Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism
Abstract
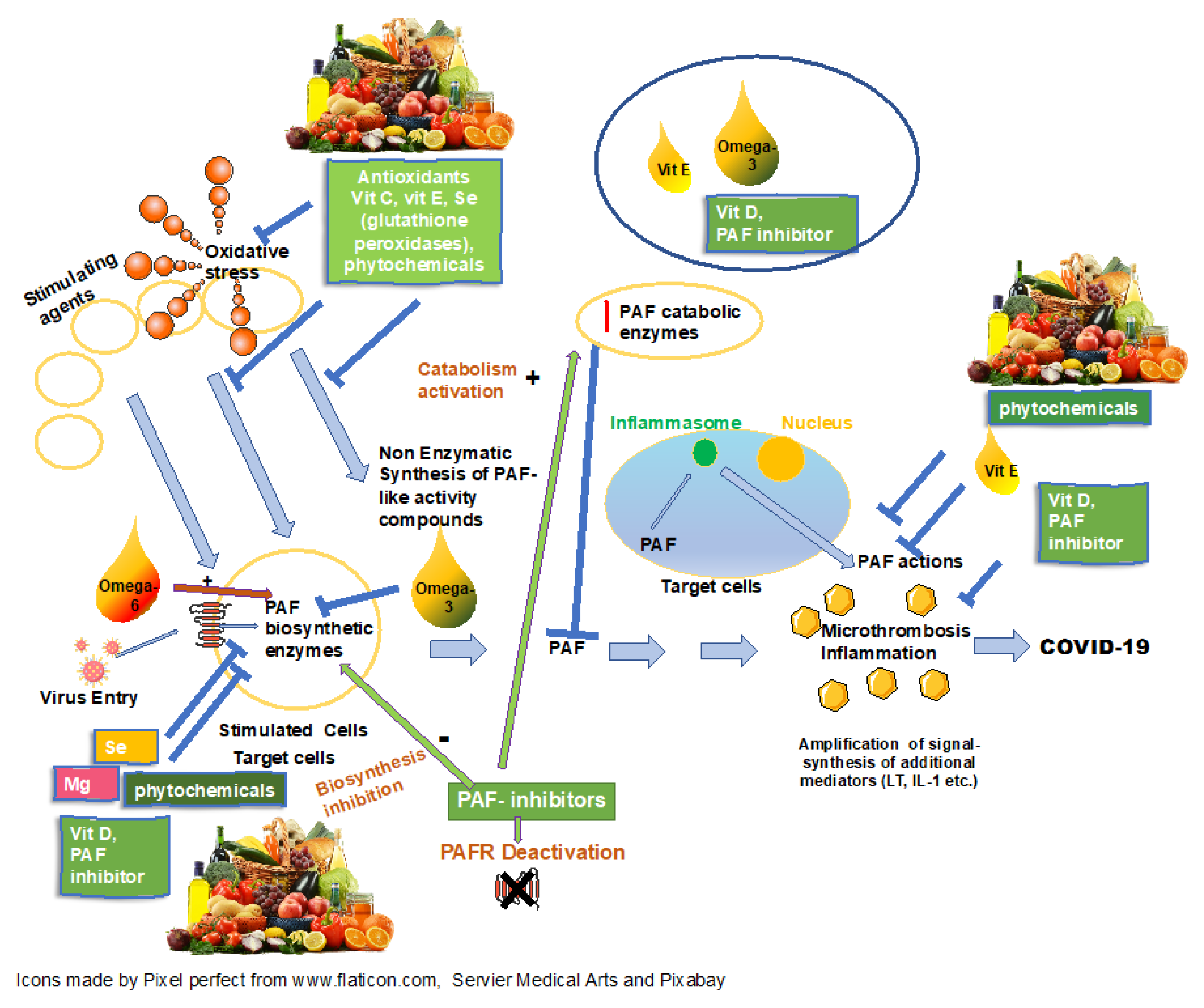
1. Introduction
2. Micronutrients, COVID-19 and PAF
2.1. Vitamin A
2.2. Vitamin C
2.3. Vitamin D
2.4. Vitamin E
2.5. Selenium
2.6. Omega-3 Fatty Acids
2.7. Zinc, Copper, Magnesium and Iron
2.8. Phytochemicals
3. Mediterranean Diet, Mediterranean Foods, COVID-19 and PAF
3.1. Olive Oil
3.2. Fish
3.3. Honey
3.4. Milk and Yogurt
3.5. Plant Foods
3.6. Wine and Its Products
4. Data from Clinical Trials
5. Hypothesis versus Epidemiological Data
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAF | platelet-activating factor |
| PAF-CPT | dithiothreitol-insensitive cholinephosphotransferase |
| CDP-choline | cytidine diphosphate-choline |
| Lp-PLA2 | lipoprotein associated phospholipase A2 |
| ACE2 | angiotensin converting enzyme 2 |
| TLR | Toll-like receptor |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| CRP | C-reactive protein |
| ALA | alpha-linolenic acid |
| AT | acetyltransferase |
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Demopoulos, C.; Antonopoulou, S.; Theocharides, T. CoVid-19, microthromboses, inflammation and platelet activating factor (PAF). Biofactors 2002, 46, 927–933. [Google Scholar]
- Zhao, X.; Li, Y.; Ge, Y.; Shi, Y.; Lv, P.; Zhang, J.; Fu, G.; Zhou, Y.; Jiang, K.; Lin, N.; et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically Ill COVID-19 Patients. J. Parenter. Enter. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- British Dietetic Association. COVID-19/Coronavirus—Advice for the General Public. 2020. Available online: https://www.bda.uk.com/resource/covid-19-corona-virus-advice-for-the-general-public.html (accessed on 14 April 2020).
