Uptake of Vitamins D2, D3, D4, D5, D6, and D7 Solubilized in Mixed Micelles by Human Intestinal Cells, Caco-2, an Enhancing Effect of Lysophosphatidylcholine on the Cellular Uptake, and Estimation of Vitamins D’ Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Differentiation
2.3. Preparation of Mixed Micelles Containing Vitamin D
2.4. Evaluation of Vitamin D Uptake by Differentiated Caco-2 Cells from Mixed Micelles
2.5. Evaluation of Facilitated Diffusion on Vitamin D Uptake by Differentiated Caco-2 Cells from Mixed Micelles
2.6. Impact of Cell-Cell Adhesion/Cell-Matrix Adhesion in Caco-2 Cells on Vitamin D Uptake
2.7. Evaluation of Cellular Cholesterol in the Differentiated Caco-2 Cells Treated with the Mixed Micelles Containing Lysophosphatidylcholine
2.8. HPLC Analysis
2.9. Statistical Methods
2.10. Estimation of Biological Activity of Vitamin D by Online Software Simulation
3. Results
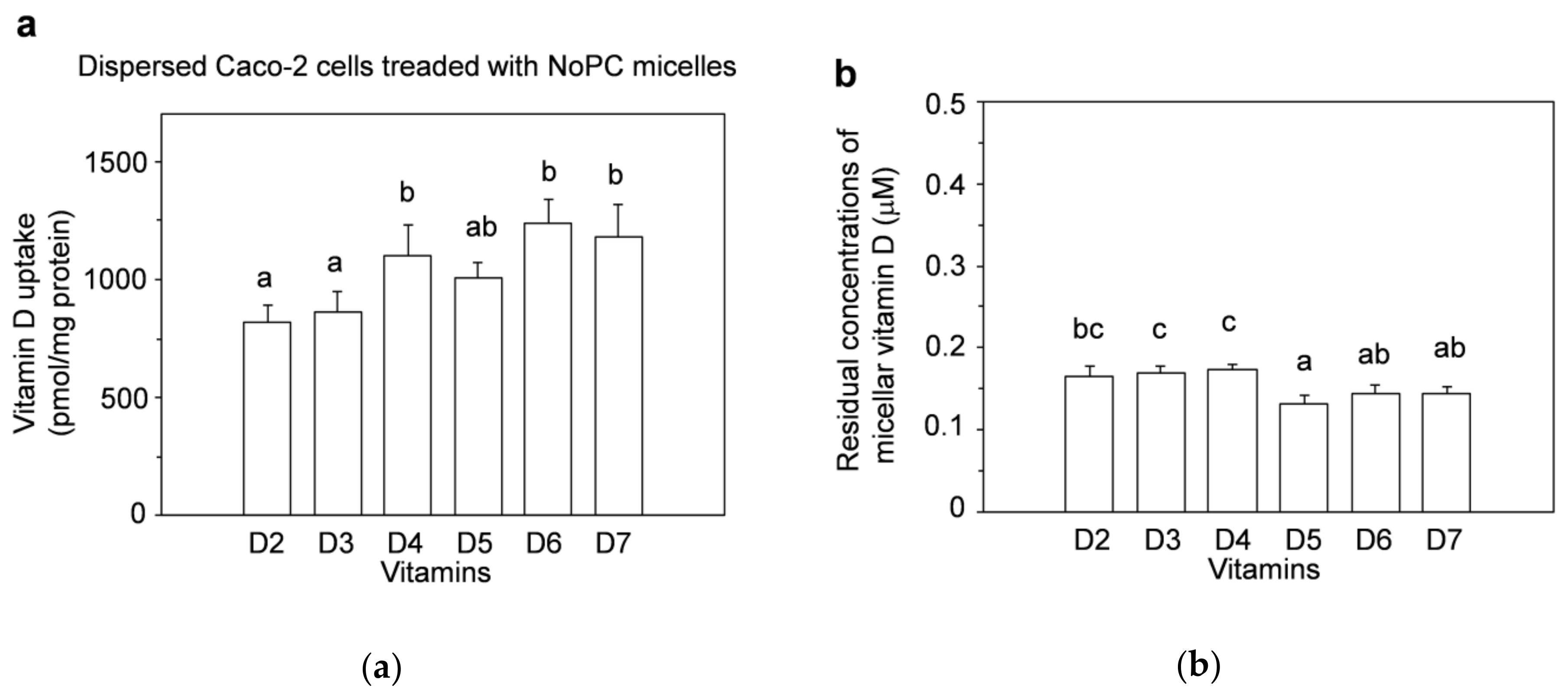
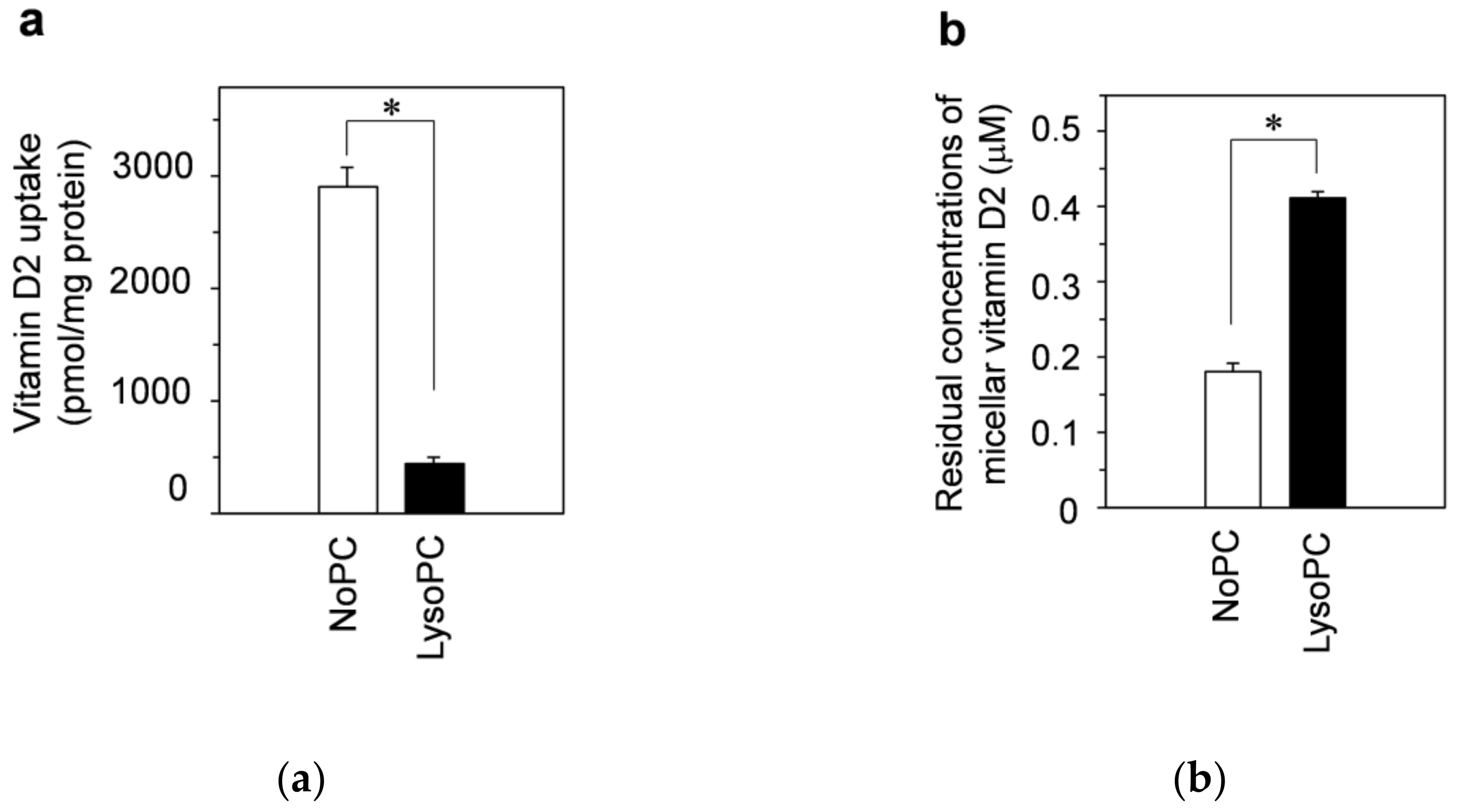
3.1. Vitamin D Uptake by Differentiated Caco-2 Cells and Effect of Lysophosphatidylcholine on Uptake
3.2. Possible Involvement of Facilitated Diffusion in Vitamin D Uptake
3.3. Impact of Cell-Cell Adhesion/Cell-Matrix Adhesion on Vitamin D Uptake by Caco-2 Cells
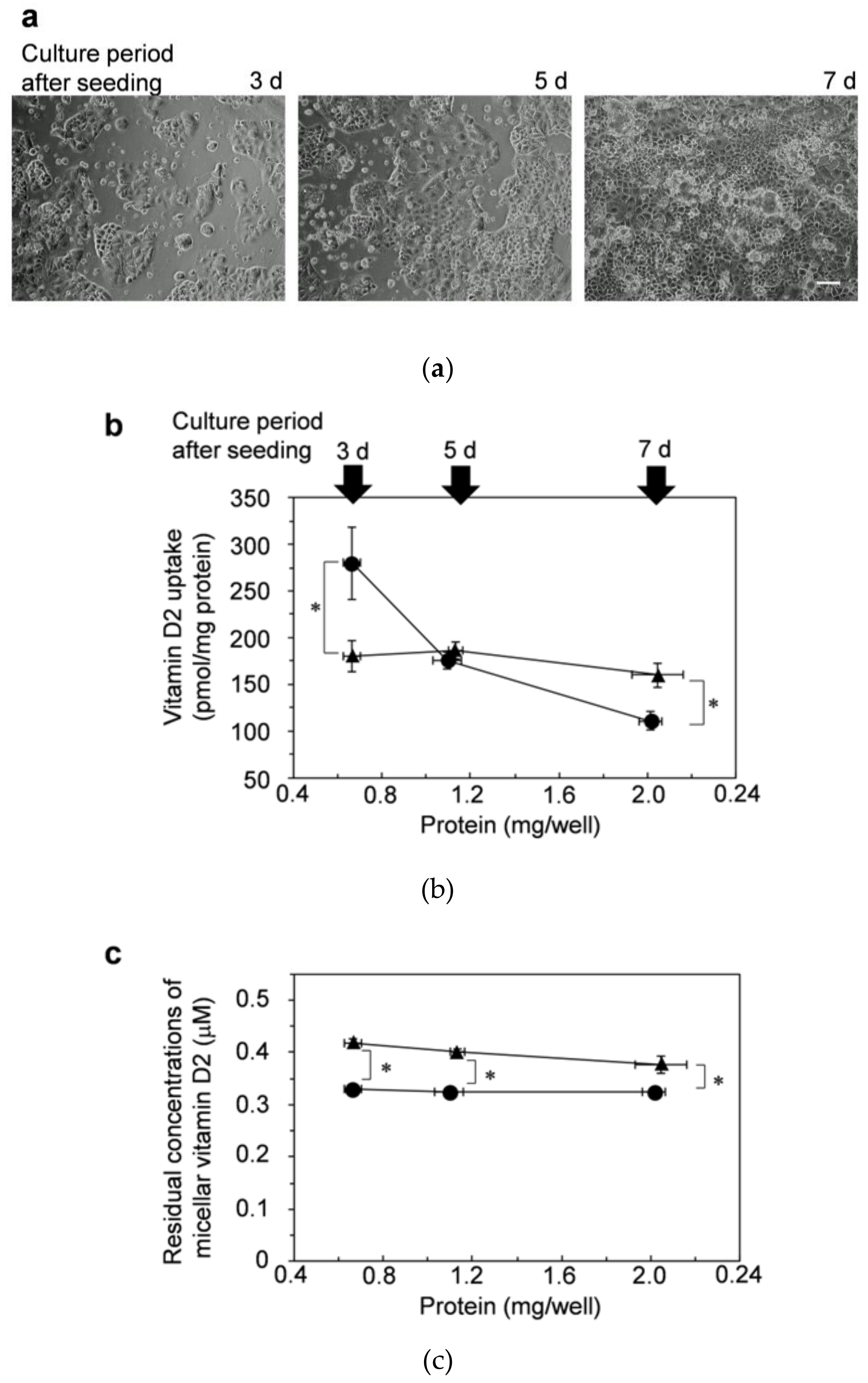
3.4. Cell-Cell Adhesion/Cell-Matrix Adhesion for Enhancing Effects of Micellar Lysophosphatidylcholine on Vitamin D Uptake: Examination in Dispersed Cells
3.5. Cell-Cell Adhesion/Cell-Matrix Adhesion for Enhancing Effects of Lysophosphatidylcholine on Vitamin D Uptake: Examination Using Adherent Caco-2 Cells with Insufficient Cell-Cell Adhesion
3.6. Effect of Lysophosphatidylcholine in Mixed Micelles on Cellular Cholesterol Amounts in Differentiated Caco-2 Cells
3.7. Estimation of Biological Activities of 1,25-Di(OH)-Vitamin D
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyauchi, M.; Hirai, C.; Nakajima, H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J. Nutr. Sci. Vitaminol. 2013, 59, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Godar, D.E.; Pope, S.J.; Grant, W.B.; Holick, M.F. Solar UV doses of young Americans and vitamin D3 production. Environ. Health Perspect. 2012, 120, 139–143. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Nordestgaard, B.G.; Bojesen, S.E. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: A prospective cohort study. J. Investig. Dermatol. 2013, 133, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Weon, K.Y.; Nam, D.Y.; Nam, J.H.; Kim, W.K. Skin protective effect of guava leaves against UV-induced melanogenesis via inhibition of ORAI1 channel and tyrosinase activity. Exp. Dermatol. 2016, 25, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Rijken, F.; Bruijnzeel-Koomen, C.A. Photoaged skin: The role of neutrophils, preventive measures, and potential pharmacological targets. Clin. Pharmacol. Ther. 2011, 89, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, H. Die Isolierung Des antirachitischen Vitamins aus Thunfischleberöl. Hoppe Seylers Z. Physiol. Chem. 1936, 241, 104–115. [Google Scholar] [CrossRef]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on experimental rickets. XXI. an experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Shipley, P.G.; Kinney, E.M.; McCollum, E.V. Studies on experimental rickets: XXIV. the effect of creation extracts of plant tissues of florid rickets. J. Biol. Chem. 1924, 59, 165–175. [Google Scholar] [CrossRef]
