Scholar]
- Rathod, R.; Baig, M.S.; Khandelwal, P.N.; Kulkarni, S.G.; Gade, P.R.; Siddiqui, S. Results of a single blind, randomized, placebo-controlled clinical trial to study the effect of intravenous L-carnitine supplementation on health-related quality of life in Indian patients on maintenance hemodialysis. Indian J. Med. Sci. 2006, 60, 143–153. [Google Scholar]
- Semeniuk, J.; Shalansky, K.F.; Taylor, N.; Jastrzebski, J.; Cameron, E.C. Evaluation of the effect of intravenous l-carnitine on quality of life in chronic hemodialysis patients. Clin. Nephrol. 2000, 54, 470–477. [Google Scholar]
- Singh, H.; Jain, D.; Bhaduri, G.; Gupta, N.; Sangwan, R. Study on effects of L-carnitine supplementation on anaemia with erythropoietin hyporesponsiveness and lipid profile in chronic kidney disease patients on maintenance haemodialysis. Indian J. Basic Appl. Med. Res. 2020, 9, 224–232. [Google Scholar]
- Fu, R.G.; Wang, L.; Zhou, J.P.; Ma, F.; Liu, X.D.; Ge, H.; Zhang, J. The effect of levocarnitine on nutritional status and lipid metabolism during long-term maintenance hemodialysis. Acad. J. Xi’an Jiaotong Univ. 2010, 22, 203–207. [Google Scholar]
- Kuwasawa-Iwasaki, M.; Io, H.; Muto, M.; Ichikawa, S.; Wakabayashi, K.; Kanda, R.; Nakata, J.; Nohara, N.; Tomino, Y.; Suzuki, Y. Effects of L-carnitine supplementation in patients receiving hemodialysis or peritoneal dialysis. Nutrients 2020, 12, 3371. [Google Scholar] [CrossRef] [PubMed]
- Emami Naini, A.; Moradi, M.; Mortazavi, M.; Amini Harandi, A.; Hadizadeh, M.; Shirani, F.; Basir Ghafoori, H.; Emami Naini, P. Effects of oral L-carnitine supplementation on lipid profile, anemia, and quality of life in chronic renal disease patients under hemodialysis: A randomized, double-blinded, placebo-controlled trial. J. Nutr. Metab. 2012, 2012, 510483. [Google Scholar] [CrossRef]
- Trovato, G.M.; Iannetti, E.; Murgo, A.M.; Carpinteri, G.; Catalano, D. Body composition and long-term levo-carnitine supplementation. Clin. Ter. 1998, 149, 209–214. [Google Scholar] [PubMed]
- Vaux, E.C.; Taylor, D.J.; Altmann, P.; Rajagopalan, B.; Graham, K.; Cooper, R.; Bonomo, Y.; Styles, P. Effects of carnitine supplementation on muscle metabolism by the use of magnetic resonance spectroscopy and near-infrared spectroscopy in end-stage renal disease. Nephron. Clin. Pract. 2004, 97, 41–48. [Google Scholar] [CrossRef]
- Chazot, C.; Blanc, C.; Hurot, J.M.; Charra, B.; Jean, G.; Laurent, G. Nutritional effects of carnitine supplementation in hemodialysis patients. Clin. Nephrol. 2003, 59, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Labonia, W.D. L-carnitine effects on anemia in hemodialyzed patients treated with erythropoietin. Am. J. Kidney Dis. 1995, 26, 757–764. [Google Scholar] [CrossRef]
- Maruyama, T.; Higuchi, T.; Yamazaki, T.; Okawa, E.; Ando, H.; Oikawa, O.; Inoshita, A.; Okada, K.; Abe, M. Levocarnitine injections decrease the need for erythropoiesis-stimulating agents in hemodialysis patients with renal anemia. Cardiorenl. Med. 2017, 7, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Mercadal, L.; Coudert, M.; Vassault, A.; Pieroni, L.; Debure, A.; Ouziala, M.; Depreneuf, H.; Fumeron, C.; Servais, A.; Bassilios, N.; et al. L-carnitine treatment in incident hemodialysis patients: The multicenter, randomized, doubleblinded, placebo-controlled CARNIDIAL trial. Clin. J. Am. Soc. Nephrol. 2012, 7, 1836–1842. [Google Scholar] [CrossRef]
- Mercadal, L.; Tezenas du Montcel, S.; Chonchol, M.B.; Debure, A.; Depreneuf, H.; Servais, A.; Bassilios, N.; Assogba, U.; Allouache, M.; Prié, D. Effects of L-carnitine on mineral metabolism in the multicentre, randomized, double blind, placebo-controlled CARNIDIAL trial. Am. J. Nephrol. 2018, 48, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A.L.; Davis, A.T.; Spry, L.; Strong, J.; Buss, M.L.; Ratkiewicz, M.M.; Weatherspoon, L.J. Carnitine treatment improved quality-of-life measure in a sample of Midwestern hemodialysis patients. JPEN J. Parenter. Enteral. Nutr. 2006, 30, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Faull, R.J.; Evans, A.M. L-carnitine supplementation in the dialysis population: Are Australian patients missing out? Nephrology 2008, 13, 3–16. [Google Scholar] [CrossRef]
- Fotiadou, E.; Georgianos, P.I.; Chourdakis, M.; Zebekakis, P.E.; Liakopoulos, V. Eating during the Hemodialysis Session: A Practice Improving Nutritional Status or a Risk Factor for Intradialytic Hypotension and Reduced Dialysis Adequacy? Nutrients 2020, 12, 1703. [Google Scholar] [CrossRef]
- Shoji, T.; Tsubakihara, Y.; Fujii, M.; Imai, E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004, 66, 1212–1220. [Google Scholar] [CrossRef]
- Casciani, C.U.; Caruso, U.; Cravotto, E.; Corsi, M.; Maccari, F. Benefitial effects of L-carnitine in post-dialysis syndrome. Curr. Ther. Res. 1982, 32, 116–127. [Google Scholar]
- van Es, A.; Henny, F.C.; Kooistra, M.P.; Lobatto, S.; Scholte, H.R. Amelioration of cardiac function by L-carnitine administration in patients on haemodialysis. Contrib. Nephrol. 1992, 98, 28–35. [Google Scholar] [PubMed]
