Impact of Diet on Symptoms of the Irritable Bowel Syndrome
Abstract
1. Prevalence and Key Features
2. Patterns of Pain and Link to Food
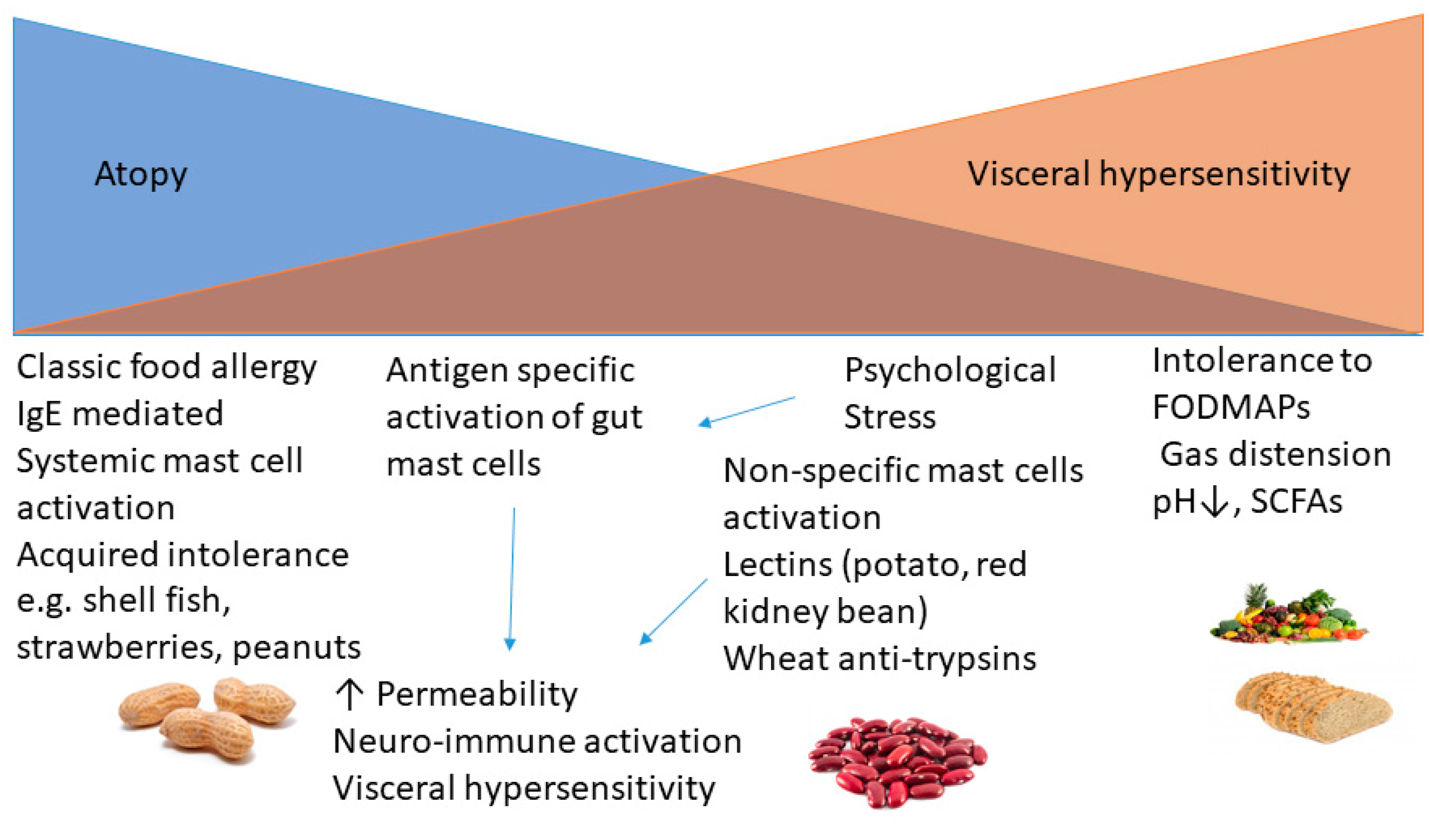
3. Immune and Non-Immune Mechanisms Underlying Postprandial Symptoms
4. Role of Mast Cells in IBS
5. Impact of Colonic Fermentation on Gut Symptoms
6. Clinical Approach
7. Eating Disorders
8. Fibre Supplements
9. Exclusion Diets: Principles and Practice
Elimination Diets
10. Exclusion Diets Based on Immunological Tests
11. Lactose and Fructose Restriction
12. NICE Diet
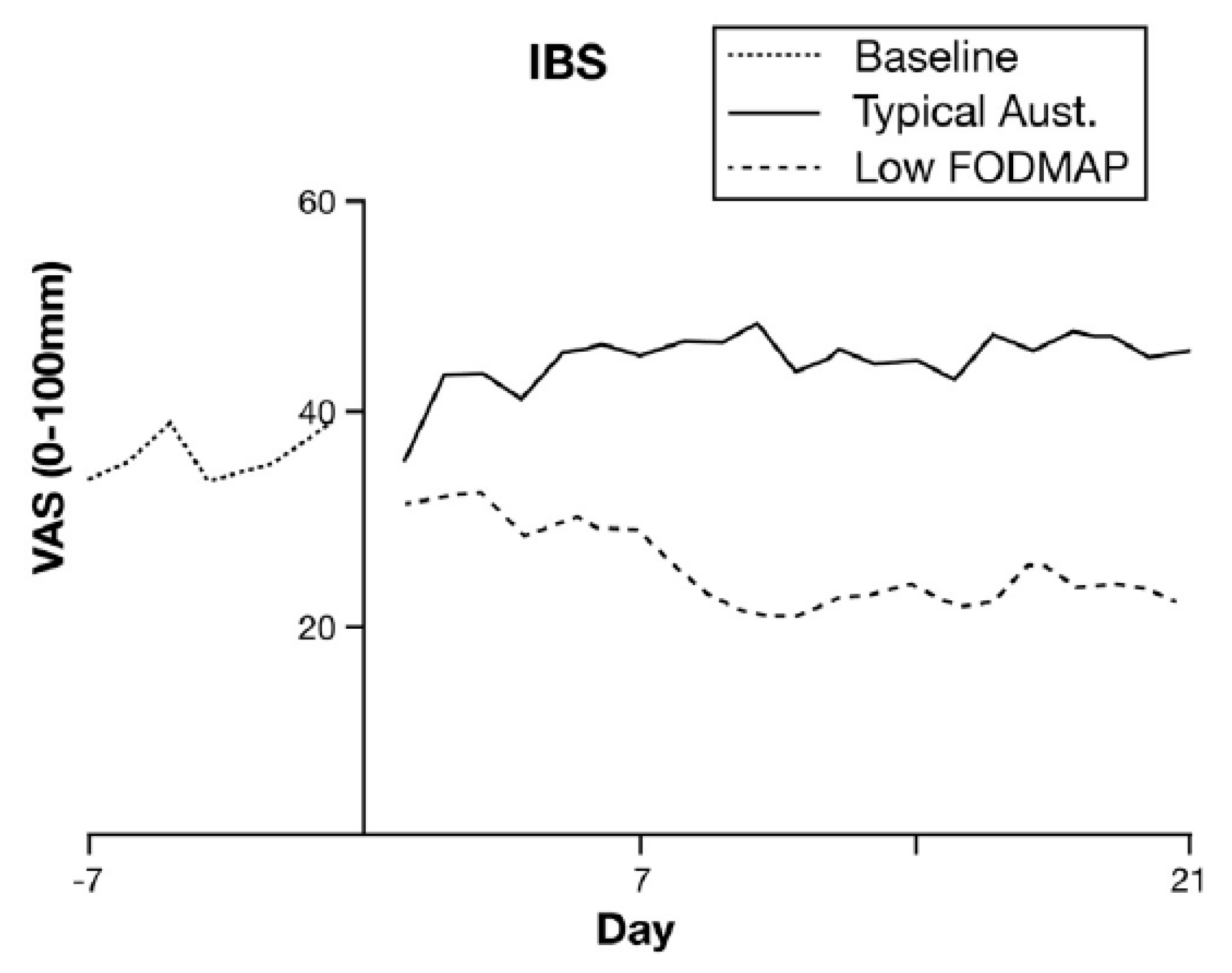
13. Low FODMAP Diet
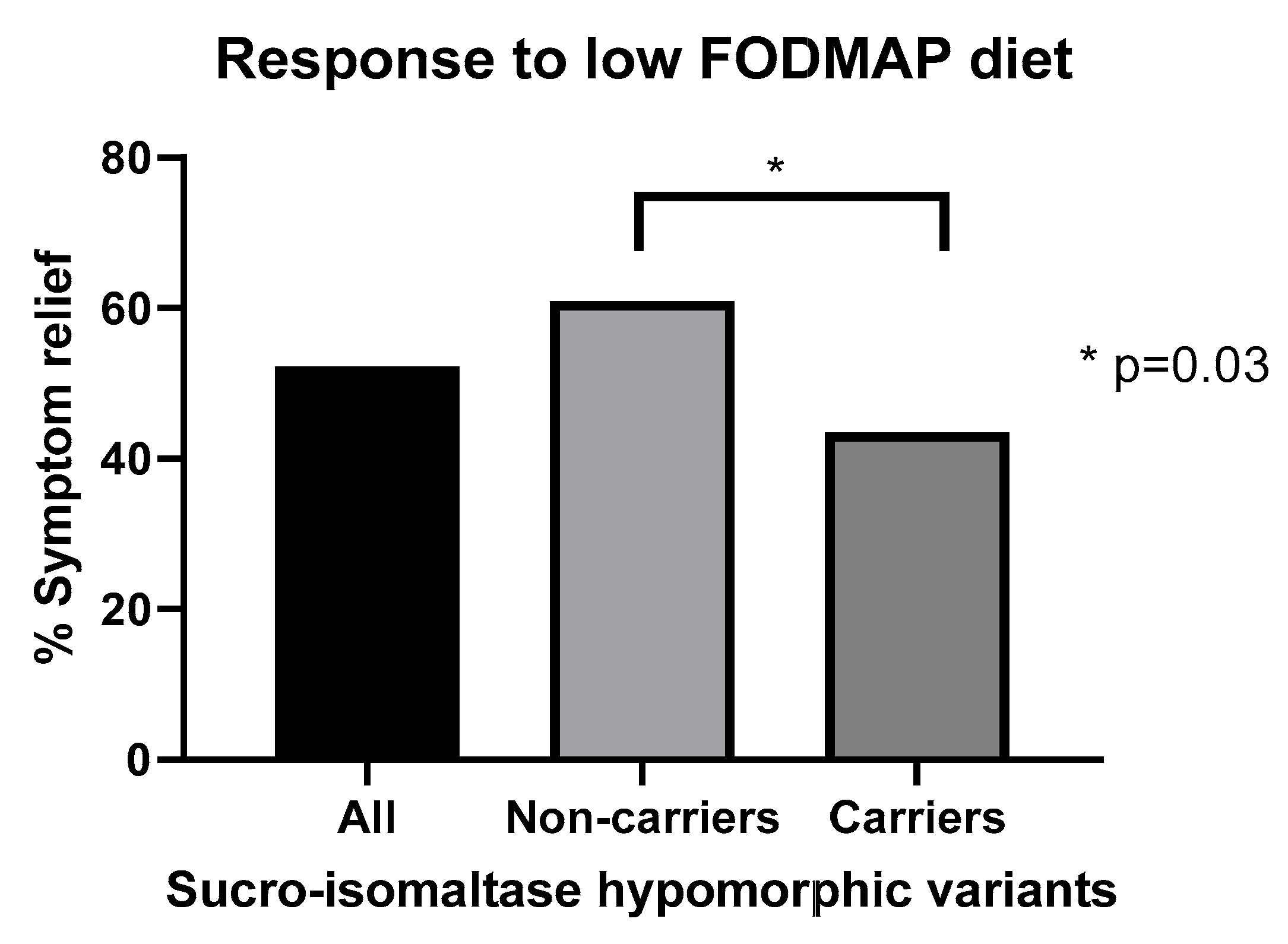
14. Predicting Response to Low FODMAP Diet
15. Impact on Microbiome
16. Wheat and Gluten-Free Diets
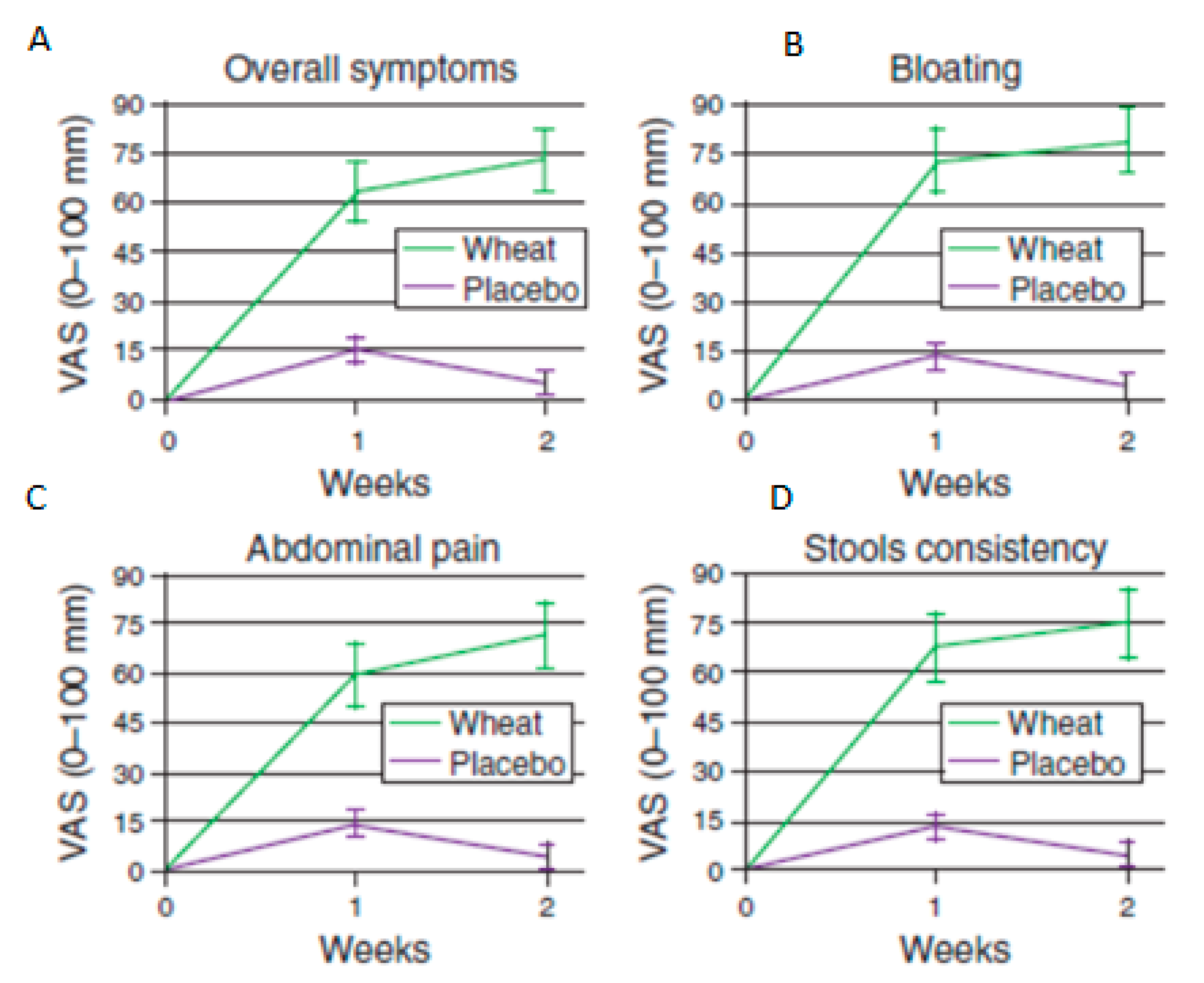
17. Non-Coeliac Wheat Sensitivity
18. Low-Histamine Diets
19. Laxative Effects of Fruit and Vegetables
20. Conclusion and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talley, N.J.; Zinsmeister, A.R.; Van Dyke, C.; Melton, L. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenteroligy 1991, 101, 927–934. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Ford, A.C.; Avila, C.A.; Verdu, E.F.; Collins, S.M.; Morgan, D.G.; Moayyedi, P.; Bercik, P. Anxiety and Depression Increase in a Stepwise Manner in Parallel with Multiple FGIDs and Symptom Severity and Frequency. Am. J. Gastroenterol. 2015, 110, 1038–1048. [Google Scholar] [CrossRef]
- Midenfjord, I.; Polster, A.V.; Sjövall, H.; Törnblom, H.; Simrén, M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol. Motil. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Simren, M.; Törnblom, H.; Palsson, O.S.; van Tilburg, M.A.L.; Van Oudenhove, L.; Tack, J.; Whitehead, W.E. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: Consistent findings from five different patient cohorts. Gut 2017, 67, 255–262. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Polster, A.V.; Palsson, O.S.; Törnblom, H.; Öhman, L.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. Subgroups of IBS patients are characterized by specific, reproducible profiles of GI and non-GI symptoms and report differences in healthcare utilization: A population-based study. Neurogastroenterol. Motil. 2019, 31, e13483. [Google Scholar] [CrossRef]
- Pimentel, M.; Hwang, L.; Melmed, G.Y.; Low, K.; Vasiliauskas, E.; Ippoliti, A.; Yang, J.; Lezcano, S.; Conklin, J.L.; Sahakian, A. New Clinical Method for Distinguishing D-IBS from Other Gastrointestinal Conditions Causing Diarrhea: The LA/IBS Diagnostic Strategy. Dig. Dis. Sci. 2009, 55, 145–149. [Google Scholar] [CrossRef]
- Palsson, O.S.; Baggish, J.S.; Turner, M.J.; Whitehead, W.E. IBS Patients Show Frequent Fluctuations between Loose/Watery and Hard/Lumpy Stools: Implications for Treatment. Am. J. Gastroenterol. 2012, 107, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Baggish, J.; Whitehead, W.E. Episodic Nature of Symptoms in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2014, 109, 1450–1460. [Google Scholar] [CrossRef]