- Hess, A.F.; Weinstock, M. Antirachitic properties imparted to inert fluids and to green vegetables by ultra-violet irradiation. J. Biol. Chem. 1924, 62, 301–313. [Google Scholar] [CrossRef]
- Hume, E.M.; Smith, H.H.; Smedley-Maclean, I. The examination of yeast-fat for the presence of vitamins A and D before irradiation and of vitamin D after irradiation. Biochem. J. 1928, 22, 27–33. [Google Scholar] [CrossRef]
- Steenbock, H.; Hart, E.B.; Hoppert, C.A.; Black, A. Fat-soluble vitamin: XXVI. The antirachitic property of milk and its increase by direct irradiation and by irradiation of the animal. J. Biol. Chem. 1925, 66, 441–449. [Google Scholar] [CrossRef]
- Steenbock, H.; Black, A. fat-soluble vitamins: XXIII. The induction of growth-promoting and calcifying properties in fats and their unsaponifiable constituents by exposure to light. J. Biol. Chem. 1925, 64, 263–298. [Google Scholar] [CrossRef]
- Redman, T. The hydrogen ion concentration and the calcium and phosphorus content of the faeces of rachitic children. Biochem. J. 1929, 23, 256–260. [Google Scholar] [CrossRef]
- Windaus, A.; Hess, A. Sterine und antirachitisches vitamin. Nachr. Ges. Wiss. Gött. 1926, 1926, 174–184. [Google Scholar]
- Hess, A.F. The rôle of activated milk in the anti-rickets campaign. Am. J. Public Health Nations Health 1932, 22, 1215–1219. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Morita, M.; Yamada, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Akune, T. Profiles of vitamin D insufficiency and deficiency in Japanese men and women: Association with biological, environmental, and nutritional factors and coexisting disorders: The ROAD study. Osteoporos. Int. 2013, 24, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Suzuki, M.; Minowa, K.; Hirai, S.; Takubo, N.; Sakamoto, Y.; Ishijima, M.; Hoshino, E.; Tokita, A.; Shimizu, T. Current vitamin D status in healthy Japanese infants and young children. J. Nutr. Sci. Vitaminol. 2018, 64, 99–105. [Google Scholar] [CrossRef]
- Yorifuji, J.; Yorifuji, T.; Tachibana, K.; Nagai, S.; Kawai, M.; Momoi, T.; Nagasaka, H.; Hatayama, H.; Nakahata, T. Craniotabes in normal newborns: The earliest sign of subclinical vitamin D deficiency. J. Clin. Endocrinol. Metab. 2008, 93, 1784–1788. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Effect of glycerophospholipid class on the beta-carotene uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2010, 74, 209–211. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Nagao, A. Effects of mixed micellar lipids on carotenoid uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2012, 76, 875–882. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Glyceroglycolipids affect uptake of carotenoids solubilized in mixed micelles by human intestinal Caco-2 cells. Lipids 2015, 50, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Lysoglyceroglycolipids improve the intestinal absorption of micellar fucoxanthin by Caco-2 cells. J. Oleo Sci. 2015, 64, 1207–1211. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Hase, M. Effect of dispersed form on the bioavailability of β-carotene from daily intake in humans. Biosci. Biotechnol. Biochem. 2020, 84, 2545–2557. [Google Scholar] [CrossRef]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Simultaneous synthesis of vitamins D2, D4, D5, D6, and D7 from commercially available phytosterol, β-sitosterol, and identification of each vitamin D by HSQC NMR. Metabolites 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Tsuzuki, W.; Nagao, A. Acyl moieties modulate the effects of phospholipids on beta-carotene uptake by Caco-2 cells. Lipids 2006, 41, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef]