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef] [PubMed]
- Cintoni, M.; Rinninella, E.; Annetta, M.G.; Mele, M.C. Nutritional management in hospital setting during SARS-CoV-2 pandemic: A real-life experience. Eur. J. Clin. Nutr. 2020, 74, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 1–2. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Maintaining a Healthy Diet during the COVID-19 Pandemic. 2020. Available online: http://www.fao.org/3/ca8380en/CA8380EN.pdf (accessed on 27 January 2021).
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The role of platelet-activating factor in chronic inflammation, immune activation, and comorbidities associated with hiv infection. Aids Rev. 2015, 17, 191–201. [Google Scholar]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation link and the role of nutrition in potential mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean diet and platelet-activating factor; a systematic review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Thrombosis and COVID-19: The Potential role of nutrition. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Antonopoulou, S.; Demopoulos, C.A. Coronavirus 2019, Microthromboses, and platelet activating factor. Clin. Ther. 2020, 42, 1850–1852. [Google Scholar] [CrossRef] [PubMed]
- Demopoulos, C.A.; Pinckard, R.N.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Nookala, V. Biochemistry, Platelet activating factor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty years since the structural elucidation of platelet-activating factor (PAF): Historical, Current, and future research perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Chrysohoou, C.; Antonopoulou, S. Platelet activating factor in heart failure: Potential role in disease progression and novel target for therapy. Curr. Heart Fail. Rep. 2013, 10, 122–129. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Patel, S.; Kulchitsky, V.A.; Romanovsky, A.A. Platelet-Activating factor: A Previously unrecognized mediator of fever. J. Physiol. 2003, 553, 221–228. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Sawa, H.; Yasuda, H. Mechanism of increased angiotensin-converting enzyme activity stimulated by platelet-activating factor. Biochim. Biophys. Acta Bioenerg. 1990, 1052, 503–508. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef]
- Yan, B.; Chu, H.; Yang, D.; Sze, K.-H.; Lai, P.-M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.T.; Chan, J.F.-W.; et al. Characterization of the Lipidomic profile of human coronavirus-infected cells: Implications for Lipid metabolism remodeling upon coronavirus replication. Viruses 2019, 11, 73. [Google Scholar] [CrossRef]
- Marathe, G. Oxidized LDL Contains inflammatory PAF-Like phospholipids. Trends Cardiovasc. Med. 2001, 11, 139–142. [Google Scholar] [CrossRef]
- Liapikos, T.A.; Antonopoulou, S.; Karabina, S.-A.P.; Tsoukatos, D.C.A.; Demopoulos, C.A.; Tselepis, A.D. Platelet-activating factor formation during oxidative modification of low-density lipoprotein when PAF-acetylhydrolase has been inactivated. Biochim. Biophys. Acta Lipids Lipid Metab. 1994, 1212, 353–360. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.C.; Wang, H.; et al. Identification of Oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zhang, J.; Zhong, J.; Zheng, Y. Elevated Levels of platelet activating factor and its acetylhydrolase indicate high risk of kawasaki disease. J. Interferon Cytokine Res. 2020, 40, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, L.A.; Michelotto, P.V.; Nunes, E.A.; Grando, F.C.C.; da Silva, F.T.; Nishiyama, A. PAF increases phagocytic capacity and superoxide anion production in equine alveolar macrophages and blood neutrophils. Res. Vet. Sci. 2012, 93, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Karagiorga, G.; Nakos, G.; Galiatsou, E.; Lekka, M.E. Biochemical parameters of bronchoalveolar lavage fluid in fat embolism. Intensive Care Med. 2005, 32, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.S.; Hsueh, W.; Sun, X.; Gidding, S.S.; Hageman, J.R. Circulating plasma platelet activating factor in persistent pulmonary hypertension of the newborn. Am. Rev. Respir. Dis. 1990, 142, 1258–1262. [Google Scholar] [CrossRef]
- Trimoreau, F.; François, B.; Desachy, A.; Besse, A.; Vignon, P.; Denizot, Y. Platelet-activating factor acetylhydrolase and haemophagocytosis in the sepsis syndrome. Mediat. Inflamm. 2000, 9, 197–200. [Google Scholar] [CrossRef]
- Falk, S.; Göggel, R.; Heydasch, U.; Brasch, F.; Muller, K.-M.; Wendel, A.; Uhlig, S. Quinolines Attenuate paf-induced pulmonary pressor responses and edema formation. Am. J. Respir. Crit. Care Med. 1999, 160, 1734–1742. [Google Scholar] [CrossRef]
- Muñoz-Cano, R.M.; Casas-Saucedo, R.; Santiago, A.V.; Bobolea, I.; Ribó, P.; Mullol, J. Platelet-Activating factor (PAF) in Allergic rhinitis: Clinical and therapeutic implications. J. Clin. Med. 2019, 8, 1338. [Google Scholar] [CrossRef]
- Tsantila, N.; Tsoupras, A.B.; Fragopoulou, E.; Antonopoulou, S.; Iatrou, C.; Demopoulos, C.A. In Vitro and in vivo effects of statins on platelet-activating factor and its metabolism. Angiology 2010, 62, 209–218. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Fragopoulou, E.; Nomikos, T.; Lioni, A.; Mangafas, N.; Demopoulos, C.A.; Antonopoulou, S.; Lazanas, M.C. Anti-platelet-activating factor effects of highly active antiretroviral therapy (HAART): A New insight in the drug therapy of HIV infection? AIDS Res. Hum. Retrovir. 2008, 24, 1079–1086. [Google Scholar] [CrossRef]