- Bampton, P.A.; Dinning, P.G.; Kennedy, M.L.; Lubowski, D.Z.; DeCarle, D.; Cook, I.J. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am. J. Gastroenterol. 2000, 95, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Battaglia, E.; De Roberto, G.; Morelli, A.; Tonini, M.; Villanacci, V. Alterations in colonic motility and relationship to pain in colonic diverticulosis. Clin. Gastroenterol. Hepatol. 2005, 3, 248–253. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Kavelock, R.; Beaty, J.; Ackerson, K.; Stumbo, P. Effects of fat and carbohydrate meals on colonic motor response. Gut 2000, 46, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Narducci, F.; Bassotti, G.; Gaburri, M.; Morelli, A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut 1987, 28, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, G.; Bodemar, G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients’ description of diarrhoea, constipation and symptom variation during a prospective 6-week study. Eur. J. Gastroenterol. Hepatol. 1998, 10, 415–422. [Google Scholar] [CrossRef]
- Miwa, H. Life style in persons with functional gastrointestinal disorders—Large-scale internet survey of lifestyle in Japan. Neurogastroenterol. Motil. 2012, 24, 464–471. [Google Scholar] [CrossRef]
- Shah, E.D.; Almario, C.V.; Spiegel, B.M.; Chey, W.D. Presentation and Characteristics of Abdominal Pain Vary by Irritable Bowel Syndrome Subtype: Results of a Nationwide Population-Based Study. Am. J. Gastroenterol. 2020, 115, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Weinland, S.R.; Morris, C.B.; Hu, Y.; Leserman, J.; Bangdiwala, S.I.; Drossman, D.A. Characterization of Episodes of Irritable Bowel Syndrome Using Ecological Momentary Assessment. Am. J. Gastroenterol. 2011, 106, 1813–1820. [Google Scholar] [CrossRef]
- Turnbull, J.L.; Adams, H.N.; Gorard, D.A. Review article: The diagnosis and management of food allergy and food intolerances. Aliment. Pharmacol. Ther. 2014, 41, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives meal-induced abdominal pain. Nat. Cell Biol. 2021, 1–6. [Google Scholar] [CrossRef]
- Santos, J.; Saperas, E.; Nogueiras, C.; Mourelle, M.; Antolín, M.; Cadahia, A.; Malagelada, J. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 1998, 114, 640–648. [Google Scholar] [CrossRef]
- Pramod, S.N.; Venkatesh, Y.P.; Mahesh, P.A. Potato lectin activates basophils and mast cells of atopic subjects by its interaction with core chitobiose of cell-bound non-specific immunoglobulin E. Clin. Exp. Immunol. 2007, 148, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Sun, J.; Schreiber, R.; König, J. Effects of dietary lectins on ion transport in epithelia. Br. J. Pharmacol. 2004, 142, 1219–1226. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology 2019, 157, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, N.G. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef]