- Catherino, W.H.; Eltoukhi, H.M.; Al-Hendy, A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin. Reprod. Med. 2013, 31, 370–379. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Simhan, H.N.; Catov, J.M.; Roberts, J.M.; Platt, R.W.; Diesel, J.C.; Klebanoff, M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Rifas-Shiman, S.L.; Kleinman, K.; Litonjua, A.A.; Huh, S.Y.; Rich-Edwards, J.W.; Camargo, C.A., Jr.; Gillman, M.W. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2012, 207, 182.e1–182.e8. [Google Scholar] [CrossRef]
- Hsieh, E.; Yin, M.T. Continued interest and controversy: Vitamin D in HIV. Curr. HIV/AIDS Rep. 2018, 15, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A., Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014, 5, 2365–2370. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Delanghe, J.R. Association between low vitamin D and COVID-19: Don’t forget the vitamin D binding protein. Aging Clin. Exp. Res. 2020, 32, 1207–1208. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.A.; Marín, C.; Mora-Plazas, M.; Villamor, E. Vitamin D deficiency associated with increased incidence of gastrointestinal and ear infections in school-age children. Pediatr. Infect. Dis. J. 2013, 32, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 2006, 35, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.M.; Sosman, J.A.; Blot, W.J. Sunlight and reduced risk of cancer: Is the real story vitamin D? J. Natl. Cancer Inst. 2005, 97, 161–163. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Vitamin D status: Ready for guiding prostate cancer diagnosis and treatment? Clin. Cancer Res. 2014, 20, 2241–2243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwartz, G.G.; Hulka, B.S. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res. 1990, 10, 1307–1311. [Google Scholar]
- Schwartz, G.G. Vitamin D and intervention trials in prostate cancer: From theory to therapy. Ann. Epidemiol. 2009, 19, 96–102. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Smedby, K.E.; Hjalgrim, H.; Melbye, M.; Torrång, A.; Rostgaard, K.; Munksgaard, L.; Adami, J.; Hansen, M.; Porwit-MacDonald, A.; Jensen, B.A.; et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J. Natl. Cancer Inst. 2005, 97, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Weihkopf, T.; Becker, N.; Nieters, A.; Mester, B.; Deeg, E.; Elsner, G.; Blettner, M.; Seidler, A. Sun exposure and malignant lymphoma: A population-based case-control study in Germany. Int. J. Cancer 2007, 120, 2445–2451. [Google Scholar] [CrossRef]
- Sluyter, J.D.; Camargo, C.A., Jr.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.T.; Thom, S.A.M.; Hametner, B.; Wassertheurer, S.; et al. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: A randomized controlled trial substudy. J. Am. Heart Assoc. 2017, 6, e006802. [Google Scholar] [CrossRef]
- Brøndum-Jacobsen, P.; Benn, M.; Jensen, G.B.; Nordestgaard, B.G. 25-Hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2794–2802. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Brøndum-Jacobsen, P.; Nordestgaard, B.G.; Schnohr, P.; Benn, M. 25-Hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann. Neurol. 2013, 73, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Lange, P.; Bojesen, S.E.; Freiberg, J.J.; Nordestgaard, B.G. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax 2014, 69, 24–31. [Google Scholar] [CrossRef]