- Sakurabayashi, T.; Takaesu, Y.; Haginoshita, S.; Takeda, T.; Aoike, I.; Miyazaki, S.; Koda, Y.; Yuasa, Y.; Sakai, S.; Suzuki, M.; et al. Improvement of myocardial fatty acid metabolism through L-carnitine administration to chronic hemodialysis patients. Am. J. Nephrol. 1999, 19, 480–484. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, M.; Ohashi, H.; Araki, H.; Tadokoro, M.; Osumi, Y.; Ito, H.; Morita, H.; Amano, I. Effects of L-carnitine supplementation on cardiac morbidity in hemodialyzed patients. Am. J. Nephrol. 2000, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sakurabayashi, T.; Miyazaki, S.; Yuasa, Y.; Sakai, S.; Suzuki, M.; Takahashi, S.; Hirasawa, Y. L-carnitine supplementation decreases the left ventricular mass in patients undergoing hemodialysis. Circ. J. 2008, 72, 926–931. [Google Scholar] [CrossRef]
- Sabry, A.A. The role of oral L-carnitine therapy in chronic hemodialysis patients. Saudi J. Kidney Dis. Transplant. 2010, 21, 454–459. [Google Scholar]
- Kudoh, Y.; Aoyama, S.; Torii, T.; Chen, Q.; Nagahara, D.; Sakata, H.; Nozawa, A. Hemodynamic stabilizing effects of L-carnitine in chronic hemodialysis patients. Cardiorenal Med. 2013, 3, 200–207. [Google Scholar] [CrossRef]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Okawa, E.; Ando, H.; Hotta, S.; Oikawa, O.; Kikuchi, F.; Okada, K.; Soma, M. Levocarnitine Improves Cardiac Function in Hemodialysis Patients with Left Ventricular Hypertrophy: A Randomized Controlled Trial. Am. J. Kidney Dis. 2016, 67, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Sifuentes, H.R.; Del Cueto-Aguilera, Á.; Gallegos-Arguijo, D.A.; Castillo-Torres, S.A.; Vera-Pineda, R.; Martínez-Granados, R.J.; Atilano-Díaz, A.; Cuellar-Monterrubio, J.E.; Pezina-Cantú, C.O.; Martínez-Guevara, E.J.; et al. Levocarnitine decreases intradialytic hypotension episodes: A randomized controlled trial. Ther. Apher. Dial. 2017, 21, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.; Rutherford, S.; Rutherford, P.A. Low carnitine levels in hemodialysis patients: Relationship with functional activity status and intra-dialytic hypotension. Clin. Nephrol. 1997, 48, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Poldermans, D.; Man in‘t Veld, A.J.; Rambaldi, R.; Van Den Meiracker, A.H.; Van Den Dorpel, M.A.; Rocchi, G.; Boersma, E.; Bax, J.J.; Weimar, W.; Roelandt, J.R.; et al. Cardiac evaluation in hypotension-prone and hypotension-resistant hemodialysis patients. Kidney Int. 1999, 56, 1905–1911. [Google Scholar] [CrossRef]
- Litwin, S.E.; Raya, T.E.; Gay, R.G.; Bedotto, J.B.; Bahl, J.J.; Anderson, P.G.; Goldman, S.; Bressler, R. Chronic inhibition of fatty acid oxidation: New model of diastolic dysfunction. Am. J. Physiol. 1990, 258, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, G.F.; Naso, A.; Carraro, G.; Lidestri, V. Beneficial effects of L-carnitine in dialysis patients with impaired left ventricular function: An observational study. Curr. Med. Res. Opin. 2002, 18, 172–175. [Google Scholar] [CrossRef]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Mizuno, M.; Okawa, E.; Ando, H.; Oikawa, O.; Okada, K.; Kikuchi, F.; Soma, M. Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: A randomized controlled trial. Nutrients 2014, 6, 5992–6004. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Som, P.; Yonekura, Y.; Knapp, F.F., Jr.; Tamaki, N.; Yamamoto, K.; Konishi, J.; Yokoyama, A. Myocardial accumulation of iodinated beta-methyl-branched fatty acid analog, [125I] (p-iodophenyl)-3-(R, S)-methylpentadecanoic acid (BMIPP), and correlation to ATP concentration--II. Studies in salt-induced hypertensive rats. Nucl. Med. Biol. 1993, 20, 163–166. [Google Scholar] [CrossRef]
- Kazmi, W.H.; Obrador, G.T.; Sternberg, M.; Lindberg, J.; Schreiber, B.; Lewis, V.; Pereira, B.J. Carnitine therapy is associated with decreased hospital utilization among hemodialysis patients. Am. J. Nephrol. 2005, 25, 106–115. [Google Scholar]
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and precachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. International Society of Renal Nutrition and Metabolism. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Kalantar-Zadeh, K.; Kopple, J.D. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J. Am. Soc. Nephrol. 2013, 24, 337–351. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Johansen, K.L.; Chertow, G.M.; Ng, A.V.; Mulligan, K.; Carey, S.; Schoenfeld, P.Y.; Kent-Braun, J.A. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000, 57, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G.; Martin, D.R. Association of patient autonomy with increased transplantation and survival among new dialysis patients in the United States. Am. J. Kidney Dis. 2005, 45, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L. The frail dialysis population: A growing burden for the dialysis community. Blood Purif. 2015, 40, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Chertow, G.M.; Jin, C.; Kutner, N.G. Significance of frailty among dialysis patients. J. Am. Soc. Nephrol. 2007, 18, 2960–2967. [Google Scholar] [CrossRef]