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019, 68, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Burta, O.; Tiuca, N.; Petrisor, D.C.; Lenghel, A.; Santos, J. Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial. United Eur. Gastroenterol. J. 2019, 7, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Walker, M.M.; Ford, A.C.; Talley, N.J. The overlap of atopy and functional gastrointestinal disorders among 23 471 patients in primary care. Aliment. Pharmacol. Ther. 2014, 40, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Vivinus-Nébot, M.; Dainese, R.; Anty, R.; Saint-Paul, M.C.; Nano, J.L.; Gonthier, N.; Marjoux, S.; Frin-Mathy, G.; Bernard, G.; Hébuterne, X.; et al. Combination of Allergic Factors Can Worsen Diarrheic Irritable Bowel Syndrome: Role of Barrier Defects and Mast Cells. Am. J. Gastroenterol. 2012, 107, 75–81. [Google Scholar] [CrossRef]
- Bashashati, M.; Moossavi, S.; Cremon, C.; Barbaro, M.R.; Moraveji, S.; Talmon, G.; Rezaei, N.; Hughes, P.A.; Bian, Z.-X.; Choi, C.H.; et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2017, 30, e13192. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.; Garsed, K.; Singh, G.; Duroudier, N.P.; Swan, C.; Hall, I.P.; Zaitoun, A.; Bennett, A.; Marsden, C.; Holmes, G.; et al. Impaired Uptake of Serotonin by Platelets From Patients With Irritable Bowel Syndrome Correlates With Duodenal Immune Activation. Gastroenterology 2011, 140, 1434–1443. [Google Scholar] [CrossRef]
- Walker, M.M.; Talley, N.J.; Prabhakar, M.; Pennaneac’h, C.J.; Aro, P.; Ronkainen, J.; Storskrubb, T.; Harmsen, W.S.; Zinsmeister, A.R.; Agreus, L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment. Pharmacol. Ther. 2009, 29, 765–773. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef]
- Barbara, G.; Wang, B.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.L.; Costigan, C.; Cox, E.F.; Lam, C.; Marciani, L.; Gowland, P.A.; Spiller, R.C. Differential Effects of FODMAPs (Fermentable Oligo-, Di-, Mono-Saccharides and Polyols) on Small and Large Intestinal Contents in Healthy Subjects Shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Lam, C.; Rehman, S.; Marciani, L.; Costigan, C.; Hoad, C.L.; Lingaya, M.R.; Banwait, R.; Bawden, S.; Gowland, P.A.; et al. Corticotropin-releasing factor increases ascending colon volume after a fructose test meal in healthy humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Pritchard, S.E.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.A.; Spiller, R.C. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals with Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Christie, S.; Cole, T.J. A study of fructo oligosaccharides in the prevention of travellers’ diarrhoea. Aliment. Pharmacol. Ther. 2001, 15, 1139–1145. [Google Scholar] [CrossRef]
- Cloetens, L.; Broekaert, W.F.; Delaedt, Y.; Ollevier, F.; Courtin, C.M.; Delcour, J.A.; Rutgeerts, P.; Verbeke, K. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: A randomised, placebo-controlled cross-over study. Br. J. Nutr. 2009, 103, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C. In Vitro Fermentation Profiles, Gas Production Rates, and Microbiota Modulation as Affected by Certain Fructans, Galactooligosaccharides, and Polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef]
