Feasibility, Acceptability, and Preliminary Validity of Self-Report Dietary Assessment in Adults with Multiple Sclerosis: Comparison with Doubly Labeled Water Measured Total Energy Expenditure
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Biologically Measured Total Energy Expenditure
2.3.2. Self-Reported Energy Intake
2.3.3. Acceptability Questionnaire
2.3.4. Demographic and Clinical Characteristics
2.4. Data Analyses
3. Results
3.1. Participants
3.2. DLW Protocol Fidelity
3.3. ASA24 Feasibility
3.4. Acceptability
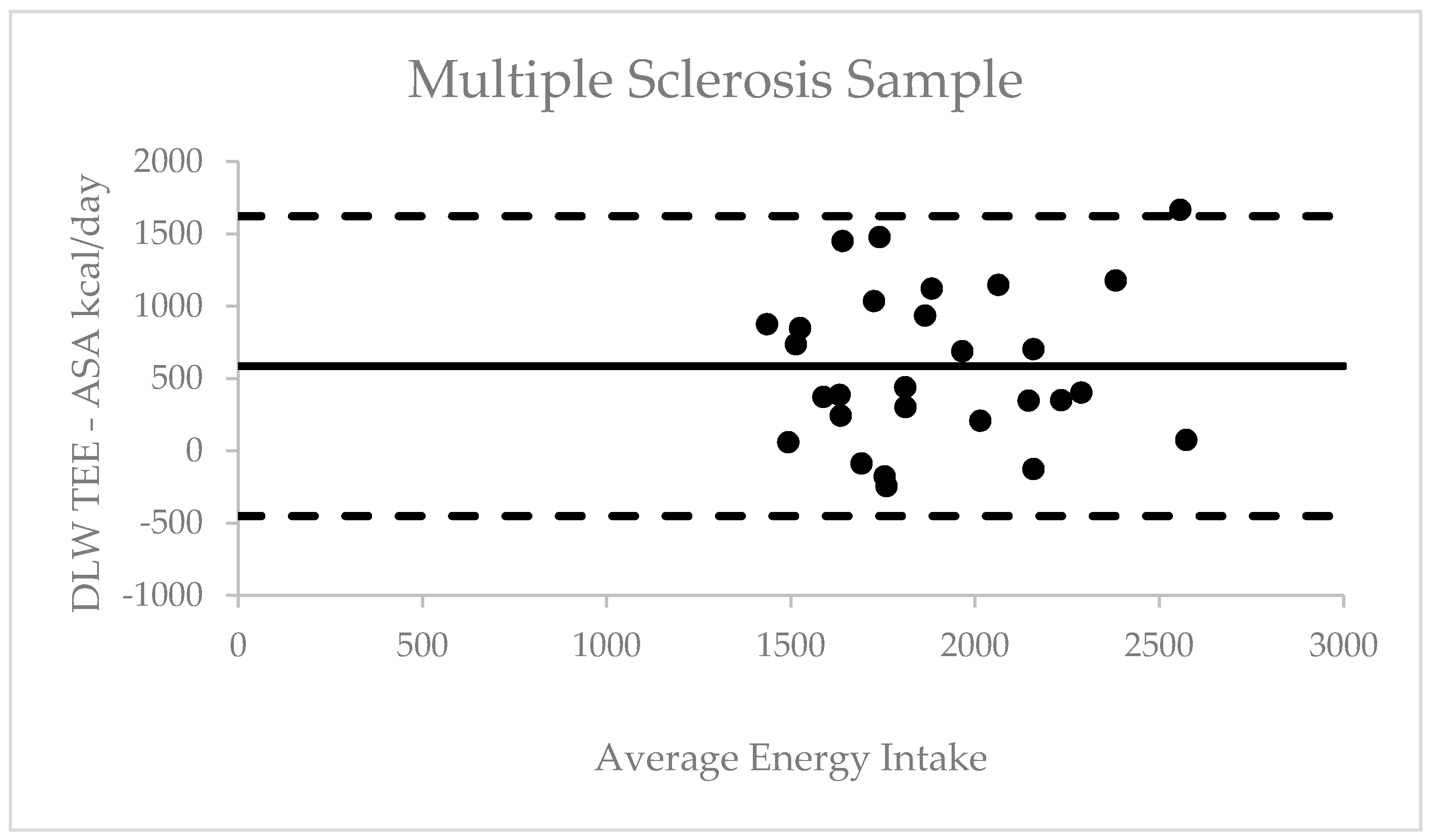
3.5. Validity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. J. 2018, 24, 96–120. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural History of Multiple Sclerosis Symptoms. Int. J. MS Care 2013, 15, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.; Bhargava, P.; Kalb, R. Your Patients with Multiple Sclerosis Have Set Wellness as a High Priority—And the National Multiple Sclerosis Society is Responding. US Neurol. 2015, 11, 80. [Google Scholar] [CrossRef]
- Motl, R.W.; Mowry, E.M.; Ehde, D.M.; LaRocca, N.G.; E Smith, K.; Costello, K.; Shinto, L.; Ng, A.V.; Sullivan, A.B.; Giesser, B.; et al. Wellness and multiple sclerosis: The National MS Society establishes a Wellness Research Working Group and research priorities. Mult. Scler. J. 2018, 24, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Sand, I.K. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Piccio, L.; Stark, J.L.; Cross, A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008, 84, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Bitarafan, S.; Harirchian, M.H.; Nafissi, S.; Sahraian, M.; Togha, M.; Siassi, F.; Saedisomeolia, A.; Alipour, E.; Mohammadpour, N.; Chamary, M.; et al. Dietary intake of nutrients and its correlation with fatigue in multiple sclero-sis patients. Iran. J. Neurol. 2014, 13, 28–32. [Google Scholar]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef]
- Hadgkiss, E.J.; A Jelinek, G.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; Van Der Meer, D.M. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr. Neurosci. 2014, 18, 125–136. [Google Scholar] [CrossRef]
- Rotstein, D.L.; Cortese, M.; Fung, T.T.; Chitnis, T.; Ascherio, A.; Munger, K.L. Diet quality and risk of multiple sclerosis in two cohorts of US women. Mult. Scler. J. 2018, 25, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Subar, A.F. Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease; Elsevier: London, UK, 2017; pp. 5–48. [Google Scholar]