- Detopoulou, P.; Fragopoulou, E.; Nomikos, T.; Yannakoulia, M.; Stamatakis, G.; Panagiotakos, D.B.; Antonopoulou, S. The relation of diet with PAF and its metabolic enzymes in healthy volunteers. Eur. J. Nutr. 2014, 54, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Mediterranean diet and its protective mechanisms against cardiovascular disease: An insight into Platelet Activating Factor (PAF) and diet interplay. Ann. Nutr. Disord. Ther. 2015, 2, 1016. [Google Scholar]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and Antiatherosclerotic Properties of olive oil and olive pomace polar extracts in rabbits. Mediat. Inflamm. 2007, 2007, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Milton-Laskíbar, I.; Trepiana, J.; Gómez-Zorita, S.; Kajarabille, N.; Léniz, A.; González, M.; Portillo, M.P. Key Aspects in nutritional management of COVID-19 Patients. J. Clin. Med. 2020, 9, 2589. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Benahmed, A.G.; Bjørklund, G. Micronutrients as immunomodulatory tools for COVID-19 management. Clin. Immunol. 2020, 220, 108545. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Sezavar, H.; Saboor-Yaraghi, A.-A.; Salehi, E.; Mottaghi, A. Whether vitamin A supplementation is effective in T-bet and IFN-ɣ gene expression reduction? Immunol. Investig. 2014, 44, 189–198. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.; Cabezuelo, M.; Torres, L.; Viña, J.; Barber, T. Vitamin A deficiency and the lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef]
- Mutoh, H.; Fukuda, T.; Kitamaoto, T.; Masushige, S.; Sasaki, H.; Shimizu, T.; Kato, S. Tissue-specific response of the human platelet-activating factor receptor gene to retinoic acid and thyroid hormone by alternative promoter usage. Proc. Natl. Acad. Sci. USA 1996, 93, 774–779. [Google Scholar] [CrossRef]
- Bazan, N.G.; Fletcher, B.S.; Herschman, H.R.; Mukherjee, P.K. Platelet-activating factor and retinoic acid synergistically activate the inducible prostaglandin synthase gene. Proc. Natl. Acad. Sci. USA 1994, 91, 5252–5256. [Google Scholar] [CrossRef]
- Claar, D.; Hartert, T.V.; Peebles, R.S. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev. Respir. Med. 2014, 9, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Willeit, J.; Knoflach, M.; Mayr, M.; Egger, G.; Notdurfter, M.; Witztum, J.L.; Wiedermann, C.J.; Xu, Q.; Kiechl, S. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: Results from the Bruneck study. Eur. Heart J. 2008, 30, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Camussi, G.; Tetta, C.; Milgrom, F.; Andres, G. Hyperacute renal allograft rejection in the rabbit. The role of platelet-activating factor and of cationic proteins derived from polymorphonuclear leukocytes and from platelets. Lab. Investig. 1984, 51, 148–161. [Google Scholar] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Hemilä, H.; Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013, 8, CD005532. [Google Scholar] [CrossRef]
- Australian Governement, Department of Health. No Evidence to Support Intravenous High-Dose Vitamin C in the Management of COVID-19; Therapeutic Group Administration: Canberra, Australia, 2020. Available online: https://www.tga.gov.au/node/904121 (accessed on 27 January 2021).
- Tousoulis, D.; Antoniades, C.; Tountas, C.; Bosinakou, E.; Kotsopoulou, M.; Toutouzas, P.; Stefanadis, C. Vitamin C affects thrombosis/ fibrinolysis system and reactive hyperemia in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2003, 26, 2749–2753. [Google Scholar] [CrossRef]
- Spittle, C.R. Vitamin C and deep vein thrombosis. Lancet 1973, 302, 199–201. [Google Scholar] [CrossRef]
- Lloberas, N.; Torras, J.; Herrero-Fresneda, I.; Cruzado, J.M.; Riera, M.; Hurtado, I.; Grinyó, J.M. Postischemic renal oxidative stress induces an inflammatory response through PAF and oxidized phospholipids: Prevention by antioxidant treatment. FASEB J. 2002, 16, 908–910. [Google Scholar] [CrossRef]
- Lewis, M.S.; Whatley, R.E.; Cain, P.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J. Clin. Investig. 1988, 82, 2045–2055. [Google Scholar] [CrossRef]
- Verouti, S.N.; Fragopoulou, E.; Karantonis, H.C.; Dimitriou, A.A.; Tselepis, A.D.; Antonopoulou, S.; Nomikos, T.; Demopoulos, C.A. PAF effects on MCP-1 and IL-6 secretion in U-937 monocytes in comparison with OxLDL and IL-1β effects. Atherosclerosis 2011, 219, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, european, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Bourbour, F.; Dahka, S.M.; Gholamalizadeh, M.; Akbari, M.E.; Shadnoush, M.; Haghighi, M.; Taghvaye-Masoumi, H.; Ashoori, N.; Doaei, S. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch. Physiol. Biochem. 2020, 1–10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Wang, W.-W.; Jiang, W.-Y.; Li, C.-Q.; Guo, J.-C.; Xun, Y. Low vitamin D levels are associated with high viral loads in patients with chronic hepatitis B: A systematic review and meta-analysis. BMC Gastroenterol. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Liu, N.Q.; Lee, T.; Schall, J.I.; Denburg, M.R.; Rutstein, R.M.; Adams, J.S.; Zemel, B.S.; Stallings, V.A.; Hewison, M. Vitamin D supplementation and antibacterial immune responses in adolescents and young adults with HIV/AIDS. J. Steroid Biochem. Mol. Biol. 2015, 148, 290–297. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Hastie, C.E.; Mackay, D.F.; Ho, F.K.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.-H.; Kwon, H.Y.; Lee, J.-S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Narahara, H.; Miyakawa, I.; Johnston, J.M. The inhibitory effect of 1,25-dihydroxyvitamin D3 on the secretion of platelet-activating factor acetylhydrolase by human decidual macrophages. J. Clin. Endocrinol. Metab. 1995, 80, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Verouti, S.N.; Tsoupras, A.B.; Alevizopoulou, F.; Demopoulos, C.A.; Iatrou, C. Paricalcitol effects on activities and metabolism of platelet activating factor and on inflammatory cytokines in hemodialysis patients. Int. J. Artif. Organs 2013, 36, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging role of vitamin d and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef]