- Chen, B.-R.; Du, L.-J.; He, H.-Q.; Kim, J.J.; Zhao, Y.; Zhang, Y.-W.; Luo, L.; Dai, N. Fructo-oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress-induced irritable bowel syndrome mouse model. World J. Gastroenterol. 2017, 23, 8321–8333. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2016, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.-I.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Washington, N.; Harris, M.; Mussellwhite, A.; Spiller, R.C. Moderation of lactulose-induced diarrhea by psyllium: Effects on motility and fermentation. Am. J. Clin. Nutr. 1998, 67, 317–321. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Østgaard, H.; Hausken, T.; Gundersen, D. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol. Med. Rep. 2012, 5, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome—Etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2005, 60, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef]
- Mego, M.; Accarino, A.; Malagelada, J.-R.; Guarner, F.; Azpiroz, F. Accumulative effect of food residues on intestinal gas production. Neurogastroenterol. Motil. 2015, 27, 1621–1628. [Google Scholar] [CrossRef]
- Brown, S.R.; Cann, P.A.; Read, N.W. Effect of coffee on distal colon function. Gut 1990, 31, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Welcher, K.; Zimmerman, B.; Stumbo, P. Is coffee a colonic stimulant? Eur. J. Gastroenterol. Hepatol. 1998, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, H.L.; Edwards, B.A.; Curran, J.F.; Boyce, S. Coffee to go? The effect of coffee on resolution of ileus following abdominal surgery: A systematic review and meta-analysis of randomised controlled trials. Clin. Nutr. 2020, 39, 1385–1394. [Google Scholar] [CrossRef]
- Boyd, C.; Abraham, S.; Kellow, J. Psychological features are important predictors of functional gastrointestinal disorders in patients with eating disorders. Scand. J. Gastroenterol. 2005, 40, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; Linde, J.A.; Feld, K.A.; Crowell, M.D.; Jeffery, R.W.; Linde, J.; Feld, K. The Association of Gastrointestinal Symptoms with Weight, Diet, and Exercise in Weight-Loss Program Participants. Clin. Gastroenterol. Hepatol. 2005, 3, 992–996. [Google Scholar] [CrossRef]
- Hussein, M.O.; Hoad, C.L.; Wright, J.; Singh, G.; Stephenson, M.C.; Cox, E.F.; Placidi, E.; Pritchard, S.E.; Costigan, C.; Ribeiro, H.; et al. Fat Emulsion Intragastric Stability and Droplet Size Modulate Gastrointestinal Responses and Subsequent Food Intake in Young Adults. J. Nutr. 2015, 145, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Sadik, R.; Björnsson, E.; Simrén, M. The relationship between symptoms, body mass index, gastrointestinal transit and stool frequency in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2010, 22, 102–108. [Google Scholar] [CrossRef]
- Dukas, L.; Willett, W.C.; Giovannucci, E.L. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am. J. Gastroenterol. 2003, 98, 1790–1796. [Google Scholar] [CrossRef]
- Moayyedi, P.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Ford, A.C. The Effect of Fiber Supplementation on Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014, 109, 1367–1374. [Google Scholar] [CrossRef]
- Nagarajan, N.; Morden, A.; Bischof, D.; King, E.A.; Kosztowski, M.; Wick, E.C.; Stein, E.M. The role of fiber supplementation in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, C.J.; De Wit, N.J.; Muris, J.W.M.; Whorwell, P.J.; Knottnerus, J.A.; Hoes, A.W. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009, 339, b3154. [Google Scholar] [CrossRef]