- Bellinghieri, G.; Santoro, D.; Calvani, M.; Savica, V. Role of carnitine in modulating acute-phase protein synthesis in hemodialysis patients. J. Ren. Nutr. 2005, 15, 13–17. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationship between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 2000, 15, 953–960. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, B.R.; Schwartz, J.; Istfan, N.W. Cytokines, muscle proteolysis, and the catabolic response to infection and inflammation. Proc. Soc. Exp. Biol. Med. 1992, 200, 220–223. [Google Scholar] [CrossRef]
- Duranay, M.; Akay, H.; Yilmaz, F.M.; Senes, M.; Tekeli, N.; Yücel, D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 3211–3214. [Google Scholar] [CrossRef] [PubMed]
- Suchitra, M.M.; Ashalatha, V.L.; Sailaja, E.; Rao, A.M.; Reddy, V.S.; Bitla, A.R.; Sivakumar, V.; Rao, P.V. The effect of L-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2011, 22, 1155–1159. [Google Scholar] [PubMed]
- Shakeri, A.; Tabibi, H.; Hedayati, M. Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int. 2010, 14, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 2003, 41, 4–12. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cammalleri, L.; Gargante, M.P.; Vacante, M.; Colonna, V.; Motta, M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: A randomized and controlled clinical trial. Am. J. Clin. Nutr. 2007, 86, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Badrasawi, M.; Shahar, S.; Zahara, A.M.; Nor Fadilah, R.; Singh, D.K. Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: A double-blind, randomized, placebocontrolled clinical trial. Clin. Interv. Aging 2016, 11, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Gargante, M.P.; Cristaldi, E.; Colonna, V.; Messano, M.; Koverech, A.; Neri, S.; Vacante, M. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch. Gerontol. Geriatr. 2008, 46, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sakurauchi, Y.; Matsumoto, Y.; Shinzato, T.; Takai, I.; Nakamura, Y.; Sato, M.; Nakai, S.; Miwa, M.; Morita, H.; Miwa, T.; et al. Effects of L-carnitine supplementation on muscular symptoms in hemodialyzed patients. Am. J. Kidney Dis. 1998, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Bellinghieri, G.; Savica, V.; Mallamace, A.; Di Stefano, C.; Consolo, F.; Spagnoli, L.G.; Villaschi, S.; Palmieri, G.; Corsi, M.; Maccari, F. Correlation between increased serum and tissue L-carnitine levels and improved muscle symptoms in hemodialyzed patients. Am. J. Clin. Nutr. 1983, 38, 523–531. [Google Scholar] [CrossRef]
- Siami, G.; Clinton, M.E.; Mrak, R.; Griffis, J.; Stone, W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron 1991, 57, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Maruyama, N.; Higuchi, T.; Nagura, C.; Takashima, H.; Kitai, M.; Utsunomiya, K.; Tei, R.; Furukawa, T.; Yamazaki, T.; et al. Efficacy of L-carnitine supplementation for improving lean body mass and physical function in patients on hemodialysis: A randomized controlled trial. Eur. J. Clin. Nutr. 2019, 73, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.S.; Kastan, B.; Rice, S.I.; Sallee, C.W.; Yuenger, N.J.; Smith, B.; Ward, R.A.; Brier, M.E.; Golper, T.A. Quality of life during and between hemodialysis treatments: Role of L-carnitine supplementation. Am. J. Kidney Dis. 1998, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, M.E.; Rylance, P.B.; Wilson, R.; De Sousa, C.; Lanigan, C.; Rose, P.E.; Howard, J.; Parsons, V. Carnitine and weakness in haemodialysis patients. Nephrol. Dial. Transplant. 1989, 4, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Giovenali, P.; Fenocchio, D.; Montanari, G.; Cancellotti, C.; D’Iddio, S.; Buoncristiani, U.; Pelagaggia, M.; Ribacchi, R. Selective trophic effect of L-carnitine in type I and IIa skeletal muscle fibers. Kidney Int. 1994, 46, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Feinfeld, D.A.; Kurian, P.; Cheng, J.T.; Dilimetin, G.; Arriola, M.R.; Ward, L.; Manis, T.; Carvounis, C.P. Effect of oral L-carnitine on serum myoglobin in hemodialysis patients. Ren. Fail. 1996, 18, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Fischer, F.P.; Mettang, T.; Pauli-Magnus, C.; Weber, J.; Kuhlmann, U. Effects of L-carnitine on leukocyte function and viability in hemodialysis patients: A double-blind randomized trial. Am. J. Kidney Dis. 1999, 34, 678–687. [Google Scholar] [CrossRef]
- Lynch, K.E.; Feldman, H.I.; Berlin, J.A.; Flory, J.; Rowan, C.G.; Brunelli, S.M. Effects of L-carnitine on dialysis-related hypotension and muscle cramps: A meta-analysis. Am. J. Kidney Dis. 2008, 52, 962–971. [Google Scholar] [CrossRef]