- Coquette, A.; Vray, B.; Vanderpas, J. Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Arch. Int. Physiol. Biochim. 1986, 94, S29–S34. [Google Scholar]
- Mileva, M.; Bakalova, R.; Tancheva, L.; Galabov, A.; Ribarov, S. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 1–11. [Google Scholar] [CrossRef]
- Hayek, M.G.; Taylor, S.F.; Bender, B.S.; Han, S.N.; Meydani, M.; Smith, D.E.; Eghtesada, S.; Meydani, S.N. Vitamin E Supplementation decreases lung virus titers in mice infected with influenza. J. Infect. Dis. 1997, 176, 273–276. [Google Scholar] [CrossRef]
- Galabov, A.S.; Mileva, M.; Simeonova, L.; Gegova, G. Combination activity of neuraminidase inhibitor oseltamivir and α-tocopherol in influenza virus A (H3N2) infection in mice. Antivir. Chem. Chemother. 2015, 24, 83–91. [Google Scholar] [CrossRef]
- Saboori, S.; Shab-Bidar, S.; Speakman, J.R.; Rad, E.Y.; Djafarian, K. Effect of vitamin E supplementation on serum C-reactive protein level: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2015, 69, 867–873. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Zhang, R.-Y.; Bai, J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int. J. Cardiol. 2020, 312, 137–138. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Kurotori, Y.; Tokumura, A.; Tsukatani, H. Vitamin E. deficiency increases the synthesis of platelet-activating factor (PAF) in rat polymorphonuclear leucocytes. Lipids 1989, 24, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Akada, S.; Iioka, H.; Moriyama, I.; Hisanaga, H.; Morimoto, K.; Ichijo, M. The role of vitamin E during pregnancy—anti-platelet aggregation activity of alpha-tocopherol. Nihon Sanka Fujinka Gakkai Zasshi 1991, 43, 523–528. [Google Scholar] [PubMed]
- Violi, F.; Pratico, D.; Ghiselli, A.; Alessandri, C.; Iuliano, L.; Cordova, C.; Balsano, F. Inhibition of cyclooxygenase-independent platelet aggregation by low vitamin E concentration. Atherosclerosis 1990, 82, 247–252. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Demopoulos, C. On the mediterranean diet. INFORM 1997, 8, 776–777. [Google Scholar]
- Kakishita, E.; Suehiro, A.; Oura, Y.; Nagai, K. Inhibitory effect of vitamin E (α-tocopherol) on spontaneous platelet aggregation in whole blood. Thromb. Res. 1990, 60, 489–499. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; De Prisco, R.; Nicolaus, B.; Pari, P.; Moriello, V.; Strazzullo, G.; Iorio, E.L.; Servillo, L.; Balestrieri, C. Lycopene in association with α-tocopherol or tomato lipophilic extracts enhances acyl-platelet-activating factor biosynthesis in endothelial cells during oxidative stress. Free. Radic. Biol. Med. 2004, 36, 1058–1067. [Google Scholar] [CrossRef]
- Larkin, E.K.; Gao, Y.-T.; Gebretsadik, T.; Hartman, T.J.; Wu, P.; Wen, W.; Yang, G.; Bai, C.; Jin, M.; Roberts, L.J.; et al. New risk factors for adult-onset incident asthma. A nested case–control study of host antioxidant defense. Am. J. Respir. Crit. Care Med. 2015, 191, 45–53. [Google Scholar] [CrossRef]
- Rainwater, D.L.; Mahaney, M.C.; VandeBerg, J.L.; Wang, X.L. Vitamin E dietary supplementation significantly affects multiple risk factors for cardiovascular disease in baboons. Am. J. Clin. Nutr. 2007, 86, 597–603. [Google Scholar] [CrossRef]
- Silbert, P.L.; Leong, L.L.L.; Sturm, M.J.; Strophair, J.; Taylor, R.R. Short term vitamin e supplementation has no effect on platelet function, plasma phospholipase a2and lyso-paf in male volunteers. Clin. Exp. Pharmacol. Physiol. 1990, 17, 645–651. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From Molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, L.; Nan, Y.; Zhu, L.Y. Protection from H1N1 Influenza virus infections in mice by supplementation with selenium: A Comparison with selenium-deficient mice. Biol. Trace Elem. Res. 2010, 141, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.L.; Hoffmann, P.R. Selenium and asthma. Mol. Asp. Med. 2012, 33, 98–106. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Cao, Y.-Z.; Cohen, Z.S.; Weaver, J.A.; Sordillo, L.M. Selenium modulates 1-O-Alkyl-2-Acetyl-sn-Glycero-3-Phosphocholine (PAF) Biosynthesis in bovine aortic endothelial cells. Antioxid. Redox Signal. 2001, 3, 1147–1152. [Google Scholar] [CrossRef]
- Hampel, G.; Watanabe, K.; Weksler, B.B.; Jaffe, E.A. Selenium deficiency inhibits prostacyclin release and enhances production of platelet activating factor by human endothelial cells. Biochim. Biophys. Acta Lipids Lipid Metab. 1989, 1006, 151–158. [Google Scholar] [CrossRef]
- Kaur, H.D.; Bansal, M.P. Studies on HDL associated enzymes under experimental hypercholesterolemia: Possible modulation on selenium supplementation. Lipids Health Dis. 2009, 8, 1–10. [Google Scholar] [CrossRef]
- Ricetti, M.M.; Guidi, G.C.; Tecchio, C.; Bellisola, G.; Rigo, A.; Perona, G. Effects of sodium selenite on in vitro interactions between platelets and endothelial cells. Int. J. Clin. Lab. Res. 1999, 29, 80–84. [Google Scholar] [CrossRef]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef]