- Snook, J.; Shepherd, H.A. Bran supplementation in the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 1994, 8, 511–514. [Google Scholar] [CrossRef]
- Parker, T.J.; Naylor, S.J.; Riordan, A.M.; Hunter, J.O. Management of patients with food intolerance in irritable bowel syndrome: The development and use of an exclusion diet. J. Human Nutr. Diet. 1995, 8, 159–166. [Google Scholar] [CrossRef]
- Nanda, R.; James, R.; Smith, H.; Dudley, C.R.; Jewell, D.P. Food intolerance and the irritable bowel syndrome. Gut 1989, 30, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated With More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Herrmann, A.; Manns, M.P. Prevalence of adverse reactions to food in patients with gastrointestinal disease. Allergy 1996, 51, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Zar, S.; Benson, M.J.; Kumar, D. Food-Specific Serum IgG4 and IgE Titers to Common Food Antigens in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2005, 100, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Ligaarden, S.C.; Lydersen, S.; Farup, P.G. IgG and IgG4 antibodies in subjects with irritable bowel syndrome: A case control study in the general population. BMC Gastroenterol. 2012, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, W.; Sheldon, T.A.; Shaath, N.; Whorwell, P.J. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomised controlled trial. Gut 2004, 53, 1459–1464. [Google Scholar] [CrossRef]
- Christopher, N.L.; Bayless, T.M. Role of the Small Bowel and Colon in Lactose-Induced Diarrhea. Gastroenterology 1971, 60, 845–852. [Google Scholar] [CrossRef]
- Ladas, S.; Papanikos, J.; Arapakis, G. Lactose malabsorption in Greek adults: Correlation of small bowel transit time with the severity of lactose intolerance. Gut 1982, 23, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Savaiano, D.A.; Levitt, M.D. Fecal hydrogen production and consumption measurements. Response to daily lactose ingestion by lactose maldigesters. Dig. Dis. Sci. 1997, 42, 348–353. [Google Scholar] [CrossRef]
- He, T.; Priebe, M.G.; Harmsen, H.J.; Stellaard, F.; Sun, X.; Welling, G.W.; Vonk, R.J. Colonic Fermentation May Play a Role in Lactose Intolerance in Humans. J. Nutr. 2006, 136, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, Y.; Chu, H.; Cong, Y.; Zhao, J.; Pohl, D.; Misselwitz, B.; Fried, M.; Dai, N.; Fox, M. Prevalence and Presentation of Lactose Intolerance and Effects on Dairy Product Intake in Healthy Subjects and Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fox, M.; Cong, Y.; Chu, H.; Zheng, X.; Long, Y.; Fried, M.; Dai, N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: The roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment. Pharmacol. Ther. 2013, 39, 302–311. [Google Scholar] [CrossRef]
- Suarez, F.L.; Savaiano, D.A.; Levitt, M.D. A Comparison of Symptoms after the Consumption of Milk or Lactose-Hydrolyzed Milk by People with Self-Reported Severe Lactose Intolerance. New Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.J.; Woolner, J.T.; Prevost, A.T.; Tuffnell, Q.; Shorthouse, M.; Hunter, J.O. Irritable bowel syndrome: Is the search for lactose intolerance justified? Eur. J. Gastroenterol. Hepatol. 2001, 13, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mishkin, S.R.; Sablauskas, L.; Mishkin, S. Fructose and/or sorbitol intolerance in a subgroup of lactose intolerant patients. Can. J. Gastroenterol. 1997, 8, 389–393. [Google Scholar] [CrossRef]