- Shoji, T.; Emoto, M.; Tabata, T.; Kimoto, E.; Shinohara, K.; Maekawa, K.; Kawagishi, T.; Tahara, H.; Ishimura, E.; Nishizawa, Y. Advanced atherosclerosis in predialysis patients with chronic renal failure. Kidney Int. 2002, 61, 2187–2192. [Google Scholar] [CrossRef]
- Shoji, T.; Masakane, I.; Watanabe, Y.; Iseki, K.; Tsubakihara, Y. Elevated non-high-density lipoprotein cholesterol (non-HDL-C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1112–1120. [Google Scholar] [CrossRef]
- Kidney Disease Outcomes Quality Initiative (K/DOQI) Group. K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. Am. J. Kidney Dis. 2003, 41, 1–91. [Google Scholar]
- Yeun, J.Y.; Levine, R.A.; Mantadilok, V.; Kaysen, G.A. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2000, 35, 469–476. [Google Scholar] [CrossRef]
- Krane, V.; Wanner, C. Statins, inflammation and kidney disease. Nat. Rev. Nephrol. 2011, 7, 385–397. [Google Scholar] [CrossRef]
- Abe, M.; Hamano, T.; Hoshino, J.; Wada, A.; Nakai, S.; Hanafusa, N.; Masakane, I.; Nitta, K.; Nakamoto, H. Predictors of outcomes in patients on peritoneal dialysis: A 2-year nationwide cohort study. Sci. Rep. 2019, 9, 3967. [Google Scholar] [CrossRef]
- Abe, M.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I.; Renal Data Registry Committee, Japanese Society for Dialysis Therapy. Effect of dialyzer membrane materials on survival in chronic hemodialysis patients: Results from the annual survey of the Japanese Nationwide Dialysis Registry. PLoS ONE 2017, 12, e0184424. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. High-performance membrane dialyzers and mortality in hemodialysis patients: A 2-year cohort study from the Annual Survey of the Japanese Renal Data Registry. Am. J. Nephrol. 2017, 46, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Liakopoulos, V.; Antoniadi, G.; Kartsios, C.; Stefanidis, I. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin. Dial. 2009, 22, 70–77. [Google Scholar] [CrossRef]
- Yu, J.; Ye, J.; Liu, X.; Han, Y.; Wang, C. Protective effect of L-carnitine against H(2)O(2)-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. Neurol. Res. 2011, 33, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Biancini, G.B.; Mescka, C.; Wayhs, C.Y.; Sitta, A.; Wajner, M.; Vargas, C.R. Oxidative stress parameters in urine from patients with disorders of propionate metabolism: A beneficial effect of L-carnitine supplementation. Cell. Mol. Neurobiol. 2012, 32, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Stulle, M.; Bianco, F.; Mengozzi, G.; Barazzoni, R.; Vasile, A.; Panzetta, G.; Guarnieri, G. Insulin action on glucose and protein metabolism during L-carnitine supplementation in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 991–997. [Google Scholar] [CrossRef]
- Katalinic, L.; Krtalic, B.; Jelakovic, B.; Basic-Jukic, N. The unexpected effects of L-carnitine supplementation on lipid metabolism in hemodialysis patients. Kidney Blood Press Res. 2018, 43, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Khatami, M.R.; Dashti-Khavidaki, S.; Lessan-Pezeshki, M.; Abdollahi, A.; Moghaddas, A. Protective effects of L-carnitine against delayed graft function in kidney transplant recipients: A pilot, randomized, double-blinded, placebo-controlled clinical trial. J. Ren. Nutr. 2017, 27, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.F.; Ranieri, F.; Toigo, G.; Vasile, A.; Ciman, M.; Rizzoli, V.; Moracchiello, M.; Campanacci, L. Lipid-lowering effect of carnitine in chronically uremic patients treated with maintenance hemodialysis. Am. J. Clin. Nutr. 1980, 33, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Vacha, G.M.; Giorcelli, G.; D’Iddio, S.; Valentini, G.; Bagiella, E.; Procopio, A.; di Donato, S.; Ashbrook, D.; Corsi, M. L-carnitine addition to dialysis fluid. A therapeutic alternative for hemodialysis patients. Nephron 1989, 51, 237–242. [Google Scholar] [CrossRef]
- Bellinghieri, G.; Savica, V.; Barbera, C.M.; Ricciardi, B.; Egitto, M.; Torre, F.; Valentini, G.; D’Iddio, S.; Bagiella, E.; Mallamace, A.; et al. L-carnitine and platelet aggregation in uremic patients subjected to hemodialysis. Nephron 1990, 55, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.J.; Choi, G.B.; Yoon, K.I. L-Carnitine in maintenance hemodialysis clinical lipid and biochemical effects. Korean J. Nephrol. 1992, 2, 260–268. [Google Scholar]
- Yderstraede, K.B.; Pedersen, F.B.; Dragsholt, C.; Trostmann, A.; Laier, E.; Larsen, H.F. The effect of L-carnitine on lipid metabolism in patients on chronic haemodialysis. Nephrol. Dial. Transplant. 1987, 1, 238–241. [Google Scholar] [PubMed]
- Weschler, A.; Aviram, M.; Levin, M.; Better, O.S.; Brook, J.G. High dose of L-carnitine increases platelet aggregation and plasma triglyceride levels in uremic patients on hemodialysis. Nephron 1984, 38, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Fatuzzo, P.; Rapisarda, F.; Neri, S.; Ferrante, M.; Oliveri Conti, G.; Fallico, R.; Di Pino, L.; Pennisi, G.; Celotta, G.; et al. A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl L-carnitine in patients with peripheral arterial disease requiring haemodialysis. Drugs Aging 2006, 23, 263–270. [Google Scholar] [CrossRef]