- Færk, G.; Çolak, Y.; Afzal, S.; Nordestgaard, B.G. Low concentrations of 25-hydroxyvitamin D and long-term prognosis of COPD: A prospective cohort study. Eur. J. Epidemiol. 2018, 33, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Shaheen, A.; Arshad, R. Frequency of hypovitaminosis D and its associated risk factors in newly diagnosed pulmonary tuberculosis patients. Pak. J. Med. Sci. 2016, 32, 480–484. [Google Scholar]
- Pal, M.; Datta, S.; Mitra, R. Tuberculosis is associated with low levels of vitamin D. World J. Pharm. Pharm. Sci. 2014, 3, 1449–1463. [Google Scholar]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 2013, 13, 22. [Google Scholar]
- Selvaraj, P.; Harishankar, M.; Afsal, K. Vitamin D: Immuno-modulation and tuberculosis treatment. Can. J. Physiol. Pharmacol. 2015, 93, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; Massa, J.; Horst, R.; Orban, T.; Ascherio, A. Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am. J. Epidemiol. 2013, 177, 411–419. [Google Scholar] [CrossRef]
- Soltesz, G.; Patterson, C.C.; Dahlquist, G. Worldwide childhood type 1 diabetes incidence—What can we learn from epidemiology? Pediatr. Diabetes 2007, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Bennett, D.A.; Millwood, I.Y.; Parish, S.; McCarthy, M.I.; Mahajan, A.; Lin, X.; Bragg, F.; Guo, Y.; Holmes, M.V.; et al. Association of vitamin D with risk of type 2 diabetes: A Mendelian randomisation study in European and Chinese adults. PLoS Med. 2018, 15, e1002566. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef]
- Biström, M.; Alonso-Magdalena, L.; Andersen, O.; Jons, D.; Gunnarsson, M.; Vrethem, M.; Hultdin, J.; Sundström, P. High serum concentration of vitamin D may protect against multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319892291. [Google Scholar] [CrossRef]
- Goldberg, P. Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence. (A viewpoint) part 1: Sunlight, dietary factors and epidemiology. Int. J. Environ. Stud. 1974, 6, 19–27. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimer’s Dement. 2014, 10, 296–302. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Menegaz, D.; Zhang, J.; Barrientos-Durán, A.; Tse, S.; Cashman, J.R.; Griffin, P.R.; Fiala, M. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J. Alzheimer’s Dis. 2012, 29, 51–62. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Muniz-Terrera, G.; Phillips, C.L.; Cherubini, A.; Ferrucci, L.; Melzer, D. Vitamin D and risk of cognitive decline in elderly persons. Arch. Intern. Med. 2010, 170, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Brunner, R.L.; Michael, Y.L.; Larson, J.C.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Liu, S.; et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am. J. Clin. Nutr. 2011, 94, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Grudet, C.; Malm, J.; Westrin, A.; Brundin, L. Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology 2014, 50, 210–219. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Shardell, M.; Corsi, A.M.; Vazzana, R.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J. Clin. Endocrinol. Metab. 2010, 95, 3225–3233. [Google Scholar] [CrossRef] [PubMed]
