ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Wine Lees
2.3. Measurement of the ACEi Activity
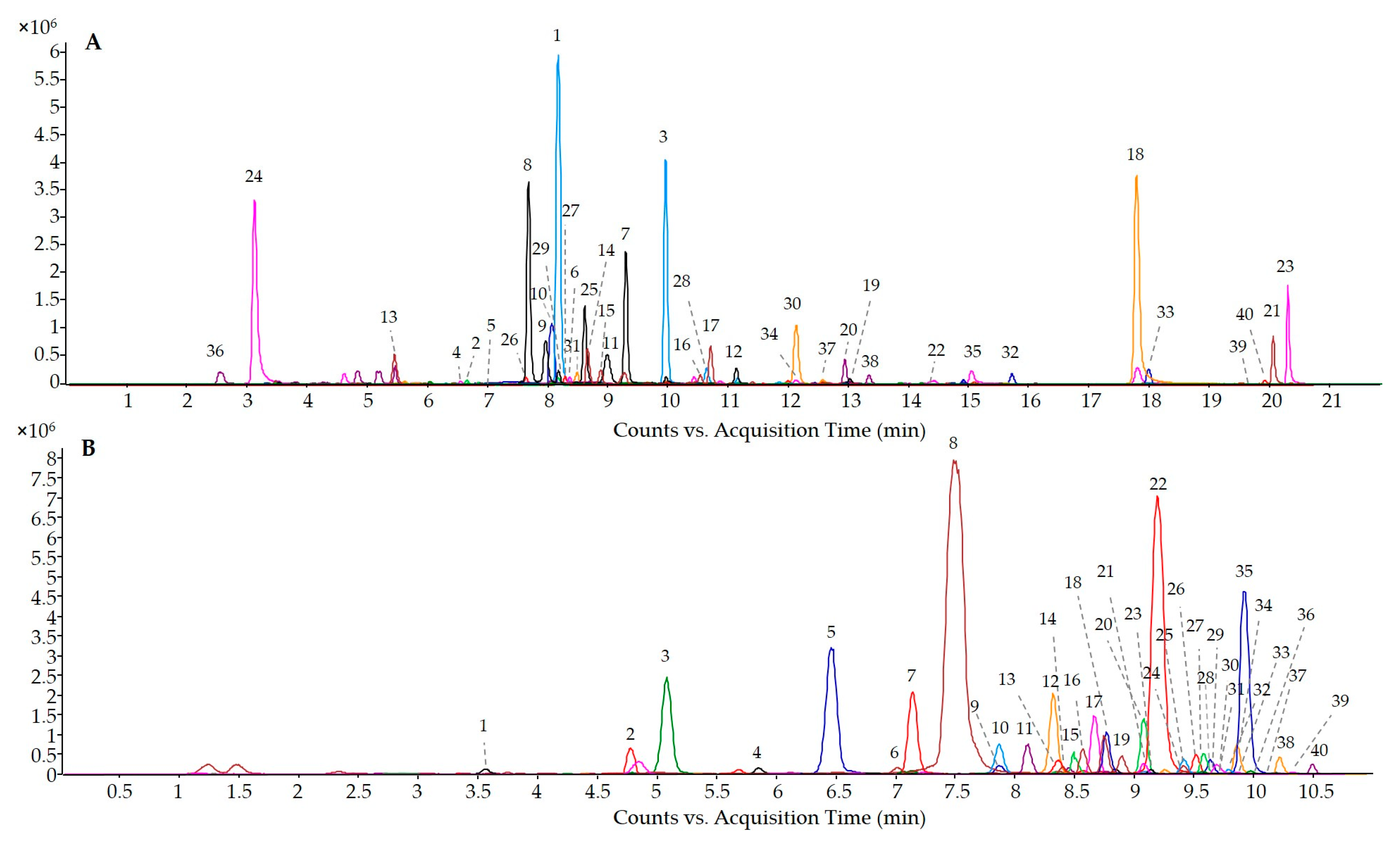
2.4. Detection and Quantification of the Phenolic Compounds from Wine Lees
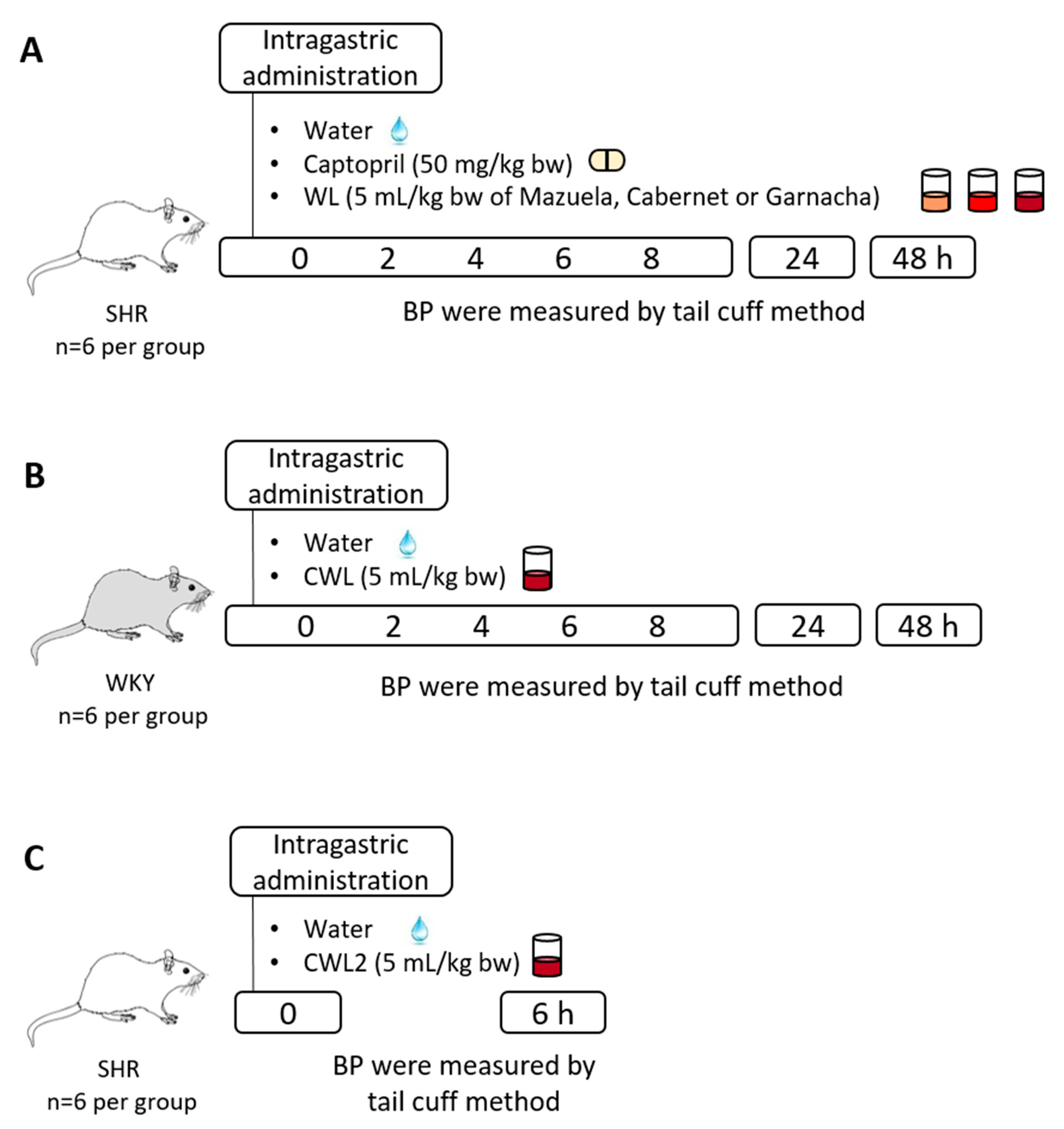
2.5. Experimental Procedure in Rats
2.6. Statistical Analysis
3. Results
3.1. Selection of the Wine Lees
3.2. Determination of the Phenolic Profile of the Three Selected Wine Lees
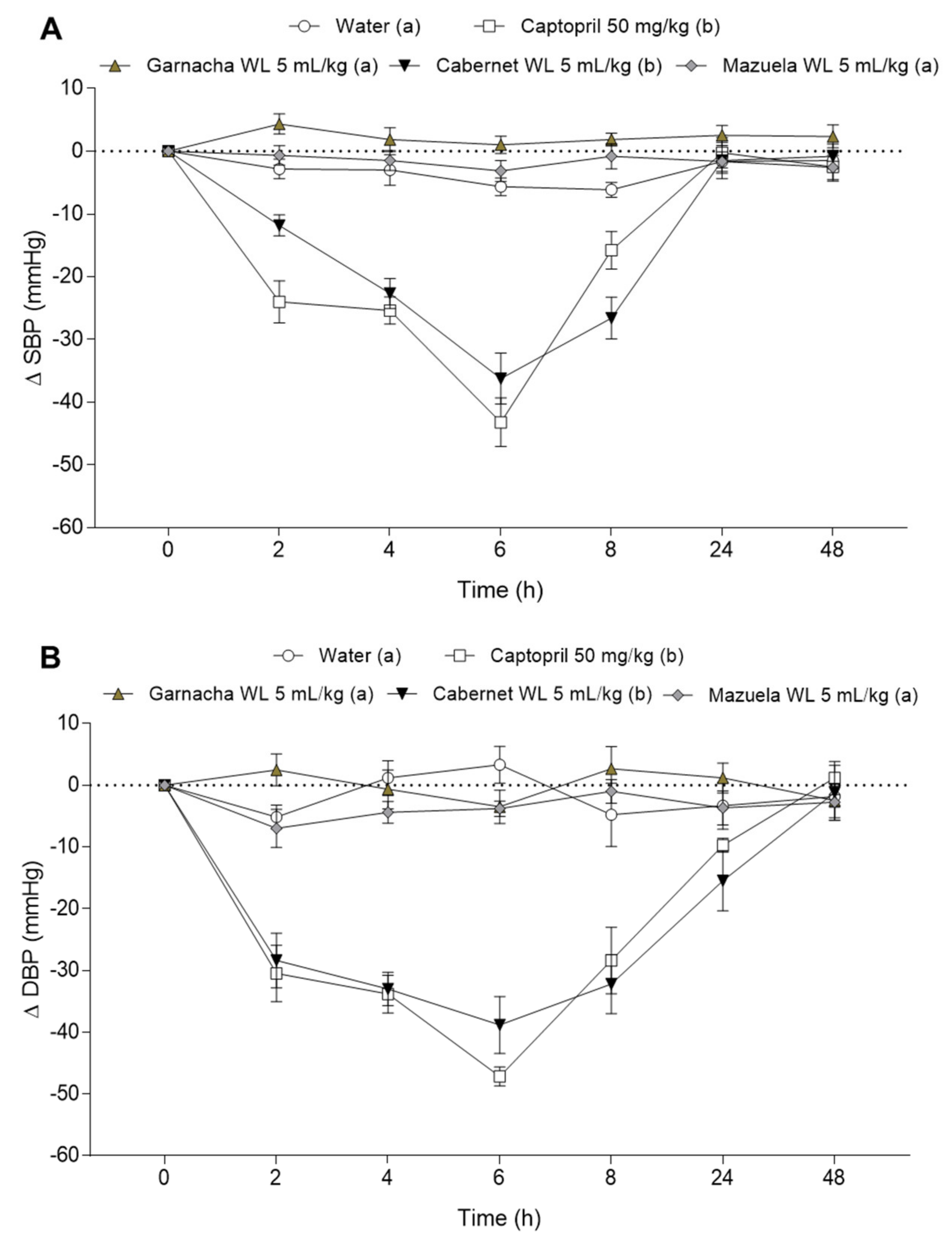
3.3. Effect of Different Wine Lees on Blood Pressure in Hypertensive Rats
3.4. Effect of Cabernet Wine Lees on Blood Pressure in Normotensive Rats
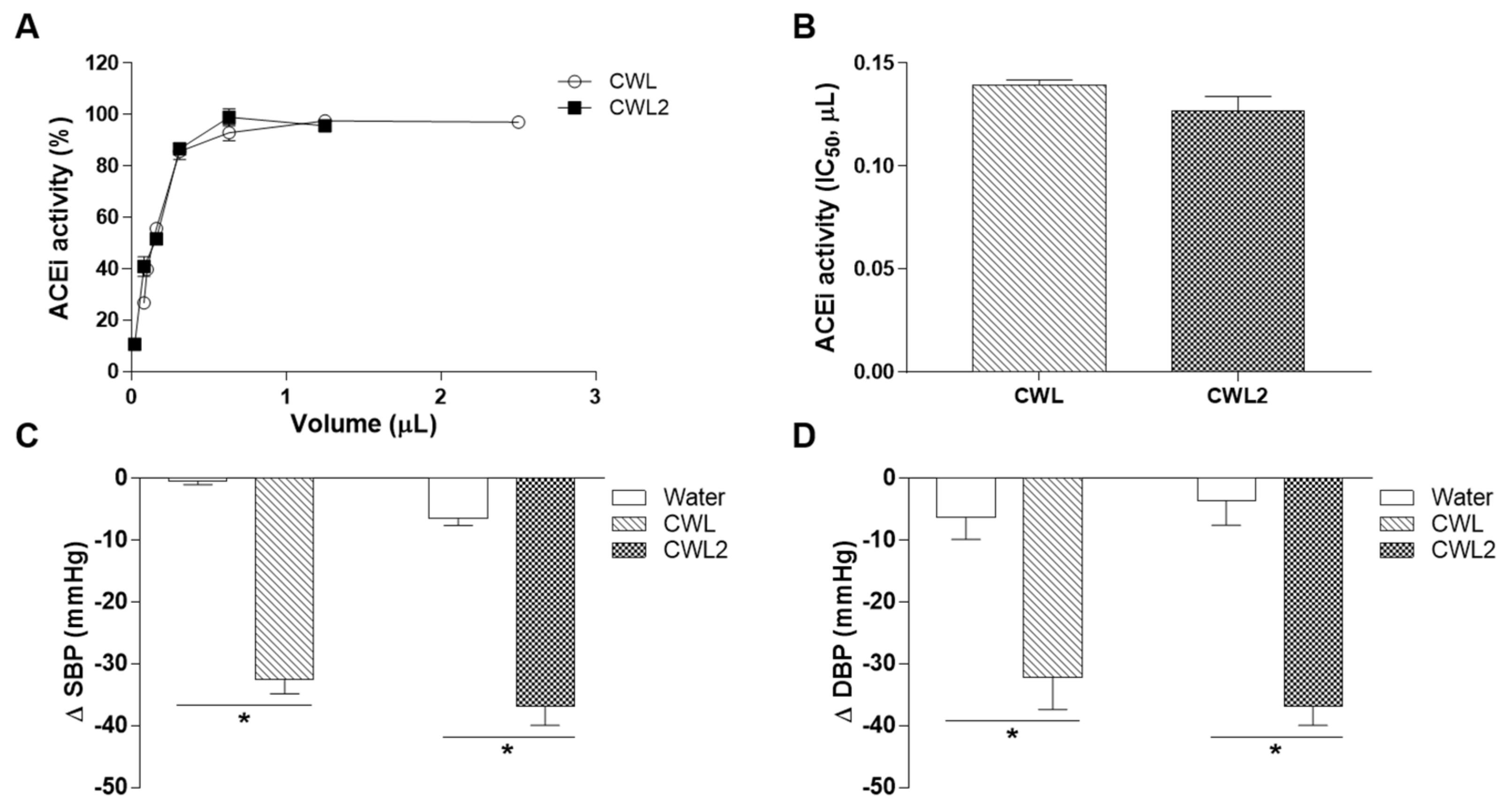
3.5. Variability between Cabernet Wine Lees from Two Different Harvests
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordans, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990–2015. JAMA J. Am. Med. Assoc. 2017, 317, 165–182. [Google Scholar] [CrossRef]
- Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 22 May 2020).
- Hedayati, S.S.; Elsayed, E.F.; Reilly, R.F. Non-pharmacological aspects of blood pressure management: What are the data? Kidney Int. 2011, 79, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, E.; Vande, W.J.; De Bruyne, P. Therapeutic efficacy and safety of ACE inhibitors in the hypertensive paediatric population: A review. Arch. Dis. Child. 2017, 102, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin System in Kidney Physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [PubMed]
- Brown, N.J.; Vaughan, D.E. Angiotensin-Converting Enzyme Inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef]
- Margalef, M.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Natural Angiotensin Converting Enzyme (ACE) Inhibitors with Antihypertensive Properties. In Natural Products Targeting Clinically Relevant Enzymes; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2017; pp. 45–67. [Google Scholar]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products –Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2019, 60, 1388–1416. [Google Scholar] [CrossRef]
- Mak, T.M.; Xiong, X.; Tsang, D.C.; Yu, I.K.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Musee, N.; Lorenzen, L.; Aldrich, C. Cellar waste minimization in the wine industry: A systems approach. J. Clean. Prod. 2007, 15, 417–431. [Google Scholar] [CrossRef]
- Vázquez-Garza, E.; Bernal-Ramírez, J.; Jerjes-Sánchez, C.; Lozano, O.; Acuña-Morín, E.; Vanoye-Tamez, M.; Ramos-González, M.R.; Chapoy-Villanueva, H.; Pérez-Plata, L.; Sánchez-Trujillo, L.; et al. Resveratrol Prevents Right Ventricle Remodeling and Dysfunction in Monocrotaline-Induced Pulmonary Arterial Hypertension with a Limited Improvement in the Lung Vasculature. Oxid. Med. Cell. Longev. 2020, 2020, 1841527. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, R.; Guo, J.; Yue, H.; Liu, Q.; Guo, L.; Zhang, Q. Resveratrol Supplementation Prevents Hypertension in Hypertensive Pregnant Rats by Increasing Sodium Excretion and Serum Nitric Oxide Level. Int. J. Hypertens. 2020, 2020, 4154010. [Google Scholar] [CrossRef]