- Hakeshzadeh, F.; Tabibi, H.; Ahmadinejad, M.; Malakoutian, T.; Hedayati, M. Effects of L-Carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren. Fail. 2010, 32, 1109–1114. [Google Scholar] [CrossRef]
- Tabibi, H.; Hakeshzadeh, F.; Hedayati, M.; Malakoutian, T. Effects of l-carnitine supplement on serum amyloid A and vascular inflammation markers in hemodialysis patients: A randomized controlled trial. J. Ren. Nutr. 2011, 21, 485–491. [Google Scholar] [CrossRef]
- Ahmadi, S.; Banadaki, S.D.; Mozaffari-Khosravi, H. Effects of oral L-carnitine supplementation on leptin and adiponectin levels and body weight of hemodialysis patients: A randomized clinical trial. Iran. J. Kidney. Dis. 2016, 10, 144–150. [Google Scholar] [PubMed]
- Alattiya, T.N.; Jaleel, N.A.; Al-Sabbag, M.S.; Jamil, N.S.; Mohammed, M.M. Effect of oral L-carnitine supplementation on the mortality markers in hemodialysis patients. Int. J. Pharm. Sci. Rev. Res. 2016, 14, 64–69. [Google Scholar]
- Yang, S.K.; Xiao, L.; Song, P.A.; Xu, X.; Liu, F.Y.; Sun, L. Effect of L-carnitine therapy on patients in maintenance hemodialysis: A systematic review and meta-analysis. J. Nephrol. 2014, 27, 317–329. [Google Scholar] [CrossRef]
- Huang, H.; Song, L.; Zhang, H.; Zhang, H.; Zhang, J.; Zhao, W. Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: A systematic review and meta-analysis. Kidney Blood Press Res. 2013, 38, 31–41. [Google Scholar] [CrossRef]
- Chen, Y.; Abbate, M.; Tang, L.; Cai, G.; Gong, Z.; Wei, R.; Zhou, J.; Chen, X. L-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 99, 408–422. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, T. The efficacy of L-carnitine in improving malnutrition in patients on maintenance hemodialysis: A meta-analysis. Biosci. Rep. 2020, 40, BSR20201639. [Google Scholar] [CrossRef] [PubMed]
| Ref | Study Design | Subjects | Dose and Route | Treatment Duration | Findings a |
|---|---|---|---|---|---|
| [77] | Two-way, parallel, double-blind | 29 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↑ RBC survival T0: 39.1 days; T6: 42.7 days (p = 0.058) |
| 29 HD patients | Placebo, IV | → RBC survival T0: 40.2 days; T6: 35.4 days (NS) | |||
| [78] | One-way, open-label | 14 HD patients (ESA-resistant) | 500 mg/day PO | 3 mo | ↑ Ht T0: 24.0% ± 2.0%; T3: 26.1% ± 2.0% (p = 0.003) |
| [71] | One-way, open-label | 15 HD patients | 30 mg/kg per Dx, IV | 3 mo | ↑ Ht T0: 30.8% ± 1.9%; T3: 34.2% ± 2.4% (p < 0.0001), ↓ Deformability of RBCs (p < 0.004) |
| [43] | One-way, open-label | 12 PD patients | 2 g/day PO | 3 mo | ↑ Ht T0: 35.4% ± 3.3%; T3: 38.1% ± 3.4% (p < 0.03), ↑ Hb T0: 11.0 ± 1.1 g/dL; T3: 11.9 ± 1.0 g/dL (p < 0.01) |
| [79] | Two-way, parallel, double-blind | 28 HD patients | 20 mg/kg per Dx, IV | 6 mo | → Ht T0: 34.1% ± 3.2%; T6: 32.8% ± 4.0% (NS) |
| 28 HD patients | Placebo | → Ht T0: 32.9% ± 3.3%; T6: 33.9% ± 2.9% (NS) | |||
| Four-way, parallel, double-blind | 32 HD patients | 10 mg/kg per Dx, IV | 6 mo | → Ht T0: 33.9% ± 3.2%; T6: 35.1% ± 4.2% (NS) | |
| 30 HD patients | 20 mg/kg per Dx, IV | → Ht T0: 33.7% ± 3.5%; T6: 33.9% ± 3.4% (NS) | |||
| 32 HD patients | 30 mg/kg per Dx, IV | → Ht T0: 33.6% ± 3.3%; T6: 33.5% ± 2.7% (NS) | |||
| 33 HD patients | Placebo | → Ht T0: 34.2% ± 3.2%; T6: 35.1% ± 4.2% (NS) | |||
| [80] | Two-way, parallel, double-blind | 48 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↑ Hb T0: 9.7 ± 1.1 g/dL; T6: 10.8 ± 1.2 g/dL (p < 0.0001) |
| 65 HD patients | Placebo, IV | → Hb T0: 9.8 ± 1.2 g/dL; T6: 9.9 ± 1.3 g/dL (NS) | |||
| [81] | Two-way, parallel, open label | 78 HD patients | 1 g/Dx, IV | 7 mo | ↑ Hb T0: 7.5 ± 1.5 g/dL; T7: 11.4 ± 1.2 g/dL (p < 0.05) |
| ↓ ERI T0: 183 ± 16 U/kg; T7: 142 ± 12 U/kg (p < 0.05) | |||||
| 78 HD patients | No treatment | → Hb T0: 7.5 ± 1.4 g/dL; T7: 9.2 ± 1.2 g/dL (NS) | |||
| → ERI T0: 185 ± 15 U/kg; T7: 160 ± 12 U/kg (NS) | |||||
| [82] | Two-way, parallel, double-blind | 18 HD patients | 15 mg/kg per Dx, IV | 6 mo | ↑ Ht T0: 24.2% ± 2.2%; T6: 32.5% ± 3.7% (p = 0.001) |
| ↑ Hb T0: 7.9 ± 0.8 g/dL; T6: 10.3 ± 1.1 g/dL (p = 0.001) | |||||
| 13 HD patients | Placebo, IV | → Ht T0: 27.5% ± 4.5%; T6: 30.2% ± 4.0% (p = 0.1) | |||
| → Hb T0: 8.0 ± 0.4 g/dL; T6: 8.7 ± 2.5 g/dL (p = 0.4) | |||||
| [83] | Two-way, parallel, single-blind | 10 HD patients | 20 mg/kg per Dx, IV | 2 mo | ↑ Hb +0.89 ± 0.56 g/dL vs. −0.47 ± 0.77 g/dL (p = 0.001) |
| 10 HD patients | Plaxevo, IV | ||||
| [84] | Double-blind, crossover, placebo-controlled | 16 HD patients | 20 mg/kg per Dx, IV | 3 mo | → ESA doses T0: 8562 ± 6762 U; T3: 8750 ± 7094 U (NS) |
| Placebo, IV | → Hb T0: 11.3 ± 1.9 g/dL T3: 11.5 ± 1.5 g/dL (NS) | ||||
| [85] | Two-way, parallel, open-label | 20 HD patients | 1 g per Dx, twice a week, IV | 6 mo | ↑ Hb T0: 6.8 ± 1.0 g/dL; T6: 7.7 ± 1.1 g/dL (p < 0.001) |
| ↓ ERI values not reported (p < 0.001) | |||||