- Valipour, G.; Saneei, P.; Esmaillzadeh, A. Serum vitamin D levels in relation to schizophrenia: A systematic review and meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 2014, 99, 3863–3872. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; van Schoor, N.M.; de Jongh, R.T.; Visser, M.; Deeg, D.J.; Lips, P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J. Clin. Endocrinol. Metab. 2013, 98, E1483–E1490. [Google Scholar] [CrossRef]
- Barassi, A.; Pezzilli, R.; Colpi, G.M.; Corsi Romanelli, M.M.; Melzi d’Eril, G.V. Vitamin D and erectile dysfunction. J. Sex. Med. 2014, 11, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Maiorino, M.I.; Olita, L.; Capuano, A.; Rafaniello, C.; Giugliano, D.; Esposito, K. Vitamin D deficiency in type 2 diabetic patients with hypogonadism. J. Sex. Med. 2014, 11, 536–542. [Google Scholar] [CrossRef]
- Daraki, V.; Roumeliotaki, T.; Koutra, K.; Chalkiadaki, G.; Katrinaki, M.; Kyriklaki, A.; Kampouri, M.; Margetaki, K.; Vafeiadi, M.; Papavasiliou, S.; et al. High maternal vitamin D levels in early pregnancy may protect against behavioral difficulties at preschool age: The Rhea mother-child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 2018, 27, 79–88. [Google Scholar] [CrossRef]
- Morales, E.; Julvez, J.; Torrent, M.; Ballester, F.; Rodríguez-Bernal, C.L.; Andiarena, A.; Vegas, O.; Castilla, A.M.; Rodriguez-Dehli, C.; Tardón, A.; et al. Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology 2015, 26, 458–465. [Google Scholar] [CrossRef]
- Sengupta, T.; Majumder, R.; Majumder, S. Role of vitamin D in treating COVID-19-associated coagulopathy: Problems and perspectives. Mol. Cell Biochem. 2021, 18, 1–7. [Google Scholar]
- Komba, S.; Kotake-Nara, E.; Machida, S. Fucoxanthin derivatives: Synthesis and their chemical properties. J. Oleo Sci. 2015, 64, 1009–1018. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Ono, S.; Matsuda, J.; Saito, A.; Yamamoto, T.; Fujimoto, W.; Shimizu, H.; Dateki, S.; Ouchi, K. A case of sitosterolemia due to compound heterozygous mutations in ABCG5: Clinical features and treatment outcomes obtained with colestimide and ezetimibe. Clin. Pediatr. Endocrinol. 2017, 26, 17–23. [Google Scholar] [CrossRef][Green Version]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Nagao, A. Absorption and metabolism of xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef]
- Keegan, R.J.; Lu, Z.; Bogusz, J.M.; Williams, J.E.; Holick, M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Derm. Endocrinol. 2013, 5, 165–176. [Google Scholar] [CrossRef]
- Prema, T.P.; Raghuramulu, N. Vitamin D3 and its metabolites in the tomato plant. Phytochemistry 1996, 42, 617–620. [Google Scholar] [CrossRef]
- Aburjaia, T.; Al-Khalil, S.; Abuirjeie, M. Vitamin D3 and its metabolites in tomato, potato, egg plant and zucchini leaves. Phytochemistry 1998, 49, 2497–2499. [Google Scholar] [CrossRef]
- Horst, R.L.; Reinhardt, T.A.; Russell, J.R.; Napoli, J.L. The isolation and identification of vitamin D2 and vitamin D3 from Medicago sativa (alfalfa plant). Arch. Biochem. Biophys. 1984, 231, 67–71. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef]
- Windaus, A.; Trautmann, G. Über das krystallisierte vitamin D4. Hoppe Seylers Z. Physiol. Chem. 1937, 247, 185–188. [Google Scholar] [CrossRef]