- Pons, Z.; Guerrero, L.; Margalef, M.; Arola, L.; Arola-Arnal, A.; Muguerza, B. Effect of low molecular grape seed proanthocyanidins on blood pressure and lipid homeostasis in cafeteria diet-fed rats. J. Physiol. Biochem. 2014, 70, 629–637. [Google Scholar] [CrossRef]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Suárez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.; De Castro, M.L. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef]
- Landeka Jurčević, I.; Dora, M.; Guberović, I.; Petras, M.; Rimac Brnčić, S.; Đikić, D. Wine Lees Polyphenols as a Novel Functional Bioactive Compound in the Protection against Oxidative Stress and Hyperlipidemia. Food Technol. Biotechnol. 2017, 55, 109. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.; Matias, A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, M.P.D.; Priego-Capote, F.; De Castro, M.D.L. Characterization and Comparison of Wine Lees by Liquid Chromatography–Mass Spectrometry in High-Resolution Mode. J. Agric. Food Chem. 2015, 63, 1116–1125. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Rodrigues, D.; Gómez-Alonso, S.; Hermosín-Gutíerrez, I.; Godoy, H.T. Occurrence of low molecular weight phenolics in Vitis vinifera red grape cultivars and their winemaking by-products from São Paulo (Brazil). Food Res. Int. 2014, 62, 500–513. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suarez, M.; Muguerza, B.; Bravo, F.I. Long-term administration of protein hydrolysate from chicken feet induces antihypertensive effect and confers vasoprotective pattern in diet-induced hypertensive rats. J. Funct. Foods 2019, 55, 28–35. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Sancho-Pardo, L.; Bravo, F.I.; Mulero, M.; Muguerza, B.; Arola-Arnal, A. Optimized extraction by response surface methodology used for the characterization and quantification of phenolic compounds in whole red grapes (Vitis vinifera). Nutrients 2018, 10, 1931. [Google Scholar] [CrossRef]
- Laitila, J.E.; Suvanto, J.; Salminen, J.P. Liquid chromatography–tandem mass spectrometry reveals detailed chromatographic fingerprints of anthocyanins and anthocyanin adducts in red wine. Food Chem. 2019, 294, 138–151. [Google Scholar] [CrossRef]
- Quinones, M.; Miguel, M.; Muguerza, B.; Aleixandre, A. Effect of a cocoa polyphenol extract in spontaneously hypertensive rats. Food Funct. 2011, 2, 649–653. [Google Scholar] [CrossRef]