| 20 HD patients | No treatment | → Hb T0: 6.7 ± 1.0 g/dL; T6: 6.9 ± 1.0 g/dL (NS), → ERI (NS) | |||
| [86] | Two-way, parallel, open-label | 20 HD patients | 1 g/Dx, IV | 3 mo | ↑ Hb T0: 7.8 ± 1.3 g/dL; T3: 9.9 ± 1.9 g/dL (p < 0.05) |
| 20 HD patients | No treatment | → Hb T0: 7.8 ± 1.1 g/dL; T12: 8.5 ± 1.2 g/dL (NS) | |||
| [87] | One-way, open-label | 62 HD patients | 600 mg/day, PO for 12 mo, then 1 g/Dx IV for 12 mo | 24 mo | ↑ Hb T0: 10.2 ± 1.2 g/dL; T12: 10.9 ± 0.9 g/dL |
| 18 PD patients | 600 mg/day, PO | 12 mo | → Hb T0: 10.6 ± 1.1 g/dL; T12: 10.6 ± 1.3 g/dL | ||
| [88] | Two-way, parallel, double-blind | 24 HD patients | 1 g/day, PO | 4 mo | → Hb T0: 10.5 ± 2.5 g/dL; T4: 11.3 ± 2.1 g/dL (NS) |
| ↓ ESA doses T0: 7250 ± 5202 U/week; T4: 2500 ± 4180 U/week (p < 0.001) | |||||
| 27 HD patients | Placebo, PO | → Hb T0: 9.5 ± 2.2 g/dL; T4: 9.9 ± 2.5 g/dL (NS) | |||
| ↓ ESA doses T0: 8000 ± 3186 U/week; T4: 6000 ± 5083 U/week (p = 0.033) | |||||
| [89] | Two-way, parallel, open-label | 25 HD patients | 1 g/Dx, IV and 1 g/non-Dx, PO | 36 mo | ↓ ESA doses T0: 5976 ± 1732 U/week; T36: 3391 ± 659 U/week (p < 0.001) |
| 35 HD patients | No treatment | → ESA doses T0: 6100 ± 1587 U/week; T36: 5519 ± 1360 U/week (NS) | |||
| [90] | Two-way, parallel, double-blind | 13 HD patients | 20 mg/kg per Dx, IV | 4 mo | → ESA doses T4: −769 ± 1739 U/week (NS), → Hb T4: −0.08 ± 0.90 g/dL (NS) |
| 13 HD patients | Placebo, PIV | → ESA doses T4: +153 ± 177 U/week (NS), → Hb T4: −0.26 ± 0.56 g/dL (NS) | |||
| [91] | Two-way, parallel, open-label | 23 HD patients | 15 mg/kg per Dx, IV | 6 mo | → ESA doses, → Ht (NS) |
| 22 HD patients | No treatment | ||||
| [92] | Two-way, parallel, double-blind | 13 HD patients | 1 g/Dx, IV | 6 mo | ↓ ERI T0: 102 ± 53 U/kg/week; T6: 63 ± 38 U/kg/week (p < 0.02) |
| 11 HD patients | Placebo, IV | → ERI T0: 79 ± 32 U/kg/week; T6: 80 ± 47 U/kg/week (NS) | |||
| [70] | Two-way, parallel, double-blind | 10 HD patients | 1 g/Dx, IV | 6 mo | ↓ ERI T0: 135 ± 79; T6: 118 ± 108 U/kg per week per %Ht (p < 0.05) |
| 11 HD patients | Placebo, IV | ↑ ERI T0: 136 ± 66; T6: 217 ± 204 U/kg per week per %Ht (p < 0.05) | |||
| [69] | Two-way, parallel, double-blind | 20 HD patients | 5 mg/kg or 25 mg/kg per Dx, IV | 4 mo | ↓ ERI T0: 16.0 ± 11.0; T4: 13.6 ± 10.5 U/kg per week per gHb (p < 0.02) |
| 20 HD patients | Placebo, IV | Values not reported | |||
| [96] | Two-way, parallel, double-blind | 13 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↓ ERI -1.62 ± 0.91 vs. +1.33 ± 0.79 U/kg per gHb (p < 0.05) |
| 14 HD patients | Placebo, IV | ||||
| [93] | Two-way, parallel, open-label | 30 HD patients | 1 g/Dx, IV | 12 mo | ↓ ERI T0: 10.7 ± 7.3; T12: 6.4 ± 3.8 U/kg per gHb per week (p < 0.0001) |
| 30 HD patients | No treatment | → ERI T0: 10.0 ± 7.9; T12: 9.6 ± 6.5 U/kg per gHb per week (NS) | |||
| [94] | Two-way, parallel, double-blind | 46 HD patients | 1 g/Dx, IV | 12 mo | → ERI T0: 20.6 ± 12.8; T12: 15.6 ± 15.9 IU/kg per gHb (p = 0.10) |
| 46 HD patients | Placebo, IV | → ERI T0: 15.8 ± 11.3; T12: 9.5 ± 5.8 IU/kg per gHb (p = 0.10) |
| Ref | Study Design | Population | Dose and Route | Treatment Duration | Findings a |
|---|---|---|---|---|---|
| [101] | Two-way, crossover, double-blind | 9 HD patients | 990 mg/day PO then placebo for 2 mo each | 2 mo | ↓ Hypotension (p < 0.001) |
| 9 HD patients | Placebo then 990 mg/day PO for 2 mo each | → Hypotension (NS) | |||
| [50] | Two-way, parallel, double-blind | 14 HD patients | 2 g/Dx, IV | 6 weeks | No difference in cardiac function (NS) |
| 14 HD patients | Placebo | ||||
| [49] | Two-way, parallel, double-blind | 38 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↓ Hypotension (p < 0.02) |
| 44 HD patients | Placebo | → Hypotension (NS) | |||
| [102] | One-way, open-label | 13 HD patients | 1 g/Dx, IV | 3 mo | ↑ LVEF T0: 42.4 ± 19.4%; T3: 48.6 ± 17.6% (p < 0.05) |
| [89] | Two-way, parallel, open-label | 25 HD patients | 1 g/Dx, IV and 1 g/non-Dx PO | 36 mo | ↑ LVEF (p < 0.05) |
| 35 HD patients | No treatment | ↓ LV end-diastolic volume (p < 0.05) | |||
| [103] | One-way, open-label | 11 HD patients | 1 g/day PO then 0.5 g/day PO for 1 mo each | 2 mo | → LVEDD, LVFS (NS) |
| ↑ Cardiac scintigraphy (p < 0.001) | |||||
| [104] | One-way, open-label | 9 HD patients (impaired LVEF) | 500 mg/day, PO | 6 mo | ↑ LVEF T0: 44.9% ± 12.2%; T6: 53.8% ± 13.8% (p = 0.005) |
| ↓ CTR T0: 56.4 ± 5.4; T6: 53.8 ± 4.0 (p = 0.042) | |||||
| [100] | One-way, open-label | 11 HD patients (impaired LVEF) | 1 g/Dx, IV | 8 mo | ↑ LVEF T0: 32.0% T8: 41.8% (p < 0.05) |
| [105] | Two-way, parallel, open-label | 10 HD patients | 10 mg/kg/day, PO | 12 mo | ↓ LVMI T0: 151.8 ± 21.2; T12: 134 ± 16 g/m2 (p < 0.01) |
| 10 HD patients | No treatment | → LVMI T0: 153.3 ± 28.2; T12: 167.1 ± 43.1 g/m2 (NS) | |||
| [106] | Two-way, parallel, double-blind | 20 HD patients | 1500 mg/day, PO | 6 mo | No difference in cardiac function (p = 0.67) |