- Sharma, S.; Chhibber, S.; Mohan, H.; Sharma, S. Dietary supplementation with omega-3 polyunsaturated fatty acids ameliorates acute pneumonia induced by Klebsiella pneumoniaein BALB/c mice. Can. J. Microbiol. 2013, 59, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, C.A.; Gonzalez-Juarbe, N.; Rahman, M.; Fernandes, G.; Orihuela, C.J.; I Restrepo, M.I. Omega-3 fatty acids in contrast to omega-6 protect against pneumococcal pneumonia. Microb. Pathog. 2020, 141, 103979. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.J.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Byleveld, P.M.; Pang, G.T.; Clancy, R.L.; Roberts, D.C.K. Fish Oil feeding delays influenza virus clearance and impairs production of interferon-γ and virus-specific Immunoglobulin A in the Lungs of mice. J. Nutr. 1999, 129, 328–335. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Rai, S.N.; Cambon, A.; Miles, R.; Jaffe, A.S.; Moser, A.B.; Jones, R.O.; Bolli, R.; Schulman, S.P. Fatty acids and TxA2 generation, in the absence of platelet-COX-1 activity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 428–433. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Takahashi, T.; Watanabe, S.; Kobayashi, T.; Okuyama, H. Possible mechanisms for the differential effects of high linoleate safflower oil and high α-linolenate perilla oil diets on platelet-activating factor production by rat polymorphonuclear leukocytes. J. Lipid Mediat. Cell Signal. 1997, 17, 207–220. [Google Scholar] [CrossRef]
- Mayer, K.; Merfels, M.; Muhly-Reinholz, M.; Gokorsch, S.; Rosseau, S.; Lohmeyer, J.; Schwarzer, N.; Krüll, M.; Suttorp, N.; Grimminger, F.; et al. ω-3 Fatty acids suppress monocyte adhesion to human endothelial cells: Role of endothelial PAF generation. Am. J. Physiol. Circ. Physiol. 2002, 283, H811–H818. [Google Scholar] [CrossRef]
- Shikano, M.; Masuzawa, Y.; Yazawa, K. Effect of docosahexaenoic acid on the generation of platelet-activating factor by eosinophilic leukemia cells, Eol-1. J. Immunol. 1993, 150, 3525–3533. [Google Scholar]
- Weber, C.; Aepfelbacher, M.; Lux, I.; Zimmer, B.; Weber, P.C. Docosahexaenoic acid inhibits PAF and LTD4 stimulated [Ca2+]i-increase in differentiated monocytic U937 cells. Biochim. Biophys. Acta Bioenerg. 1991, 1133, 38–45. [Google Scholar] [CrossRef]
- Sirivongrangson, P.; Kulvichit, W.; Payungporn, S.; Pisitkun, T.; Chindamporn, A.; Peerapornratana, S.; Pisitkun, P.; Chitcharoen, S.; Sawaswong, V.; Worasilchai, N.; et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med. Exp. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Takahashi, T.; Tanabe, A.; Watanabe, S.; Okuyama, H. Dietary α-linolenate suppresses endotoxin-induced platelet-activating factor production in rat kidney. Lipids 1999, 34, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Akisu, M.; Huseyinov, A.; Baka, M.; Yalaz, M.; Kultursay, N. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the generation of platelet-activating factor and leukotriene B4 in hypoxic–ischemic brain in young mice. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.B.; Koenig, W.; Khuseyinova, N.; Christensen, J.H. Lipoprotein-associated phospholipase A2 concentrations in plasma are associated with the extent of coronary artery disease and correlate to adipose tissue levels of marine n-3 fatty acids. Atherosclerosis 2008, 196, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.W.; Koenig, W.; Christensen, J.H.; Schmidt, E.B. The effect of marine n-3 fatty acids in different doses on plasma concentrations of Lp-PLA2 in healthy adults. Eur. J. Nutr. 2009, 48, 1–5. [Google Scholar] [CrossRef]
- Gajos, G.; Zalewski, J.; Mostowik, M.; Konduracka, E.; Nessler, J.; Undas, A. Polyunsaturated omega-3 fatty acids reduce lipoprotein-associated phospholipase A2 in patients with stable angina. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 434–439. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Alaupovic, P.; Kris-Etherton, P.M.; West, S.G. Dose-response effects of marine omega-3 fatty acids on apolipoproteins, apolipoprotein-defined lipoprotein subclasses, and Lp-PLA2 in individuals with moderate hypertriglyceridemia. J. Clin. Lipidol. 2015, 9, 360–367. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Detopoulou, P.; Alepoudea, E.; Nomikos, T.; Kalogeropoulos, N.; Antonopoulou, S. Associations between red blood cells fatty acids, desaturases and metabolism of Platelet Activating Factor in healthy volunteers. Prostaglandins Leukotrienes Essential Fatty Acids 2021, 164, 2021. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.W.T.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ Inhibits coronavirus and arterivirus RNA Polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Walker, C.F.; Black, R.E. Zinc and the risk for infectious disease. Annu. Rev. Nutr. 2004, 24, 255–275. [Google Scholar] [CrossRef]
- Kiabi, F.H.; Alipour, A.; Darvishi-Khezri, H.; Aliasgharian, A.; Zeydi, A.E. Zinc supplementation in adult mechanically ventilated trauma patients is associated with decreased occurrence of ventilator-associated pneumonia: A secondary analysis of a prospective, observational study. Indian J. Crit. Care Med. 2017, 21, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.-Q. Chloroquine is a zinc ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Fischer, J.G.; Kays, S.E. Is copper an antioxidant nutrient? Crit. Rev. Food Sci. Nutr. 1992, 32, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.; O’Connor, J.M.; Hannigan, B.M.; Strain, J.J. The immune system as a physiological indicator of marginal copper status? Br. J. Nutr. 2002, 87, 393–403. [Google Scholar] [CrossRef]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. JBIC J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- De Silva, A.; Atukorala, S.; Weerasinghe, I.; Ahluwalia, N. Iron supplementation improves iron status and reduces morbidity in children with or without upper respiratory tract infections: A randomized controlled study in Colombo, Sri Lanka. Am. J. Clin. Nutr. 2003, 77, 234–241. [Google Scholar] [CrossRef]