- Costa, G.N.S.; Tonon, R.V.; Mellinger-Silva, C.; Galdeano, M.C.; Iacomini, M.; A Santiago, M.C.P.; Almeida, E.L.; Freitas, S.P. Grape seed pomace as a valuable source of antioxidant fibers. J. Sci. Food Agric. 2019, 99, 4593–4601. [Google Scholar] [CrossRef] [PubMed]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.; Cabral, L.M. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Zhang, H.; Huang, Y.; Yang, G.; Du, M.; Zhu, M.-J. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol. Nutr. Food Res. 2013, 57, 2253–2257. [Google Scholar] [CrossRef]
- Hernández-Salinas, R.; Decap, V.; Leguina, A.; Cáceres, P.; Perez, D.; Urquiaga, I.; Iturriaga, R.; Velarde, V. Antioxidant and antihyperglycemic role of wine grape powder in rats fed with a high fructose diet. Biol. Res. 2015, 48, 53. [Google Scholar] [CrossRef] [PubMed]
- Diebolt, M.; Bucher, B.; Andriantsitohaina, R. Wine Polyphenols Decrease Blood Pressure, Improve NO Vasodilatation, and Induce Gene Expression. Hypertension 2001, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Viana, F.S.C.; Souza, M.A.V.; Kovary, K.; Guedes, D.C.; Oliveira, E.P.B.; Rubenich, L.M.S.; Carvalho, L.C.R.M.; Oliveira, R.M.; Tano, T.; et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J. Pharm. Pharmacol. 2002, 54, 1515–1520. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lu, Y.L.; Wang, G.J.; Chen, L.G.; Wen, C.L.; Hou, W.C. Ethanolic extracts and isolated compounds from small-leaf grape (Vitis thunbergii var. taiwaniana) with antihypertensive activities. J. Agric. Food Chem. 2012, 60, 7435–7441. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2019, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Tzakos, A.G.; Naqvi, N.; Comporozos, K.; Pierattelli, R.; Theodorou, V.; Husain, A.; Gerothanassis, I.P. The molecular basis for the selection of captopril cis and trans conformations by angiotensin I converting enzyme. Bioorganic Med. Chem. Lett. 2006, 16, 5084–5087. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, A.M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Hidalgo, J.M.; Martínez-Rodríguez, A.J.; Martin-Álvarez, P.J.; Pueyo, E. Influence of the elaboration process on the peptide fraction with angiotensin I-converting enzyme inhibitor activity in sparkling wines and red wines aged on lees. Food Chem. 2008, 111, 965–969. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Alcaide, J.M.; Polo, M.C.; Pueyo, E. Angiotensin I-converting enzyme inhibitory compounds in white and red wines. Food Chem. 2007, 100, 43–47. [Google Scholar] [CrossRef]
- Fuglsang, A.; Nilsson, D.; Nyborg, N.C.B. Cardiovascular effects of fermented milk containing angiotensin-converting enzyme inhibitors evaluated in permanently catheterized, spontaneously hypertensive rats. Appl. Environ. Microbiol. 2002, 68, 3566–3569. [Google Scholar] [CrossRef]