- Windaus, A.; Schenck, F.; Werder, F.V. Über das antirachitisch wirksame Bestrahlungsprodukt aus 7-dehydro-cholesterin. Hoppe Seylers Z. Physiol. Chem. 1936, 241, 100–103. [Google Scholar] [CrossRef]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Villette, C.; Delecolle, J.; Olsen, C.E.; Motawia, M.S.; Geoffroy, P.; Miesch, M.; Jensen, P.E.; Heintz, D.; Schaller, H. Vitamin D 5 in Arabidopsis thaliana. Sci. Rep. 2018, 8, 16348. [Google Scholar] [CrossRef] [PubMed]
- Seckbach, J.; Ikan, R. Sterols and chloroplast structure of Cyanidium caldarium. Plant Physiol. 1972, 49, 457–459. [Google Scholar] [CrossRef][Green Version]
- Karmakar, T.; Chakraborty, D.P. 7-Dehydrositosterol from Rauwolfia serpentina. Phytochemistry 1983, 22, 608–609. [Google Scholar] [CrossRef]
- Korn, E.D.; Von Brand, T.; Tobie, E.J. The sterols of Trypanosoma cruzi and Crithidia fasciculata. Comp. Biochem. Physiol. 1969, 30, 601–610. [Google Scholar] [CrossRef]
- Smith, F.R.; Korn, E.D. 7-Dehydrostigmasterol and ergosterol: The major sterols of an amoeba. J. Lipid Res. 1968, 9, 405–408. [Google Scholar] [CrossRef]
- Schoenheimer, R. New contributions in sterol metabolism. Science 1931, 74, 579–584. [Google Scholar] [CrossRef]
- Ajagbe, B.O.; Othman, R.A.; Myrie, S.B. Plant sterols, stanols, and sitosterolemia. J. AOAC Int. 2015, 98, 716–723. [Google Scholar] [CrossRef]
- Biancuzzo, R.M.; Young, A.; Bibuld, D.; Cai, M.H.; Winter, M.R.; Klein, E.K.; Ameri, A.; Reitz, R.; Salameh, W.; Chen, T.C.; et al. Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am. J. Clin. Nutr. 2010, 91, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Biancuzzo, R.M.; Chen, T.C.; Klein, E.K.; Young, A.; Bibuld, D.; Reitz, R.; Salameh, W.; Ameri, A.; Tannenbaum, A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008, 93, 677–681. [Google Scholar] [CrossRef]
- Trang, H.M.; Cole, D.E.; Rubin, L.A.; Pierratos, A.; Siu, S.; Vieth, R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am. J. Clin. Nutr. 1998, 68, 854–858. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A. Vitamin D3 is more potent than vitamin D2 in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Takeuchi, A.; Okano, T.; Tanda, M.; Kobayashi, T. Possible origin of extremely high contents of vitamin D3 in some kinds of fish liver. Comp. Biochem. Physiol. 1991, 100, 483–487. [Google Scholar]
- Mattila, P.; Piironen, V.; Haapala, R.; Hirvi, T.; Uusi-Rauva, E. Possible factors responsible for the high variation in the cholecalciferol contents of fish. J. Agric. Food Chem. 1997, 45, 3891–3896. [Google Scholar] [CrossRef]
- Imrie, M.H.; Neville, P.F.; Snellgrove, A.W.; DeLuca, H.F. Metabolism of vitamin D2 and vitamin D3 in the rachitic chick. Arch. Biochem. Biophys. 1967, 120, 525–532. [Google Scholar] [CrossRef]
- De Luca, H.F.; Weller, M.; Blunt, J.W.; Neville, P.F. Synthesis, biological activity, and metabolism of 22,23-3H vitamin D4. Arch. Biochem. Biophys. 1968, 124, 122–128. [Google Scholar] [CrossRef]
- Ito, N.; Ohtsubo, T.; Kusu, F.; Hakamata, H. An ultra performance liquid chromatographic method for determining phytosterol uptake by Caco-2 cells. Anal. Biochem. 2012, 421, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Haikal, Z.; Play, B.; Landrier, J.F.; Giraud, A.; Ghiringhelli, O.; Lairon, D.; Jourdheuil-Rahmani, D. NPC1L1 and SR-BI are involved in intestinal cholesterol absorption from small-size lipid donors. Lipids 2008, 43, 401–408. [Google Scholar] [CrossRef]