- Irish, A.K.; Erickson, C.M.; Wahls, T.L.; Snetselaar, L.G.; Darling, W.G. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: A pilot study. Degener Neurol. Neuromuscul. Dis. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Masullo, L.; Papas, M.A.; Cotugna, N.; Baker, S.; Mahoney, L.; Trabulsi, J. Complementary and Alternative Medicine Use and Nutrient Intake Among Individuals with Multiple Sclerosis in the United States. J. Community Health 2014, 40, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Krekels, M.; Grevendonk, L.; Eijnde, B.O. Multiple Sclerosis Affects Skeletal Muscle Characteristics. PLoS ONE 2014, 9, e108158. [Google Scholar] [CrossRef]
- Goran, M.I. Variation in Total Energy Expenditure in Humans. Obes. Res. 1995, 3, 59–66. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J.; Chen, K.Y.; Leibel, R.L.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Ravussin, E. Methodologic considerations for measuring energy expenditure differences between di-ets varying in carbohydrate using the doubly labeled water method. Am. J. Clin. Nutr. 2019, 109, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Coward, W.A. The doubly-labelled-water (2H218O) method: Principles and practice. Proc. Nutr. Soc. 1988, 47, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Yamada, Y.; Sagayama, H.; Berman, E.S.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; et al. A standard calculation methodology for human doubly labeled water studies. Cell Rep. Med. 2021, 2, 100203. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, E.F.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The automated self-administered 24-hour dietary recall (ASA24): A resource for researchers, clinicians, and educators from the national cancer institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Cheng, F.W.; Still, C.D.; Jensen, G.L. A Validation of Automated Self-Administered 24-Hour Dietary Recalls (ASA24) Relative to Interviewer-Administered Recalls using the Nutrition Data System for Research (NDSR). FASEB J. 2016, 30, 43.3. [Google Scholar]
- Mishra, S.; Barnard, N.D.; Gonzales, J.; Xu, J.; Agarwal, U.; Levin, S. Nutrient intake in the GEICO multicenter trial: The ef-fects of a multicomponent worksite intervention. Eur. J. Clin. Nutr. 2013, 67, 1066–1071. [Google Scholar] [CrossRef]
- Silveira, S.L.; Winter, L.L.; Clark, R.; Ledoux, T.; Robinson-Whelen, S. Baseline Dietary Intake of Individuals with Spinal Cord Injury Who Are Overweight or Obese. J. Acad. Nutr. Diet. 2019, 119, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.C.; Motl, R.W.; Sandroff, B.M.; Pula, J.H.; Cadavid, D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.L.; Ho, Y.Y.; Rollo, M.E.; Collins, C.E. Validity of Dietary Assessment Methods When Compared to the Method of Doubly Labeled Water: A Systematic Review in Adults. Front. Endocrinol. 2019, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Kroke, A.; Klipstein-Grobusch, K.; Voss, S.; Möseneder, J.; Thielecke, F.; Noack, R.; Boeing, H. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am. J. Clin. Nutr. 1999, 70, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bagert, B.; Camplair, P.; Bourdette, D. Cognitive Dysfunction in Multiple Sclerosis. CNS Drugs 2002, 16, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.M.; Grafman, J.; DiGiulio, D.; Mittenberg, W.; Bernardin, L.; Leo, G.J.; Luchetta, T.; Unverzagt, F. Memory dysfunction in multiple sclerosis: Its relation to working memory, semantic encoding, and implicit learning. Neuropsychology 1993, 7, 364. [Google Scholar] [CrossRef]

| Variable | Healthy Control (HC) Sample (n = 15) | Multiple Sclerosis (MS) Sample (n = 30) | p Value |
|---|---|---|---|
| Age, years ± SD | 34.53 ± 8.77 | 40.47 ± 9.64 | 0.05 |
| Sex, n (%) | 0.77 | ||
| Female | 13(86) | 25(83) | |
| Male | 2(13) | 5(17) | |
| Marital Status, n (%) | 0.39 | ||
| Married | 5(33) | 14(47) | |
| Single | 8(53) | 11(37) | |
| Divorced/Separated | 2(13) | 4(13) | |
| Widower | 0(0) | 1(3) | |
| Employment, n (%) | 0.22 | ||
| Yes | 13(86) | 21(70) | |
| No | 2(13) | 9(30) | |
| Race, n (%) | 0.06 | ||
| Caucasian | 11(73) | 13(43) | |
| African American | 4(27) | 16(53) | |
| Other | 0(0) | 1(3) | |
| Education, n (%) | 0.50 | ||
| Less than college degree | 6(40) | 9(30) | |
| College degree or more | 9(60) | 21(70) | |
| MS Duration, years ± SD | N/A | 10.00 ± 6.24 | N/A |
| Type MS, n (%) | N/A | N/A | |
| Relapsing Remitting | 28(93) | ||
| Progressive | 2(7) | ||
| Patient Determined Disease Steps, Median (IQR) | N/A | 0.5(2.0) | N/A |
| Question Response Options | Healthy Control (HC) Sample n(%) | Multiple Sclerosis (MS) Sample n(%) | Chi-Square X2 Value | p Value |
|---|---|---|---|---|
| Baseline Appointment Questions | ||||
| How difficult was it to remember everything you ate yesterday? | 5.41 | 0.14 | ||
| Not at all difficult | 11(73) | 12(40) | ||
| A little difficult | 4(27) | 13(43) | ||
| Moderately difficult | ˗ | 3(10) | ||
| Very difficult | ˗ | 2(7) | ||
| How did you feel using the ASA24 online system? | 2.23 | 0.14 | ||
| Very comfortable | 11(73) | 15(50) | ||
| Comfortable | 4(27) | 15(50) | ||
| Uncomfortable | ˗ | ˗ | ||
| Very uncomfortable | ˗ | ˗ | ||
| 14-Day Appointment Questions | ||||
| How difficult was it to remember everything you ate on previous days? | 3.83 | 0.28 | ||
| Not at all difficult | 6(40) | 14(47) | ||
| A little difficult | 9(60) | 11(37) | ||
| Moderately difficult | ˗ | 3(10) | ||
| Very difficult | ˗ | 2(7) | ||
| How easy was it to access the ASA24 online system? | 1.28 | 0.73 | ||
| Not at all easy | ˗ | 2(7) | ||
| A little easy | 1(7) | 3(10) | ||
| Moderately easy | 2(13) | 3(10) | ||
| Very easy | 12(80) | 22(73) | ||
| How did you feel using the ASA24 online system? | 4.43 | 0.22 | ||
| Very comfortable | 8(53) | 16(53) | ||