- Ledochowski, M.; Widner, B.; Bair, H.; Probst, T.; Fuchs, D. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand. J. Gastroenterol. 2000, 35, 1048–1052. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kraft, N.; Zimmerman, B.; Jackson, M.; Rao, S.S. Fructose Intolerance in IBS and Utility of Fructose-Restricted Diet. J. Clin. Gastroenterol. 2008, 42, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Stróżyk, A.; Horvath, A.; Meyer, R.; Szajewska, H. Efficacy and safety of hydrolyzed formulas for cow’s milk allergy management: A systematic review of randomized controlled trials. Clin. Exp. Allergy 2020, 50, 766–779. [Google Scholar] [CrossRef]
- Turpeinen, A.; Kautiainen, H.; Tikkanen, M.-L.; Sibakov, T.; Tossavainen, O.; Myllyluoma, E. Mild protein hydrolysation of lactose-free milk further reduces milk-related gastrointestinal symptoms. J. Dairy Res. 2016, 83, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, R.; Salmenkari, H.; Sibakov, T.; Vapaatalo, H.; Turpeinen, A. Randomised Controlled Trial: Partial Hydrolysation of Casein Protein in Milk Decreases Gastrointestinal Symptoms in Subjects with Functional Gastrointestinal Disorders. Nutrients 2020, 12, 2140. [Google Scholar] [CrossRef]
- Kristjánsson, G.; Venge, P.; Hällgren, R. Mucosal reactivity to cow’s milk protein in coeliac disease. Clin. Exp. Immunol. 2007, 147, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, J.; Bullock, I. Diagnosis and management of irritable bowel syndrome in adults in primary care: Summary of NICE guidance. BMJ 2008, 336, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.M.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. Review article: FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment. Pharmacol. Ther. 2020, 52, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPS Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Hosadurg, D.; Martin, L.; Zarate-Lopez, N.; Passananti, V.; Emmanuel, A. Adherence with a low-FODMAP diet in irritable bowel syndrome: Are eating disorders the missing link? Eur. J. Gastroenterol. Hepatol. 2019, 31, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; De Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A Low-FODMAP Diet for Irritable Bowel Syndrome: Some Answers to the Doubts from a Long-Term Follow-Up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Cope, J.L.; Hollister, E.B.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Versalovic, J.; Shulman, R.J. Randomised clinical trial: Gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 42, 418–427. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile Organic Compounds in Feces Associate With Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Olesen, S.S.; Materna, A.; Drewes, A.M. Predictors of response to a low-FODMAP diet in patients with functional gastrointestinal disorders and lactose or fructose intolerance. Aliment. Pharmacol. Ther. 2017, 45, 1094–1106. [Google Scholar] [CrossRef]