- Muguerza, B.; Ramos, M.; Sánchez, E.; Manso, M.; Miguel, M.; Aleixandre, A.; Delgado, M.; Recio, I. Antihypertensive activity of milk fermented by Enterococcus faecalis strains isolated from raw milk. Int. Dairy J. 2006, 16, 61–69. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B. A comparative study on the bioavailability of phenolic compounds from organic and nonorganic red grapes. Food Chem. 2019, 299, 125092. [Google Scholar] [CrossRef] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola, A.; Muguerza, B. Acute administration of single oral dose of grape seed polyphenols restores blood pressure in a rat model of metabolic syndrome: Role of nitric oxide and prostacyclin. Eur. J. Nutr. 2016, 55, 749–758. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Pons, Z.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients 2018, 10, 1295. [Google Scholar] [CrossRef]
- Valls, R.M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; Lopes-Miranda, J.; Giralt, M.; Covas, M.I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hyper-tensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet 2003, 362, 1527–1535. [Google Scholar] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Zanchetti, A. Hypertension: Lower or higher blood-pressure targets for high-risk patients? Nat. Rev. Cardiol. 2016, 13, 637–638. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Buscialà, A.; Bosisio, E. Vascular effects of wine polyphenols. Cardiovasc. Res. 2004, 63, 593–602. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Li, S.-H.; Zhao, P.; Tian, H.-B.; Chen, L.-H.; Cui, L.-Q. Effect of Grape Polyphenols on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0137665. [Google Scholar] [CrossRef] [PubMed]
- Al-Dashti, Y.A.; Holt, R.R.; Stebbins, C.L.; Keen, C.L.; Hackman, R.M. Dietary Flavanols: A Review of Select Effects on Vascular Function, Blood Pressure, and Exercise Performance. J. Am. Coll. Nutr. 2018, 37, 553–567. [Google Scholar] [CrossRef]
- Alañón, M.; Castle, S.; Serra, G.; Lévèques, A.; Poquet, L.; Actis-Goretta, L.; Spencer, J. Acute study of dose-dependent effects of (−)-epicatechin on vascular function in healthy male volunteers: A randomized controlled trial. Clin. Nutr. 2020, 39, 746–754. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Weber, T.; Skene, S.S.; Ottaviani, J.I.; Crozier, A.; Kelm, M.; Schroeter, H.; Heiss, C. Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: Randomized, controlled, double-masked intervention trial. Am. J. Clin. Nutr. 2018, 108, 1229–1237. [Google Scholar] [CrossRef]
- Quiñones, M.; Margalef, M.; Arola-Arnal, A.; Muguerza, B.; Miguel, M.; Aleixandre, A. The blood pressure effect and related plasma levels of flavan-3-ols in spontaneously hypertensive rats. Food Funct. 2015, 6, 3479–3489. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.-M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; MacGregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bo, Y.; Wang, X.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. The Effect of Anthocyanins on Blood Pressure. Med. 2016, 95, e3380. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, E.C.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2019, 74, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Calfío, C.; Huidobro-Toro, J.P. Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis microphylla) with Nutraceutical Potential. Molecules 2019, 24, 2700. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