| 35 HD patients | No treatment | Cardiac function was not investigated. | |||
| [107] | Two-way, parallel, double-blind | 10 HD patients | 900 mg/day, PO | 3 mo | ↑ LVEF T0: 61.8% ± 16.0% T3: 64.4% ± 13.8% (p < 0.05) |
| ↓ Hypotension T0: 4.0 ± 1.7; T3: 1.3 ± 0.9 times/mo (p < 0.05) | |||||
| 8 HD patients | Placebo | → LVEF (NS) | |||
| [108] | Two-way, parallel, open-label | 75 HD patients | 20 mg/kg/day, PO | 12 mo | ↑ LVEF T0: 53.1% ± 5.3% T12: 58.6% ± 5.5% (p < 0.001) |
| ↓ LVMI T0: 112 ± 26; T12: 107 ± 24 g/m2 (p < 0.001) | |||||
| 73 HD patients | No treatment | → LVEF, LVMI (NS) | |||
| [109] | Two-way, parallel, double-blind | 18 HD patients | 30 mg/kg/before Dx, IV | 3 mo | ↓ Hypotension 9.3% vs. 33.1% (p < 0.0001) |
| 15 HD patients | Placebo, IV |
| Ref | Study Design | Subjects | Dose and Route | Treatment Duration | Findings a |
|---|---|---|---|---|---|
| [101] | Double-blind, cross-over, placebo-controlled | 18 HD patients | 990 mg/day, PO Placebo, PO | 2 mo | ↓ Cramps (p < 0.001), ↓ Asthenia (p < 0.001), ↓ Dyspnea (p < 0.001) |
| [140] | Double-blind, cross-over, placebo-controlled | 14 HD patients | 2 g/day, PO Placebo, PO | 2 mo | ↑ Exercise time (p = 0.01), ↓ Asthenia (p = 0.01), ↓ Muscle cramps (p = 0.01) |
| [50] | Two-way, parallel, double-blindl | 14 HD patients | 2 g/Dx, IV | 1.5 mo | No difference in muscular status (NS) |
| 14 HD patients | Placebo, IV | ||||
| [144] | One-way, open-label | 6 HD patients | 2 g/day, PO | 1.5 mo | No difference in muscular function (NS) |
| [49] | Two-way, parallel, double-blind | 38 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↓ Cramps (p = 0.02), ↓ Asthenia postdialysis (p = 0.04), ↑ O2 consumption (p = 0.03) |
| 44 HD patients | Placebo, IV | ||||
| [145] | One-way, open-label | 26 HD patients | 2 g/dialysate (n = 11), 2 g/day PO (n = 6), 2 g/Dx IV (n = 9) | 6 mo | ↓ Cramps (p = 0.04), ↓ Pain (p = 0.04), ↑ Isometric force (p = 0.001) |
| [146] | One-way, open-label | 6 HD patients | 2 g/day, PO | 2 mo | ↓ Cramps (p = 0.01), ↓ Weakness (p = 0.001), ↓ Fatigue (p = 0.05) |
| [139] | Two-way, parallel, open-label | 30 HD patients | 500 mg/day, PO | 3 mo | ↓ Weakness (p < 0.005), ↓ Fatigue (p < 0.005), ↓ Cramps/aches (p < 0.05) |
| 21 HD patients | No treatment | ||||
| [147] | Two-way, parallel, double-blind | 9 HD patients | 10 mg/kg per Dx, IV | 4 mo | No difference in muscle cramps, uremic pruritus, physical strength, and general well-being |
| 8 HD patients | Placebo, IV | ||||
| [143] | Two-way, parallel, double-blind | 101 HD patients | 1 g/day, PO | 6 mo | 1.5 mo, ↑ QOL (p = 0.02); 3 mo, ↑ QOL (p = 0.015); >4.5 mo, ↓ QOL (p = 0.013) |
| Placebo, PO | |||||
| [141] | Two-way, parallel, double-blind | 7 HD patients | 2 g/Dx, IV for 6 mo, then 1 g/Dx, IV for 10 mo | 16 mo | → Daily activity score T0: 3.5; T6: 2.0 (NS) |
| 7 HD patients | No treatment for 6 mo, then 1 g/Dx, IV for 10 mo | → Daily activity score T0: 3.4; T6: 3.1 (NS) | |||
| [84] | Double-blind, cross-over, placebo-controlled | 16 HD patients | 20 mg/kg per Dx, IV | 3 mo | No changes in muscle parameters and QOL scores |
| Placebo, IV | |||||
| [96] | Two-way, parallel, double-blind | 13 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↑ SF-36 scores T0: 33.9 ± 1.9; T6: 43.2 ± 3.0 (p < 0.05) |
| 14 HD patients | Placebo, IV | → SF-36 scores T0: 40.6 ± 2.6; T6: 40.1 ± 3.0 (NS) | |||
| [83] | Two-way, parallel, single-blind | 10 HD patients | 20 mg/kg per Dx, IV | 2 mo | ↑ SF-36 scores T2: +18.3 ± 12.7 vs. −6.4 ± 16.4 (p = 0.001) |
| 10 HD patients | Placebo, IV | ||||
| [142] | Two-way, parallel, open-label | 42 HD patients | 1 g/Dx, IV | 12 mo | ↑ AMA: +2.11% vs. −4.11% (p < 0.01); ↑ LBM 0.70% vs. −2.22% (p < 0.001); ↑ HGS: +1.58% vs. −2.69% (p < 0.05) |
| 42 HD patients | No treatment | ||||
| [87] | One-way, open-label | 62 HD patients | 600 mg/day, PO for 12 mo, then 1 g/Dx IV for 12 mo | 24 mo | ↓ Muscle spasms in patients who had undergone HD for >4 years (p-value not reported) |
| 18 PD patients | 600 mg/day, PO | 12 mo |
| Ref | Study Design | Subjects | Dose and Route | Treatment Duration | Findings a |
|---|---|---|---|---|---|
| [163] | Two-way, parallel, open-label | 8 HD patients | 0.5 g/Dx IV for 2 mo, then 1.0 g/Dx IV for 1.5 mo | 3.5 mo | ↓ TG T0: 336 ± 56 mg/dL; T3.5: 244 ± 82 mg/dL (p < 0.05) |
| 8 HD patients | Placebo, IV | 3.5 mo | → TG T0: 329 ± 72 mg/dL; T3.5: 444 ± 82 mg/dL (NS) | ||
| [164] | Two-way, parallel, open-label | 11 HD patients | 1 g/Dx, IV for 1 mo then 2 g/Dx dialysate for 3 mo | 4 mo | ↓ TG, ↑ HDL (p-values not reported) |
| 11 HD patients | 1 g/Dx, IV for 1 mo then 4 g/Dx dialysate for 3 mo | ||||
| [165] | Two-way, crossover, double-blind | 9 HD patients | 1 g t.i.d. PO then placebo for 5 wk each | 5 wk | No difference in plasma lipid levels (NS) |
| 9 HD patients | Placebo then 1 g t.i.d. PO for 5 wk each | 5 wk | |||
| [48] | Two-way, parallel, double-blind | 38 HD patients | 20 mg/kg per Dx, IV | 6 mo | No difference in plasma lipid levels (NS) |