| Comfortable | 4(27) | 11(37) | ||
| Uncomfortable | ˗ | 2(7) | ||
| Very uncomfortable | 3(20) | 1(3) | ||
| What was the HARDEST part of completing the ASA24 online dietary recall? | 0.2 | 0.98 | ||
| Remembering to complete the questionnaire | 2(13) | 5(17) | ||
| Remembering the food you ate | 9(60) | 16(53) | ||
| Using the website | 1(7) | 2(7) | ||
| Other | 3(20) | 7(23) | ||
| What was the EASIEST part of completing the ASA24 online dietary recall? | 0.83 | 0.66 | ||
| Remembering to complete the questionnaire | 6(40) | 8(27) | ||
| Remembering the food you ate | 2(13) | 5(17) | ||
| Using the website | 7(47) | 17(57) | ||
| How helpful did you find the e-mail/text reminders? | 0.53 | 0.77 | ||
| Not at all helpful | ˗ | ˗ | ||
| A little helpful | ˗ | ˗ | ||
| Moderately helpful | ˗ | 1(3) | ||
| Very helpful | 6(40) | 11(37) | ||
| Extremely helpful | 9(60) | 18(60) | ||
| How did you complete MOST of your ASA24 dietary recalls? | 0.87 | 0.65 | ||
| Smartphone | 9(60) | 16(53) | ||
| Tablet | 1(7) | 5(17) | ||
| Desktop computer | 5(33) | 9(30) | ||
| Did you need someone to help you complete the dietary recalls? | 1.61 | 0.21 | ||
| Yes | ˗ | 3(10) | ||
| No | 15(100) | 27(90) | ||
| Which method do you think is best to complete a 24 h dietary recall? | 2.81 | 0.25 | ||
| In-person with an interviewer | ˗ | 2(7) | ||
| Over the phone with an interviewer | ˗ | 3(10) | ||
| Online using ASA24 | 15(100) | 25(83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, S.L.; Jeng, B.; Gower, B.A.; Motl, R.W. Feasibility, Acceptability, and Preliminary Validity of Self-Report Dietary Assessment in Adults with Multiple Sclerosis: Comparison with Doubly Labeled Water Measured Total Energy Expenditure. Nutrients 2021, 13, 1198. https://doi.org/10.3390/nu13041198
Silveira SL, Jeng B, Gower BA, Motl RW. Feasibility, Acceptability, and Preliminary Validity of Self-Report Dietary Assessment in Adults with Multiple Sclerosis: Comparison with Doubly Labeled Water Measured Total Energy Expenditure. Nutrients. 2021; 13(4):1198. https://doi.org/10.3390/nu13041198
Chicago/Turabian StyleSilveira, Stephanie L., Brenda Jeng, Barbara A. Gower, and Robert W. Motl. 2021. "Feasibility, Acceptability, and Preliminary Validity of Self-Report Dietary Assessment in Adults with Multiple Sclerosis: Comparison with Doubly Labeled Water Measured Total Energy Expenditure" Nutrients 13, no. 4: 1198. https://doi.org/10.3390/nu13041198
APA StyleSilveira, S. L., Jeng, B., Gower, B. A., & Motl, R. W. (2021). Feasibility, Acceptability, and Preliminary Validity of Self-Report Dietary Assessment in Adults with Multiple Sclerosis: Comparison with Doubly Labeled Water Measured Total Energy Expenditure. Nutrients, 13(4), 1198. https://doi.org/10.3390/nu13041198