- Zheng, T.; Eswaran, S.; Photenhauer, A.L.; Merchant, J.L.; Chey, W.D.; D’Amato, M. Reduced efficacy of low FODMAPs diet in patients with IBS-D carrying sucrase-isomaltase (SI) hypomorphic variants. Gut 2020, 69, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Abbeele, P.V.D.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.-M.; Van De Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Rossi, M.; Corradini, C.; Amaretti, A.; Nicolini, M.; Pompei, A.; Zanoni, S.; Matteuzzi, D. Fermentation of Fructooligosaccharides and Inulin by Bifidobacteria: A Comparative Study of Pure and Fecal Cultures. Appl. Environ. Microbiol. 2005, 71, 6150–6158. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Composition and Metabolic Activities of Bacterial Biofilms Colonizing Food Residues in the Human Gut. Appl. Environ. Microbiol. 2006, 72, 6204–6211. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Efficacy of an Encapsulated Probiotic Bifidobacterium infantis 35624 in Women with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Bruggencate, S.J.M.T.; Bovee-Oudenhoven, I.M.J.; Lettink-Wissink, M.L.G.; Katan, M.B.; Van Der Meer, R. Dietary Fructooligosaccharides Affect Intestinal Barrier Function in Healthy Men. J. Nutr. 2006, 136, 70–74. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- O’Leary, C.; Wieneke, P.; Buckley, S.; O’Regan, P.; Cronin, C.C.; Quigley, E.M.; Shanahan, F. Celiac disease and irritable bowel-type symptoms. Am. J. Gastroenterol. 2002, 97, 1463–1467. [Google Scholar] [CrossRef]
- Sanders, D.S.; Carter, M.J.; Hurlstone, D.P.; Pearce, A.; Ward, A.M.; McAlindon, M.E.; Lobo, A.J. Association of adult coeliac disease with irritable bowel syndrome: A case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet 2001, 358, 1504–1508. [Google Scholar] [CrossRef]
- Lewis, N.R.; Scott, B.B. Meta-analysis: Deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment. Pharmacol. Ther. 2010, 31, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-Celiac Wheat Sensitivity Diagnosed by Double-Blind Placebo-Controlled Challenge: Exploring a New Clinical Entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef]
- Wahnschaffe, U.; Ullrich, R.; Riecken, E.; Schulzke, J. Celiac disease–like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology 2001, 121, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, U.; Schulzke, J.; Zeitz, M.; Ullrich, R. Predictors of Clinical Response to Gluten-Free Diet in Patients Diagnosed with Diarrhea-Predominant Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2007, 5, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Vazquez–Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A Controlled Trial of Gluten-Free Diet in Patients with Irritable Bowel Syndrome-Diarrhea: Effects on Bowel Frequency and Intestinal Function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten Causes Gastrointestinal Symptoms in Subjects Without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients with Self-Reported Non-Celiac Gluten Sensitivity After Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E. Fructan, Rather Than Gluten, Induces Symptoms in Patients with Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Kanny, G.; Bauza, T.; Frémont, S.; Guillemin, F.; Blaise, A.; Daumas, F.; Cabanis, J.C.; Nicolas, J.P.; Moneret-Vautrin, D.A. Histamine content does not influence the tolerance of wine in normal subjects. Allerg. Immunol. 1999, 31, 45–48. [Google Scholar] [CrossRef]