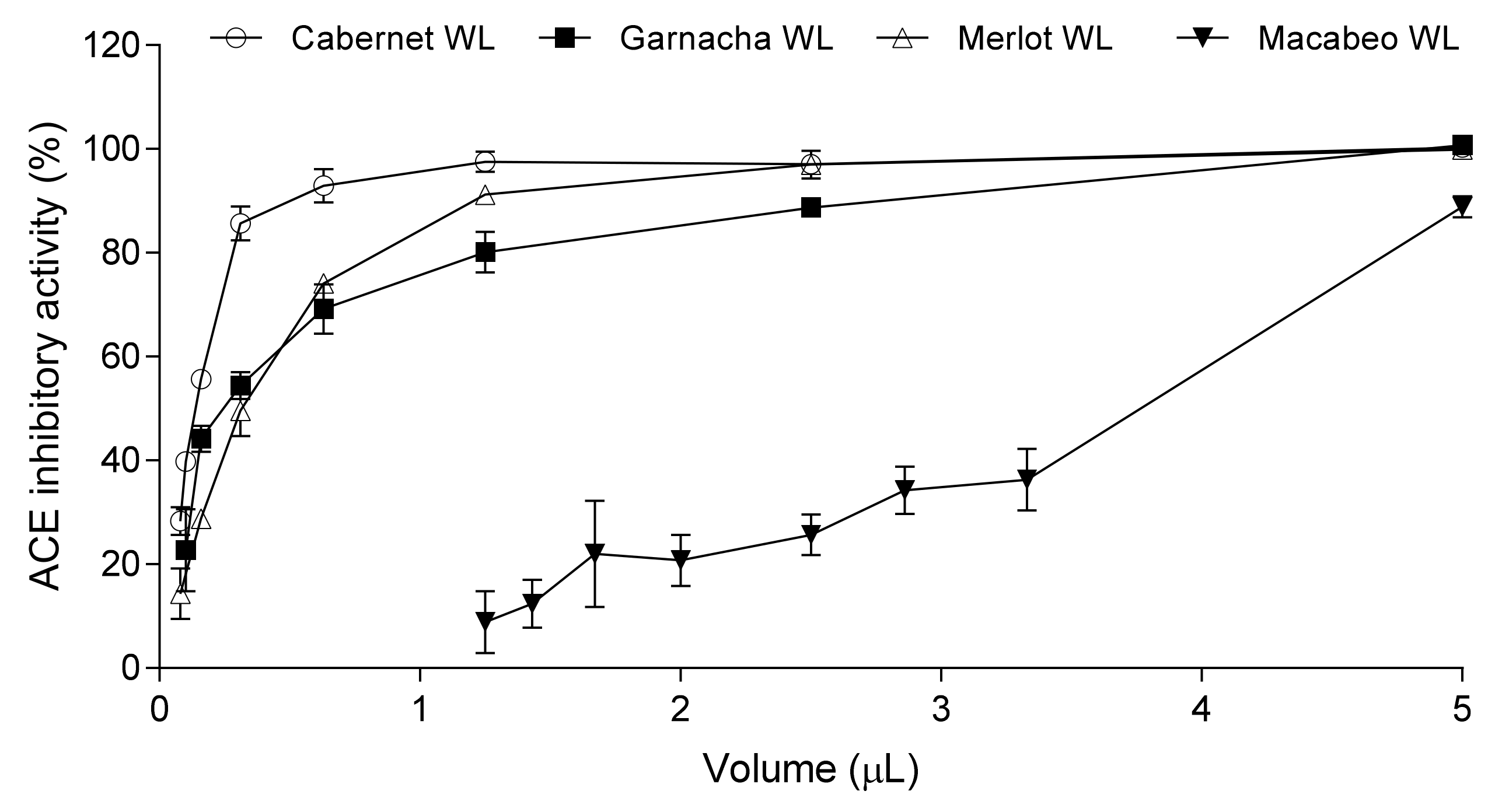

| Grape Variety | ACEi activity | |
|---|---|---|
| %* | IC50 (µL) | |
| Cabernet | 55.69 ± 1.92 | 0.15 ± 0.01 |
| Garnacha | 44.16 ± 2.54 | 0.22 ± 0.01 |
| Mazuela | 50.70 ± 8.10 | 0.21 ± 0.03 |
| Merlot | 28.76 ± 0.34 | 0.32 ± 0.02 |
| Macabeo | < 10 | 3.74 ± 0.05 |
| Phenolic Compounds | Cabernet WL (mg/L) | Mazuela WL (mg/L) | Garnacha WL (mg/L) |
|---|---|---|---|
| Flavanols | 331.11 | 122.63 | 154.60 |
| Flavonols | 57.62 | 44.83 | 57.28 |
| Phenolic acids | 133.54 | 132.75 | 103.05 |
| Stilbenes | 14.73 | 20.08 | 13.59 |
| Anthocyanins | 153.53 | 74.99 | 51.12 |
| Total | 690.63 | 395.28 | 379.64 |
| Compounds | R.T. (min) | [M-H]- | Fragment (m/z) | Cabernet WL (mg/L) | Mazuela WL (mg/L) | Garnacha WL (mg/L) | |
|---|---|---|---|---|---|---|---|
| Flavanols | |||||||
| 1 | Catechin | 8.17 | 289.0718 | 97.63 ± 0.62 a | 39.27 ± 0.25 b | 56.65 ± 0.36 c | |
| 2 | Catechin gallate 1 | 6.66 | 441.0827 | 289.07209 | 0.80 ± 0.01 a | 1.53 ± 0.03 b | 0.79 ± 0.01 a |
| 3 | Epicatechin | 9.96 | 289.0718 | 43.48 ± 0.23 a | 12.94 ± 0.07 b | 20.16 ± 0.11 b | |
| 4 | (Epi)catechin O-glucoside iso1 2 | 6.55 | 451.1246 | 289.0721 | 0.50 ± 0.00 a | 0.50 ± 0.00 a | 0.90 ± 0.00 a |
| 5 | (Epi)catechin O-glucoside iso2 2 | 7.41 | 451.1246 | 289.0721 | 0.33 ± 0.00 a | 0.29 ± 0.00 a | 0.43 ± 0.00 a |
| 6 | (Epi)catechin O-glucoside iso3 2 | 8.37 | 451.1246 | 289.0721 | 1.47 ± 0.03 a | 0.77 ± 0.01 b | 1.64 ± 0.03 a |
| 7 | Procyanidin dimer B2 | 9.30 | 577.1387 | 289.0733 | 34.60 ± 0.01 a | 9.61 ± 0.00 b | 9.09 ± 0.00 c |
| 8 | Procyanidin dimer iso1 3 | 7.68 | 577.1387 | 289.0733 | 64.21 ± 0.34 a | 32.11 ± 0.17 b | 32.37 ± 0.17 b |
| 9 | Procyanidin dimer iso2 3 | 7.97 | 577.1387 | 289.0733 | 14.26 ± 0.09 a | 4.19 ± 0.03 b | 5.81 ± 0.04 c |
| 10 | Procyanidin dimer iso3 3 | 8.18 | 577.1387 | 289.0733 | 2.96 ± 0.02 a | 0.84 ± 0.01 b | 1.50 ± 0.01 c |
| 11 | Procyanidin dimer iso4 3 | 8.99 | 577.1387 | 289.0733 | 12.80 ± 0.00 a | 2.71 ± 0.00 b | 3.79 ± 0.00 c |
| 12 | Procyanidin dimer iso5 3 | 11.14 | 577.1387 | 289.0733 | 4.32 ± 0.04 a | 1.54 ± 0.02 b | 1.97 ± 0.02 b |
| 13 | Procyanidin trimer iso1 3 | 5.46 | 865.2016 | 577.1369 | 16.28 ± 0.29 a | 5.14 ± 0.09 b | 7.91 ± 0.14 c |
| 14 | Procyanidin trimer iso2 3 | 8.67 | 865.2016 | 577.1369 | 14.35 ± 0.71 a | 4.62 ± 0.23 b | 5.15 ± 0.25 c |
| 15 | Procyanidin trimer iso3 3 | 8.89 | 865.2016 | 577.1369 | 6.11 ± 0.03 a | 2.37 ± 0.01 b | 2.55 ± 0.01 b |