- Neves, J.; Haider, T.; Gassmann, M.; Muckenthaler, M.U. Iron homeostasis in the lungs—a balance between health and disease. Pharmaceuticals 2019, 12, 5. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving Iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr. Clin. Microbiol. Rep. 2020, 7, 13–19. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Roulia, M.; Ferentinos, E.; Stamatopoulos, I.; Demopoulos, C.A.; Kyritsis, P. Structurally diverse metal coordination compounds, bearing imidodiphosphinate and diphosphinoamine ligands, as potential inhibitors of the platelet activating factor. Bioinorg. Chem. Appl. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A Review on platelet activating factor inhibitors: Could a new class of potent metal-based anti-inflammatory drugs induce anticancer properties? Bioinorg. Chem. Appl. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wykle, R.L.; Malone, B.; Snyder, F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J. Biol. Chem. 1980, 255, 10256–10260. [Google Scholar] [CrossRef]
- Ambrosio, G.; Oriente, A.; Napoli, C.; Palumbo, G.; Chiariello, P.; Marone, G.; Condorelli, M.; Triggiani, M. Oxygen radicals inhibit human plasma acetylhydrolase, the enzyme that catabolizes platelet-activating factor. J. Clin. Investig. 1994, 93, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.D.; Erickson, K.L. Alteration of Macrophage responsiveness to platelet-activating factor by interferon-γ and lipopolysaccharide. Cell. Immunol. 1996, 174, 155–164. [Google Scholar] [CrossRef]
- Huang, Y.C.; Kennedy, T.P.; Su, Y.F.; Watkins, W.D.; Whorton, A.R.; Piantadosi, C.A. Protection against platelet-activating factor-induced injury by interferon inducer in perfused rabbit lung. J. Appl. Physiol. 1993, 74, 251–258. [Google Scholar] [CrossRef]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Renzo, L.; Romeo, F. Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxidative Med. Cell. Longev. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Vázquez-Calvo, Á.; de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses west nile virus, zika virus, and dengue virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. Preprints 2020, 2020, 12. [Google Scholar]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A Novel combination of vitamin c, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: A Perspective from system biology analysis. Nutrients 2020, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors 2020, 46, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Hensel, A.; Bauer, R.; Heinrich, M.; Spiegler, V.; Kayser, O.; Hempel, G.; Kraft, K. Challenges at the Time of COVID-19: Opportunities and Innovations in antivirals from nature. Planta Med. 2020, 86, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Nomikos, T.; Karantonis, H.C.; Apostolakis, C.; Pliakis, E.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S. Biological activity of acetylated phenolic compounds. J. Agric. Food Chem. 2007, 55, 80–89. [Google Scholar] [CrossRef]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef]
- Keihanian, F.; Sahebkar, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Singh, A.; Shafi, Z.; Mahto, S.K.; Yadav, S.; Sankhwar, R. Role and application of curcumin as an alternative therapeutic agent. Adv. Microb. Res. 2020. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, ddt-insensitive cholinephosphotransferase, of human mesangial cells. Mediat. Inflamm. 2007, 2007, 1–10. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Asimakopoulos, D.; Antonopoulou, S.; Demopoulos, C.A.; Fragopoulou, E. Effect of robola and cabernet sauvignon extracts on platelet activating factor enzymes activity on U937 cells. Food Chem. 2014, 165, 50–59. [Google Scholar] [CrossRef]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet activating factor (PAF) biosynthesis is inhibited by phenolic compounds in U-937 cells under inflammatory conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–183. [Google Scholar] [CrossRef]
- Yanoshita, R.; Chang, H.W.; Son, K.H.; Kudo, I.; Samejima, Y. Inhibition of lyso PAF acetyltransferase activity by flavonoids. Inflamm. Res. 1996, 45, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Hartisch, C.; Kolodziej, H.; von Bruchhausen, F. Dual Inhibitory activities of tannins from Hamamelis virginiana and related polyphenols on 5-Lipoxygenase and Lyso-PAF: Acetyl-CoA acetyltransferase1. Planta Med. 1997, 63, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, M.L.; Castaldo, D.; Balestrieri, C.; Quagliuolo, L.; Giovane, A.; Servillo, L. Modulation by flavonoids of PAF and related phospholipids in endothelial cells during oxidative stress. J. Lipid Res. 2003, 44, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, S.; Fukuju, A.; Takayama, M.; Nagai, M.; Yanoshita, R.; Samejima, Y. Inhibition of LysoPAF Acetyltransferase activity by components of licorice root. Biol. Pharm. Bull. 1999, 22, 1144–1146. [Google Scholar] [CrossRef]
- Shen, T.Y. Chemical and biochemical characterization of lignan analogs as novel PAF receptor antagonists. Lipids 1991, 26, 1154–1156. [Google Scholar] [CrossRef]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and asthma: Is it time to adapt our message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.N.; Tousoulis, D.; et al. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: Correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Panossian, A.; Brendler, T. The role of adaptogens in prophylaxis and treatment of viral respiratory infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Fragopoulou, E.; Karantonis, H.C.; Mitsou, E.; Sitara, M.; Rementzis, J.; Mourelatos, A.; Ginis, A.; Phenekos, C. Effect of Traditional greek mediterranean meals on platelet aggregation in normal subjects and in patients with type 2 diabetes mellitus. J. Med. Food 2006, 9, 356–362. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Fragopoulou, E.; Antonopoulou, S.; Rementzis, J.; Phenekos, C.; Demopoulos, C.A. Effect of fast-food Mediterranean-type diet on type 2 diabetics and healthy human subjects’ platelet aggregation. Diabetes Res. Clin. Pract. 2006, 72, 33–41. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Bellastella, G.; Longo, M.; Caruso, P.; Esposito, K. Mediterranean Diet and COVID-19: Hypothesizing Potential benefits in people with diabetes. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, C.E.; Konsta, M.; Dradaki, V.; Roumpou, A.; Dri, I.; Papaioannou, I. Effects of Mediterranean diet on hospital length of stay, medical expenses, and mortality in elderly, hospitalized patients: A 2-year observational study. Nutrition 2020, 80, 110868. [Google Scholar] [CrossRef] [PubMed]