- Komericki, P.; Klein, G.; Reider, N.; Hawranek, T.; Strimitzer, T.; Lang, R.; Kranzelbinder, B.; Aberer, W. Histamine intolerance: Lack of reproducibility of single symptoms by oral provocation with histamine: A randomised, double-blind, placebo-controlled cross-over study. Wien. Klin. Wochenschr. 2011, 123, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schäfer, C.; et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J. Int. 2017, 26, 72–79. [Google Scholar] [CrossRef]
- Wilkinson-Smith, V.; Major, G.; Ashleigh, L.; Murray, K.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. In vivo assessment of foods that stimulate intestinal secretions using magnetic resonance imaging: Implications for dietary advice in ileostomy care. Neurogastroenterol. Motil. 2017, 29, 40. [Google Scholar]
- Thomson, T.J.; Runcie, J.; Khan, A. The effect of diet on ileostomy function. Gut 1970, 11, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, S.; Lomer, M.; Marciani, L.; Hoad, C.L.; Pritchard, S.; Paul, J.; Gowland, P.A.; Spiller, R. Processing Apples to Puree or Juice Speeds Gastric Emptying and Reduces Postprandial Intestinal Volumes and Satiety in Healthy Adults. J. Nutr. 2020, 150, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Smith, V.; Dellschaft, N.; Ansell, J.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers. Aliment. Pharmacol. Ther. 2019, 49, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.; Butts, C.A.; Paturi, G.; Eady, S.L.; Wallace, A.J.; Hedderley, D.; Gearry, R.B. Kiwifruit-derived supplements increase stool frequency in healthy adults: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2015, 35, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.O. Increasing dietary fiber intake in terms of kiwifruit improves constipation in Chinese patients. World J. Gastroenterol. 2007, 13, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Lin, Y.-T.; Lu, Y.-T.; Liu, Y.-S.; Liu, J.-F. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac. J. Clin. Nutr. 2010, 19, 451–457. [Google Scholar] [PubMed]
| Onions | 35% |
| Milk | 32% |
| Wheat | 30% |
| Chocolate | 28% |
| Butter | 25% |
| Yoghurt | 25% |
| Coffee | 24% |
| Eggs | 23% |
| Nuts | 18% |
| Citrus | 18% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiller, R. Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients 2021, 13, 575. https://doi.org/10.3390/nu13020575
Spiller R. Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients. 2021; 13(2):575. https://doi.org/10.3390/nu13020575
Chicago/Turabian StyleSpiller, Robin. 2021. "Impact of Diet on Symptoms of the Irritable Bowel Syndrome" Nutrients 13, no. 2: 575. https://doi.org/10.3390/nu13020575
APA StyleSpiller, R. (2021). Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients, 13(2), 575. https://doi.org/10.3390/nu13020575