| 16 | Procyanidin trimer iso4 3 | 10.55 | 865.2016 | 577.1369 | 3.22 ± 0.14 a | 1.38 ± 0.06 b | 1.19 ± 0.06 b |
| 17 | Procyanidin trimer iso5 3 | 10.71 | 865.2016 | 577.1369 | 13.79 ± 0.23 a | 2.82 ± 0.05 b | 2.70 ± 0.05 b |
| Flavonols | |||||||
| 18 | Quercetin | 17.80 | 301.0372 | 36.78 ± 0.17 a | 28.62 ± 0.13 b | 25.35 ± 0.12 c | |
| 19 | Quercetin-3-O-glucoside 4 | 13.00 | 463.0904 | 301.0361 | 1.63 ± 0.04 a | 2.73 ± 0.08 b | 7.68 ± 0.21 c |
| 20 | Quercetin-3-O-glucuronide 4 | 12.95 | 477.0702 | 301.0369 | 2.42 ± 0.01 a | 5.62 ± 0.03 b | 4.86 ± 0.02 c |
| 21 | Kaempferol 4 | 20.07 | 285.0405 | 5.15 ± 0.02 a | 1.63 ± 0.01 b | 9.35 ± 0.04 c | |
| 22 | kaempferol-3-O-glucuronide 4 | 14.22 | 461.0763 | 285.0412 | 0.48 ± 0.01 a | 1.31 ± 0.02 b | 3.24 ± 0.05 c |
| 23 | Isorhamnetin 4 | 20.31 | 315.0531 | 11.16 ± 0.12 a | 4.92 ± 0.05 b | 6.80 ± 0.07 c | |
| Phenolic acids | |||||||
| 24 | Gallic acid | 3.13 | 169.0193 | 120.87 ± 3.67 a | 121.19 ± 3.68 a | 96.11 ± 2.92 b | |
| 25 | Caffeic acid | 8.63 | 179.0401 | 3.27 ± 0.04 a | 2.18 ± 0.02 a | 1.09 ± 0.01 a | |
| 26 | Caffeic acid O-glucoside iso1 5 | 7.64 | 341.0878 | 179.0350 | 0.55 ± 0.02 a | 1.21 ± 0.05 a | 0.13 ± 0.01 a |
| 27 | Caffeic acid O-glucoside iso2 5 | 8.29 | 341.0878 | 179.0350 | 0.66 ± 0.03 a | 1.13 ± 0.05 a | 0.20 ± 0.01 a |
| 28 | p-Coumaric acid | 10.65 | 163.0439 | 3.44 ± 0.03 a | 3.73 ± 0.04 a | 1.15 ± 0.01 a | |
| 29 | 4-Hydroxybenzoic acid | 8.17 | 137.0243 | 1.67 ± 0.05 a | 0.89 ± 0.03 a | 1.19 ± 0.04 a | |
| 30 | Ferulic acid | 12.00 | 193.0506 | 0.75 ± 0.01 a | 0.27 ± 0.00 a | 0.51 ± 0.01 a | |
| 31 | Vanillic acid | 8.51 | 167.0350 | 2.33 ± 0.07 a | 2.15 ± 0.08 a | 2.67 ± 0.04 a | |
| Stilbenes | |||||||
| 32 | trans-Resveratrol 6 | 15.73 | 227.0714 | 4.60 ± 0.02 a | 3.63 ± 0.02 b | 3.12 ± 0.01 c | |
| 33 | Resveratrol iso16 | 18.00 | 227.0714 | 2.95 ± 0.01 a | 2.56 ± 0.01 b | 0.97 ± 0.00 c | |
| 34 | Resveratrol O-glucoside iso1 6 | 12.44 | 389.1242 | 227.0721 | 0.27 ± 0.00 a | 1.22 ± 0.02 b | 1.17 ± 0.02 b |
| 35 | Resveratrol O-glucoside iso2 6 | 14.92 | 389.1242 | 227.0721 | 1.35 ± 0.02 a | 6.01 ± 0.10 b | 3.32 ± 0.05 c |
| 36 | Piceatannol 6 | 2.59 | 243.0663 | 203.0727 | 4.20 ± 0.05 a | 4.96 ± 0.06 b | 4.04 ± 0.05 c |
| 37 | Piceatannol 3-O-glucoside iso1 6 | 12.89 | 405.1208 | 243.0670 | 0.22 ± 0.01 a | 0.14 ± 0.00 b | 0.04 ± 0.00 c |
| 38 | Piceatannol 3-O-glucoside iso2 6 | 13.15 | 405.1208 | 243.0670 | 0.06 ± 0.00 a | 0.18 ± 0.00 b | 0.05 ± 0.00 a |
| 39 | Viniferin-iso1 6 | 19.53 | 453.1344 | 116.9291 | 0.27 ± 0.01 a | 0.33 ± 0.01 a | 0.15 ± 0.00 b |
| 40 | Viniferin-iso2 6 | 19.92 | 453.1344 | 116.9291 | 0.81 ± 0.02 a | 1.05 ± 0.03 b | 0.73 ± 0.02 c |
| Anthocyanins | R.T. (min) | [M-H]+ | Fragment (m/z) | Cabernet WL (mg/L) | Mazuela WL (mg/L) | Garnacha WL (mg/L) | |
|---|---|---|---|---|---|---|---|