| 44 HD patients | Placebo, IV | 6 mo | |||
| [166] | Two-way, parallel, double-blind | 15 HD patients | 1–1.5 g/Dx, IV | 2 mo | No difference in plasma lipid levels (NS) |
| 15 HD patients | Placebo | 2 mo | |||
| [167] | Two-way, parallel, double-blind | 11 HD patients | 100 μmol/L dialysate | 6 mo | No difference in plasma lipid levels (NS) |
| 10 HD patients | Placebo | 6 mo | |||
| [168] | Two-way, parallel, open-label | 6 HD patients | 900 mg t.i.d. PO | 1 mo | ↑ TG T0: 180 ± 66 mg%; T1: 219 ± 88 mg% (p < 0.05) |
| 4 HD patients | Placebo | 1 mo | → TG T0: 222 ± 35 mg%; T1: 222 ± 35 mg% (NS) | ||
| [131] | Two-way, parallel, open-label | 21 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↓ TG T0: 1.6 ± 0.6; T6: 1.5 ±0.7 mmol/L (p = 0.001), ↑ TP T0: 6.4 ± 0.5; T6: 6.9 ± 0.5 g/dL (p < 0.001), ↑ Alb T0: 3.6 ± 0.3; T6: 4.1 ± 0.3 g/dL (p < 0.001), ↑ Tf T0: 1.2 ± 0.2; T6: 1.6 ± 0.4 g/L (p < 0.001), ↑ BMI T0: 23.4 ± 4.0; T6: 23.7 ± 4.0 (p < 0.001) |
| 21 HD patients | No treatment | → TG, TP, Alb, Tf, BMI (NS) | |||
| [132] | Two-way, parallel, double-blind | 20 HD patients | 1 g/Dx, IV | 6 mo | ↓ CRP: T0: 2.1 ± 0.6 mg/dL; T6: 0.67 ± 0.1 mg/dL (p = 0.02), → TC, HDL, LDL, TG (NS) |
| 15 HD patients | No treatment | → CRP, TC, HDL, LDL, TG (NS) | |||
| [88] | Two-way, parallel, double-blind | 24 HD patients | 1 g/day, PO | 4 mo | ↓ TG T0: 166 ± 71 mg/dL; T4: 138 ± 54 mg/dL (p = 0.001) |
| ↑ HDL T0: 30 ± 7 mg/dL; T4: 34 ± 7 mg/dL (p < 0.001) | |||||
| 27 HD patients | Placebo, PO | ↑ TG T0: 142 ± 58 mg/dL; T4: 151 ± 48 mg/dL (p = 0.029) | |||
| → HDL | |||||
| [92] | Two-way, parallel, double-blind | 13 HD patients | 1 g/Dx, IV | 6 mo | → TC, HDL, TG (NS) |
| 11 HD patients | Placebo, IV | → TC, HDL, TG (NS) | |||
| [90] | Two-way, parallel, double-blind | 13 HD patients | 20 mg/kg per Dx, IV | 4 mo | → TC, TG (NS) |
| 13 HD patients | Placebo, PIV | → TC, TG (NS) | |||
| [169] | Two-way, parallel, double-blind | 32 HD patients | 600 mg/Dx, IV | 12 mo | ↓ MDA T0: 2.2 ± 0.7 μmol/mL; T3: 1.5 ± 0.7 μmol/mL (p < 0.001) |
| ↑ ABI T0: 0.71 ± 0.06; T3: 0.78± 0.08 (p < 0.001) | |||||
| 32 HD patients | Placebo, IV | ↑ MDA T0: 1.94 ± 0.5 μmol/mL; T3: 1.9 ± 0.7 μmol/mL (p < 0.01) | |||
| ↓ ABI T0: 0.75 ± 0.08; T3: 0.72 ± 0.01 (p < 0.001) | |||||
| [85] | Two-way, parallel, open-label | 20 HD patients | 1 g/Dx, twice a week, IV | 6 mo | ↓ TC (p < 0.001),↑ HDL (p < 0.001), ↓ TG (p < 0.001) |
| 20 HD patients | No treatment | ↑ TC (p < 0.001), ↓ HDL(p < 0.01), → TG (NS) | |||
| [86] | Two-way, parallel, open-label | 20 HD patients | 1 g/Dx, IV | 3 mo | ↓ TG T0: 190 ± 69 mg/dL; T3: 179 ± 51 mg/dL (p < 0.05) |
| ↓ LDL 119± 21 mg/dL; T3: 98 ± 19 mg/dL (p < 0.05) | |||||
| ↓ CRP T0: 20.8 ± 1.7 μM; T3: 16.5± 1.3 μM (p < 0.05) | |||||
| 20 HD patients | No treatment | → TG, LDL, CRP (NS) | |||
| [161] | One-way, open-label | 50 HD patients | 1 g/Dx, IV | 12 mo | ↑ LDL (p = 0.005), ↓ HDL (p = 0.001), → TG (NS) |
| [133] | Two-way, parallel, open-label | 18 HD patients | 1 g/day, PO | 3 mo | ↓ CRP T3: −1.6 ± 2.3 mg/L (p < 0.05), ↓ IL-6 T3: −5.5 ± 3.6 ng/L (p < 0.001), ↓ IL-1β T3: −0.6 ± 0.6 ng/L (p < 0.001) |
| 18 HD patients | No treatment | → CRP, IL-6, IL-1β (NS) | |||
| [170] | Two-way, parallel, double-blind | 18 HD patients | 1 g/day, PO | 3 mo | ↓ CRP T0: 7.5 ± 5.5 mg/L; T3: 4.4 ± 3.3 mg/L (p < 0.05) |
| 18 HD patients | Placebo, PO | → CRP T0: 6.5 ± 5 mg/L; T3: 6.3 ± 3.1 mg/L (NS) | |||
| [171] | Two-way, parallel, double-blind | 18 HD patients | 1 g/day, PO | 3 mo | ↓ SAA T3: −32% (p < 0.001) |
| 18 HD patients | Placebo, PO | → SAA (NS) | |||
| [172] | Two-way, parallel, open-label | 17 HD patients | 1 g/day, PO | 3 mo | → BMI, Leptin, Adiponectin (NS) |
| 25 HD patients | No treatment | → BMI, Leptin, Adiponectin (NS) | |||
| [173] | Two-way, parallel, open-label | 20 HD patients | 1 g/day, PO | 2 mo | → Alb T0: 3.37 ± 0.40 g/dL; T2: 3.38 ± 0.43 g/dL (NS) |
| 20 HD patients | No treatment | → Alb T0: 3.35 ± 0.34 g/dL; T2: 3.40 ± 0.38 g/dL (NS) | |||
| [80] | Two-way, parallel, double-blind | 48 HD patients | 20 mg/kg per Dx, IV | 6 mo | ↓ CRP T0: 1.8 ± 1.2 mg/dL; T6: 1.2 ± 0.2 (p < 0.002), ↑ Alb T0: 3.6 ± 0.3 g/dL; T6: 3.9 ± 0.4 g/dL (p < 0.0001), ↑ BMI T0: 20.5 ± 0.1; T6: 21.2 ± 0.5 (p < 0.0001) |
| 65 HD patients | Placebo, IV | → CRP (NS), ↓ Alb (p < 0.0001), ↓ BMI (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219. https://doi.org/10.3390/nu13041219
Takashima H, Maruyama T, Abe M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients. 2021; 13(4):1219. https://doi.org/10.3390/nu13041219
Chicago/Turabian StyleTakashima, Hiroyuki, Takashi Maruyama, and Masanori Abe. 2021. "Significance of Levocarnitine Treatment in Dialysis Patients" Nutrients 13, no. 4: 1219. https://doi.org/10.3390/nu13041219
APA StyleTakashima, H., Maruyama, T., & Abe, M. (2021). Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients, 13(4), 1219. https://doi.org/10.3390/nu13041219