- Kiourtzidis, M.; Kühn, J.; Brandsch, C.; Stangl, G.I. Vitamin D status of mice deficient in scavenger receptor class B type 1, cluster determinant 36 and ATP-binding cassette proteins G5/G8. Nutrients 2020, 12, 2169. [Google Scholar] [CrossRef]
- Nakano, T.; Inoue, I.; Alpers, D.H.; Akiba, Y.; Katayama, S.; Shinozaki, R.; Kaunitz, J.D.; Ohshima, S.; Akita, M.; Takahashi, S.; et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G207–G214. [Google Scholar] [CrossRef]
- Sawai, T.; Usui, N.; Dwaihy, J.; Drongowski, R.A.; Abe, A.; Coran, A.G.; Harmon, C.M. The effect of phospholipase A2 on bacterial translocation in a cell culture model. Pediatr. Surg. Int. 2000, 16, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Sawai, T.; Drongowski, R.A.; Lampman, R.W.; Coran, A.G.; Harmon, C.M. The effect of phospholipids and fatty acids on tight-junction permeability and bacterial translocation. Pediatr. Surg. Int. 2001, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sawai, T.; Lampman, R.; Hua, Y.; Segura, B.; Drongowski, R.A.; Coran, A.G.; Harmon, C.M. Lysophosphatidylcholine alters enterocyte monolayer permeability via a protein kinase C/Ca2+ mechanism. Pediatr. Surg. Int. 2002, 18, 591–594. [Google Scholar] [CrossRef]
- Doi, N.; Tomita, M.; Hayashi, M. Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab. Pharmacokinet. 2011, 26, 162–170. [Google Scholar] [CrossRef]
- Lambert, D.; O’Neill, C.A.; Padfield, P.J. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem. J. 2005, 387, 553–560. [Google Scholar] [CrossRef]
- Lambert, D.; O’Neill, C.A.; Padfield, P.J. Methyl-beta-cyclodextrin increases permeability of Caco-2 cell monolayers by displacing specific claudins from cholesterol rich domains associated with tight junctions. Cell. Physiol. Biochem. 2007, 20, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; LeCluyse, E.L.; Thakker, D.R. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J. Pharm. Sci. 1999, 88, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Morris-Natschke, S.L.; Kucera, L.S.; Ishaq, K.S.; Thakker, D.R. Structure-activity relationships for enhancement of paracellular permeability by 2-alkoxy-3-alkylamidopropylphosphocholines across Caco-2 cell monolayers. J. Pharm. Sci. 1999, 88, 1169–1174. [Google Scholar] [CrossRef]
- Muir, L.V.; Born, E.; Mathur, S.N.; Field, F.J. Lysophosphatidylcholine increases 3-Hydroxy-3-methylglutaryl-coenzyme A reductase gene expression in CaCo-2 cells. Gastroenterology 1996, 110, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L.; Fivizzani, M.A.; Schnoes, H.K.; DeLuca, H.F. Synthesis of vitamin D5: Its biological activity relative to vitamins D3 and D2. Arch. Biochem. Biophys. 1979, 197, 119–125. [Google Scholar] [CrossRef]
- Windaus, A.; Langer, R. Über das 22-dihydro-ergosterin. Eur. J. Org. Chem. 1933, 508, 105–114. [Google Scholar] [CrossRef]
- Grab, W. Die Auswertung der antirachitischen Wirksamkeit neuer Sterinderivate im Versuch an Ratten und Küken. Hoppe Seylers Z. Physiol. Chem. 1936, 243, 63–89. [Google Scholar] [CrossRef]
- Wunderlich, W. Über das 7-Dehydro-sitosterin. Hoppe Seylers Z. Physiol. Chem. 1936, 241, 116–124. [Google Scholar] [CrossRef]
- Haslewood, G.A. The action of light on substances related to ergosterol. Biochem. J. 1939, 33, 454–456. [Google Scholar] [CrossRef]
- Linsert, O. Über das 7-Dehydro-stigmasterin. Hoppe Seylers Z. Physiol. Chem. 1936, 241, 125–128. [Google Scholar] [CrossRef]
- Ruigh, W.L. 7-Dehydrocampesterol, a new provitamin D. J. Am. Chem. Soc. 1942, 64, 1900–1902. [Google Scholar] [CrossRef]