- Lo Buglio, A.; Bellanti, F.; Capurso, C.; Paglia, A.; Vendemiale, G. Adherence to Mediterranean diet, malnutrition, length of stay and mortality in elderly patients hospitalized in internal medicine wards. Nutrients 2019, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Sciorsci, R.L.; Magrone, T.; Jirillo, E. Exploitation of some natural products for prevention and/or nutritional treatment of SARS-CoV2 infection. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic lipid minor constituents from vegetable oils. comparison between olive oils and others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Karantonis, H.C.; Tsantila, N.; Stamatakis, G.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S.; Demopoulos, C.A. Bioactive polar lipids in olive oil, pomace and waste byproducts. J. Food Biochem. 2008, 32, 443–459. [Google Scholar] [CrossRef]
- Detopoulou, M.; Fragopoulou, E.; Mikellidi, A.; Vlachogianni, Ι.; Xanthopoulou, Μ.; Argyrou, C.; Nomikos, T.; Yannakoulia, M.; Antonopoulou, S. Cardioprotective properties of a novel enriched yogurt with inhibitors of Platelet activating factor (PAF). Proc. Nutr. Soc. 2020, 79. [Google Scholar] [CrossRef]
- Panayiotou, A.; Samartzis, D.; Nomikos, T.; Fragopoulou, E.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Lipid fractions with aggregatory and antiaggregatory activity toward platelets in fresh and fried cod (Gadus morhua): Correlation with platelet-activating factor and atherogenesis. J. Agric. Food Chem. 2000, 48, 6372–6379. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.; Zabetakis, I. Comparison of antiatherogenic properties of lipids obtained from wild and cultured sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem. 2007, 100, 560–567. [Google Scholar] [CrossRef]
- Nomikos, T.; Karantonis, H.C.; Skarvelis, C.; Demopoulos, C.A.; Zabetakis, I. Antiatherogenic properties of lipid fractions of raw and fried fish. Food Chem. 2006, 96, 29–35. [Google Scholar] [CrossRef]
- Rementzis, J.; Antonopoulou, S.; Demopoulos, C.A. Identification and Study of gangliosides from Scomber scombrus muscle. J. Agric. Food Chem. 1997, 45, 611–615. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids Health Dis. 2011, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Karantonis, H.C.; Andriotis, M.; Demopoulos, C.A.; Zabetakis, I. Antibacterial and anti-PAF activity of lipid extracts from sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem. 2008, 111, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, A.; Verhagen, H.; Moschandreas, J.; Apostolaki, I.; Westerop, J.J.M.V. Mediterranean Diet of Crete. J. Am. Diet. Assoc. 2000, 100, 1487–1493. [Google Scholar] [CrossRef]
- Skiadas, P.; Lascaratos, J. Dietetics in ancient Greek philosophy: Plato’s concepts of healthy diet. Eur. J. Clin. Nutr. 2001, 55, 532–537. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Ab Hamid, H.; Khaza’Ai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 1–17. [Google Scholar] [CrossRef]
- Pimentel, R.B.; Da Costa, C.A.; Albuquerque, P.M.; Junior, S.D. Antimicrobial activity and rutin identification of honey produced by the stingless bee Melipona compressipes manaosensis and commercial honey. BMC Complement. Altern. Med. 2013, 13, 1–151. [Google Scholar] [CrossRef]
- Mustafa, M.Z.; Shamsuddin, S.H.; Sulaiman, S.A.; Abdullah, J.M. Anti-inflammatory properties of stingless bee honey may reduce the severity of pulmonary manifestations in COVID-19 Infections. Malays. J. Med Sci. 2020, 27, 165–169. [Google Scholar] [CrossRef]
- Hossain, K.S.; Hossain, M.G.; Moni, A.; Rahman, M.M.; Rahman, U.H.; Alam, M.; Kundu, S.; Rahman, M.M.; Hannan, M.A.; Uddin, M.J. Prospects of honey in fighting against COVID-19: Pharmacological insights and therapeutic promises. Heliyon 2020, 6, e05798. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.E. IN Silico approach of some selected honey constituents as sars-cov-2 main protease (COVID-19) inhibitors. Eurasian J. Med. Oncol. 2020. [Google Scholar] [CrossRef]
- Ahmed, A.; Khan, R.A.; Azim, M.K.; Saeed, S.A.; Mesaik, M.A.; Ahmed, S.; Imran, I. Effect of natural honey on human platelets and blood coagulation proteins. Pak. J. Pharm. Sci. 2011, 24, 389–397. [Google Scholar] [PubMed]
- Koussissis, G.; Semidalas, E.; Hadjistavrou, E.; Kalyvas, V.; Antonopoulou, S.; Demopoulos, C.A. PAF antagonists in food: Isolation and identification of PAF antagonists in honey and wax. Rev. Fr. Corps Gras 1994, 5/6, 127–132. [Google Scholar]
- Mardani, R.; Alamdary, A.; Nasab, S.M.; Gholami, A.; Ahmadi, N. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020, 289, 198148. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.H.; Majumder, R.; Torabi, R.; Saeg, F.; Hoffman, R.; Cirillo, J.D.; Greiffenstein, P. Vitamin D Insufficiency is prevalent in severe Covid-19. MedRxiv 2020. [Google Scholar]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020, 93, 508–511. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Semidalas, C.E.; Koussissis, S.; Demopoulos, C.A. Platelet-Activating factor (PAF) Antagonists in foods: A study of lipids with paf or anti-paf-like activity in cow’s milk and yogurt. J. Agric. Food Chem. 1996, 44, 3047–3051. [Google Scholar] [CrossRef]