| 1 | Gallocatechin-Malvidin-3-glucoside dimer 1 | 3.58 | 797.2035 | 0.25 ± 0.01 a | 0.10 ± 0.00 a | 0.16 ± 0.00 a | |
| 2 | Malvidin-3-glucoside-(epi) catechin 1 | 4.84 | 781.1974 | 1.11 ± 0.01 a | 0.53 ± 0.00 b | 0.50 ± 0.00 b | |
| 3 | Delphinidin-3-glucoside 2 | 5.06 | 465.1028 | 303.0511 | 3.69 ± 0.04 a | 2.98 ± 0.03 b | 1.39 ± 0.01 c |
| 4 | Cyanidin-3-glucoside 2 | 5.85 | 449.1078 | 287.0531 | 0.23 ± 0.01 a | 0.20 ± 0.01 a | 0.16 ± 0.01 a |
| 5 | Petunidin-3-glucoside 3 | 6.47 | 479.1184 | 317.0669 | 5.03 ± 0.06 a | 4.90 ± 0.06 a | 2.18 ± 0.03 b |
| 6 | Petunidin-3-glucoside-pyruvic acid 3 | 7.05 | 547.1082 | 385.0547 | 0.09 ± 0.00 a | 0.06 ± 0.00 a | 0.03 ± 0.00 a |
| 7 | Peonidin-3-glucoside 3 | 7.14 | 463.1235 | 301.0717 | 2.72 ± 0.04 a | 1.83 ± 0.03 b | 2.48 ± 0.04 c |
| 8 | Malvidin-3-glucoside 1 | 7.48 | 493.1341 | 331.0843 | 60.67 ± 0.68 a | 43.90 ± 0.49 b | 26.78 ± 0.30 c |
| 9 | Peonidin-3-glucoside-pyruvic acid 3 | 7.81 | 531.1133 | 369.0607 | 0.04 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| 10 | Delphinidin-(6-acetyl)-3-glucoside 2 | 7.87 | 507.1133 | 303.0496 | 0.91 ± 0.02 a | 0.09 ± 0.00 b | 0.02 ± 0.00 b |
| 11 | Visitin A (malvidin-3-glucoside-pyruvic acid) 1 | 8.11 | 561.1239 | 399.0730 | 1.23 ± 0.01 a | 0.63 ± 0.01 b | 0.35 ± 0.00 c |
| 12 | Visitin B (malvidin-3-glucoside-acetaldehyde) 1 | 8.32 | 517.1341 | 355.0826 | 3.06 ± 0.08 a | 4.92 ± 0.12 b | 5.11 ± 0.00 b |
| 13 | Malvidin-3-glucoside-ethyl-(epi) catechin 1 | 8.40 | 809.2287 | 0.37 ± 0.00 a | 0.09 ± 0.00 b | 0.31 ± 0.13 a | |
| 14 | Cyanidin-(6-acetyl)-3-glucoside 2 | 8.45 | 491.1184 | 491.1189 | 0.20 ± 0.00 a | 0.02 ± 0.00 b | 0.01 ± 0.00 b |
| 15 | Acetylvisitin A 1 | 8.50 | 603.1344 | 399.0718 | 0.79 ± 0.03 a | 0.10 ± 0.00 b | 0.15 ± 0.00 b |
| 16 | Malvidin-3-glucoside-ethyl-(epi) catechin 1 | 8.57 | 809.2287 | 1.38 ± 0.02 a | 0.51 ± 0.01 b | 1.65 ± 0.00 c | |
| 17 | Petunidin-(6-acetyl)-3-glucoside 3 | 8.66 | 521.1378 | 317.0667 | 1.29 ± 0.04 a | 0.16 ± 0.01 b | 0.04 ± 0.02 b |
| 18 | Malvidin-3-glucoside-ethyl-(epi) catechin 1 | 8.75 | 809.2287 | 2.04 ± 0.06 a | 0.80 ± 0.03 b | 2.63 ± 0.00 c | |
| 19 | Acetylvisitin B 1 | 8.77 | 559.1446 | 355.0813 | 1.66 ± 0.05 a | 0.47 ± 0.01 b | 0.27 ± 0.08 b |
| 20 | Peonidin-(6-acetyl)-3-glucoside 3 | 9.08 | 505.1341 | 301.0714 | 1.32 ± 0.03 a | 0.13 ± 0.00 b | 0.08 ± 0.01 b |
| 21 | Delphinidin-(6-coumaroyl)-3-glucoside 2 | 9.08 | 611.1395 | 303.0508 | 0.44 ± 0.01 a | 0.55 ± 0.01 a | 0.09 ± 0.00 b |
| 22 | Malvidin-(6-acetyl)-3-glucoside 1 | 9.13 | 535.1446 | 331.0836 | 28.39 ± 0.03 a | 2.57 ± 0.00 b | 0.79 ± 0.00 c |
| 23 | Coumaroylvisitin A 1 | 9.29 | 707.1607 | 399.0718 | 0.20 ± 0.00 a | 0.13 ± 0.00 b | 0.04 ± 0.00 b |
| 24 | Malvidin-(6-caffeoyl)-3-glucoside 1 | 9.41 | 655.1657 | 331.0808 | 0.36 ± 0.02 a | 0.10 ± 0.00 b | 0.04 ± 0.00 b |
| 25 | Cyanidin-(6-coumaroyl)-3-glucoside 2 | 9.42 | 595.1446 | 287.0560 | 0.10 ± 0.00 a | 0.11 ± 0.00 a | 0.03 ± 0.00 b |