- Mehta, R.G.; Moriarty, R.M.; Mehta, R.R.; Penmasta, R.; Lazzaro, G.; Constantinou, A.; Guo, L. Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1alpha-hydroxyvitamin D5. J. Natl. Cancer Inst. 1997, 89, 212–218. [Google Scholar] [CrossRef]
- Strugnell, S.; Byford, V.; Makin, H.L.; Moriarty, R.M.; Gilardi, R.; LeVan, L.W.; Knutson, J.C.; Bishop, C.W.; Jones, G. 1 alpha,24(S)-dihydroxyvitamin D2: A biologically active product of 1 alpha-hydroxyvitamin D2 made in the human hepatoma, Hep3B. Biochem. J. 1995, 310, 233–241. [Google Scholar] [CrossRef]
- Liu, G.; Oettel, K.; Ripple, G.; Staab, M.J.; Horvath, D.; Alberti, D.; Arzoomanian, R.; Marnocha, R.; Bruskewitz, R.; Mazess, R.; et al. Phase I trial of 1alpha-hydroxyvitamin d(2) in patients with hormone refractory prostate cancer. Clin. Cancer Res. 2002, 8, 2820–2827. [Google Scholar]
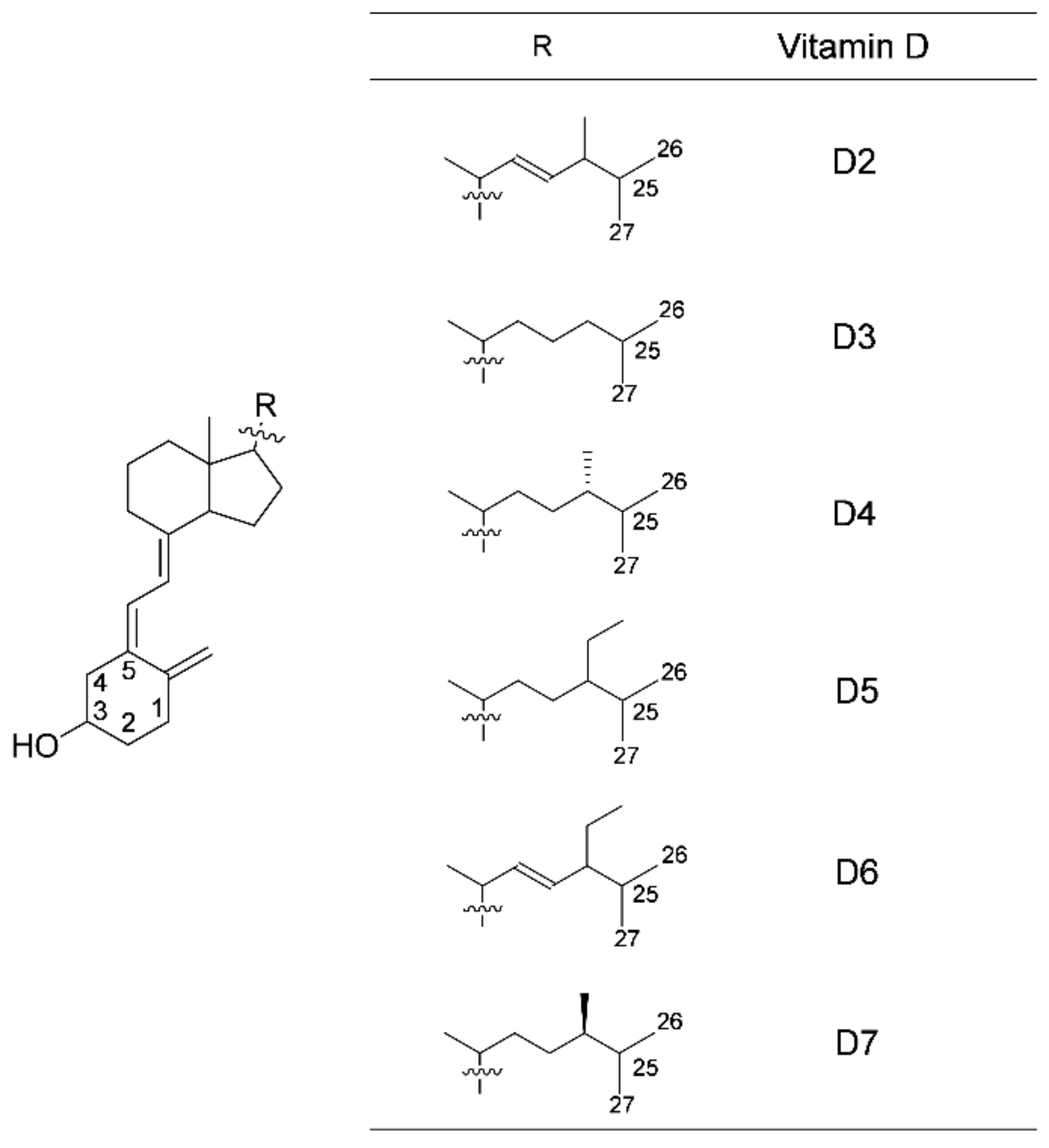



| Vitamin D2 | Vitamin D3 | Vitamin D4/D7 ∗ | Vitamin D5 | Vitamin D6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Activity | Pa 1 | Pi 2 | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi |
| Anti-osteoporotic | 0.982 a | 0.003 | 0.973 b | 0.003 | 0.969 | 0.003 | 0.965 | 0.003 | 0.970 | 0.003 |
| Bone diseases treatment | 0.981 a | 0.003 | 0.976 b | 0.003 | 0.968 | 0.003 | 0.966 | 0.003 | 0.966 | 0.003 |
| Vitamin | 0.977 b | 0.000 | 0.975 | 0.000 | 0.936 | 0.000 | 0.978 a | 0.000 | 0.976 | 0.000 |
| Hyperparathyroidism treatment | 0.946 a | 0.000 | 0.933 b | 0.000 | 0.883 | 0.000 | 0.882 | 0.000 | 0.901 | 0.000 |
| Calcium regulator | 0.902 a | 0.001 | 0.876 | 0.002 | 0.869 | 0.002 | 0.870 | 0.002 | 0.880 b | 0.001 |
| Vitamin D-like | 0.869 a | 0.000 | 0.757 | 0.000 | 0.578 | 0.000 | 0.791 | 0.000 | 0.844 b | 0.000 |
| Vitamin D receptor agonist | 0.816 a | 0.000 | 0.693 | 0.000 | 0.680 | 0.000 | 0.686 | 0.000 | 0.738 b | 0.000 |
| Bone formation stimulant | 0.578 | 0.004 | 0.598 | 0.004 | 0.628 b | 0.003 | 0.634 a | 0.003 | 0.627 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotake-Nara, E.; Komba, S.; Hase, M. Uptake of Vitamins D2, D3, D4, D5, D6, and D7 Solubilized in Mixed Micelles by Human Intestinal Cells, Caco-2, an Enhancing Effect of Lysophosphatidylcholine on the Cellular Uptake, and Estimation of Vitamins D’ Biological Activities. Nutrients 2021, 13, 1126. https://doi.org/10.3390/nu13041126
Kotake-Nara E, Komba S, Hase M. Uptake of Vitamins D2, D3, D4, D5, D6, and D7 Solubilized in Mixed Micelles by Human Intestinal Cells, Caco-2, an Enhancing Effect of Lysophosphatidylcholine on the Cellular Uptake, and Estimation of Vitamins D’ Biological Activities. Nutrients. 2021; 13(4):1126. https://doi.org/10.3390/nu13041126
Chicago/Turabian StyleKotake-Nara, Eiichi, Shiro Komba, and Megumi Hase. 2021. "Uptake of Vitamins D2, D3, D4, D5, D6, and D7 Solubilized in Mixed Micelles by Human Intestinal Cells, Caco-2, an Enhancing Effect of Lysophosphatidylcholine on the Cellular Uptake, and Estimation of Vitamins D’ Biological Activities" Nutrients 13, no. 4: 1126. https://doi.org/10.3390/nu13041126
APA StyleKotake-Nara, E., Komba, S., & Hase, M. (2021). Uptake of Vitamins D2, D3, D4, D5, D6, and D7 Solubilized in Mixed Micelles by Human Intestinal Cells, Caco-2, an Enhancing Effect of Lysophosphatidylcholine on the Cellular Uptake, and Estimation of Vitamins D’ Biological Activities. Nutrients, 13(4), 1126. https://doi.org/10.3390/nu13041126