| 26 | Catechin-ethyl-Malvidin-3-acetylglucoside dimer 1 | 9.43 | 851.2511 | 0.88 ± 0.03 a | 0.03 ± 0.00 b | 0.06 ± 0.00 b | |
| 27 | Petunidin-(6-coumaroyl)-3-glucoside 3 | 9.52 | 625.1552 | 317.0662 | 0.74 ± 0.03 a | 0.78 ± 0.01 a | 0.16 ± 0.00 b |
| 28 | Pinotin A (malvidin-3-glucoside-vinylcatechol) 1 | 9.53 | 625.1552 | 463.0998 | 0.84 ± 0.02 a | 0.88 ± 0.02 a | 0.18 ± 0.00 b |
| 29 | Malvidin-glucoside-vinyl-catechin 1 | 9.56 | 805.1974 | 0.15 ± 0.00 a | 0.08 ± 0.00 b | 0.16 ± 0.00 a | |
| 30 | Coumaroylvisitin B 1 | 9.58 | 663.1708 | 355.0822 | 0.91 ± 0.03 a | 1.08 ± 0.04 b | 1.12 ± 0.04 b |
| 31 | Malvidin-3-glucoside-vinylguaiacol 1 | 9.63 | 639.1708 | 331.0823 | 0.59 ± 0.01 a | 0.37 ± 0.01 b | 0.17 ± 0.00 b |
| 32 | Catechin-ethyl-malvidin-3-coumaroylglucoside dimer 1 | 9.70 | 955.2785 | 0.68 ± 0.01 a | 0.21 ± 0.00 b | 0.51 ± 0.01 a | |
| 33 | Catechin-ethyl-malvidin-3-acetylglucoside dimer 1 | 9.81 | 851.2511 | 0.14 ± 0.00 a | 0.02 ± 0.00 b | 0.02 ± 0.00 b | |
| 34 | Peonidin-(6coumaroyl)-3-glucoside 3 | 9.87 | 609.1603 | 301.0716 | 0.94 ± 0.03 a | 0.60 ± 0.02 b | 0.42 ± 0.01 c |
| 35 | Malvidin-(6-coumaroyl)-3-glucoside 1 | 9.92 | 639.1708 | 331.0823 | 10.77 ± 0.02 a | 4.43 ± 0.01 b | 2.31 ± 0.01 c |
| 36 | Malvidin-glucoside-vinyl-catechin 1 | 9.99 | 805.1974 | 0.16 ± 0.00 a | 0.06 ± 0.00 b | 0.14 ± 0.00 a | |
| 37 | Acetyl-pinotin A 1 | 10.19 | 667.1657 | 0.01 ± 0.00 a | 0.00 ± 0.00 b | 0.01 ± 0.00 a | |
| 38 | Malvidin 3-O-glucoside 4-vinylphenol (Pigment A) 1 | 10.22 | 609.1603 | 447.1079 | 0.64 ± 0.01 a | 0.44 ± 0.00 b | 0.44 ± 0.00 b |
| 39 | Catechin-ethyl-malvidin-3-coumaroylglucoside dimer 1 | 10.33 | 955.2785 | 0.12 ± 0.00 a | 0.04 ± 0.00 b | 0.10 ± 0.00 a | |
| 40 | Malvidin acetyl 3-O-glucoside 4-vinylphenol (Acetyl-pigment A) 1 | 10.50 | 651.1708 | 447.1076 | 0.38 ± 0.01 a | 0.03 ± 0.00 b | 0.02 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Margalef, M.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile. Nutrients 2021, 13, 679. https://doi.org/10.3390/nu13020679
López-Fernández-Sobrino R, Soliz-Rueda JR, Margalef M, Arola-Arnal A, Suárez M, Bravo FI, Muguerza B. ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile. Nutrients. 2021; 13(2):679. https://doi.org/10.3390/nu13020679
Chicago/Turabian StyleLópez-Fernández-Sobrino, Raúl, Jorge R. Soliz-Rueda, Maria Margalef, Anna Arola-Arnal, Manuel Suárez, Francisca I. Bravo, and Begoña Muguerza. 2021. "ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile" Nutrients 13, no. 2: 679. https://doi.org/10.3390/nu13020679
APA StyleLópez-Fernández-Sobrino, R., Soliz-Rueda, J. R., Margalef, M., Arola-Arnal, A., Suárez, M., Bravo, F. I., & Muguerza, B. (2021). ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile. Nutrients, 13(2), 679. https://doi.org/10.3390/nu13020679