- Lordan, R.; Vidal, N.P.; Pham, T.H.; Tsoupras, A.; Thomas, R.H.; Zabetakis, I. Yoghurt fermentation alters the composition and antiplatelet properties of milk polar lipids. Food Chem. 2020, 332, 127384. [Google Scholar] [CrossRef]
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The effect of ovine milk fermentation on the antithrombotic properties of polar lipids. J. Funct. Foods 2019, 54, 289–300. [Google Scholar] [CrossRef]
- Megalemou, K.; Sioriki, E.; Lordan, R.; Dermiki, M.; Nasopoulou, C.; Zabetakis, I. Evaluation of sensory and in vitro anti-thrombotic properties of traditional Greek yogurts derived from different types of milk. Heliyon 2017, 3, e00227. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghi, N. Molecular Docking Study of Novel COVID-19 Protease with Low Risk Terpenoides Compounds of Plants. Chemrixiv 2020. [Google Scholar] [CrossRef]
- Wang, K.-L.; Li, Z.-Q.; Cao, Z.-Y.; Ke, Z.-P.; Cao, L.; Wang, Z.-Z.; Xiao, W. Effects of ginkgolide A, B and K on platelet aggregation. Zhongguo Zhong Yao Za Zhi 2017, 42, 4722–4726. [Google Scholar] [PubMed]
- Detopoulou, P.; Aggeli, M.; Andrioti, E.; Detopoulou, M. Macronutrient content and food exchanges for 48 Greek Mediterranean dishes. Nutr. Diet. 2016, 74, 200–209. [Google Scholar] [CrossRef]
- Phillips, C.; Poyser Norman, L. Inhibition of platelet aggregation by onion extracts. Lancet 1978, 311, 1051–1052. [Google Scholar] [CrossRef]
- Lim, H.; Kubota, K.; Kobayashi, A.; Seki, T.; Ariga, T. Inhibitory effect of sulfur-containing compounds in Scorodocarpus borneensis Becc. on the Aggregation of rabbit platelets. Biosci. Biotechnol. Biochem. 1999, 63, 298–301. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Detopoulou, P.; Nomikos, T.; Pliakis, E.; Panagiotakos, D.; Antonopoulou, S. Mediterranean wild plants reduce postprandial platelet aggregation in patients with metabolic syndrome. Metabolism 2012, 61, 325–334. [Google Scholar] [CrossRef]
- Wong, W.-T.; Ismail, M.; Imam, M.U.; Zhang, Y.-D. Modulation of platelet functions by crude rice (Oryza sativa) bran policosanol extract. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Rungratanawanich, W.; Cenini, G.; Mastinu, A.; Sylvester, M.; Wilkening, A.; Abate, G.; Bonini, S.; Aria, F.; Marziano, M.; Maccarinelli, G.; et al. γ-Oryzanol improves cognitive function and modulates hippocampal proteome in mice. Nutrients 2019, 11, 753. [Google Scholar] [CrossRef]
- Bousquest, J.; Cristol, J.-P.; Czarlewski, W.; Anto, J.M.; Martineau, A.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; Fiocchi, A.; et al. Nrf2-interacting nutrients and COVID-19: Time for research to develop adaptation strategies. Clin. Transl. Allergy 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Bayin, E.C.; Genc, S.; Genc, K. The Nrf2/ARE Pathway: A Promising target to counteract mitochondrial dysfunction in parkinson’s disease. Park. Dis. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine consumption reduced postprandial platelet sensitivity against platelet activating factor in healthy men. Eur. J. Nutr. 2016, 56, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on platelet activating factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S.; Demopoulos, C.A. Biologically active lipids with antiatherogenic properties from white wine and must. J. Agric. Food Chem. 2002, 50, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Nomikos, T.; Antonopoulou, S.; Mitsopoulou, C.A.; Demopoulos, C.A. Separation of biologically active lipids from red wine. J. Agric. Food Chem. 2000, 48, 1234–1238. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Tsantila, N.; Mitropoulou, A.; Zabetakis, I.; Demopoulos, C.A. Biological activity of total lipids from red and white wine/must. J. Agric. Food Chem. 2001, 49, 5186–5193. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S.; Nomikos, T.; Demopoulos, C.A. Structure elucidation of phenolic compounds from red/white wine with antiatherogenic properties. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1632, 90–99. [Google Scholar] [CrossRef]
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Τhomaidis, Ν.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of anti-platelet activity of grape pomace extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef]
- Mori, T.A.; Beilin, L.J.; Burke, V.; Morris, J.; Ritchie, J. Interactions between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. Arter. Thromb. Vasc. Biol. 1997, 17, 279–286. [Google Scholar] [CrossRef]
- Grimminger, F.; Mayser, P.; Papavassilis, C.; Thomas, M.; Schlotzer, E.; Heuer, K.-U.; Führer, D.; Hinsch, K.-D.; Walmrath, D.; Schill, W.-B.; et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis. J. Mol. Med. 1993, 71, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Gavriil, L.; Detopoulou, M.; Petsini, F.; Antonopoulou, S.; Fragopoulou, E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: A randomised, double-blind and placebo-controlled trial. Br. J. Nutr. 2019, 121, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Dent, G.; McCusker, M.; Guinot, P.; Page, C.; Barnes, P.J. Effect of a ginkgolide mixture (bn 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet 1987, 329, 248–251. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Szerlip, H.M.; Ballantyne, C.M.; Bays, H.E.; Philip, S.; Doyle, R.T.; Juliano, R.A.; Granowitz, C. Icosapent ethyl reduces atherogenic markers in high-risk statin-treated patients with stage 3 chronic kidney disease and high triglycerides. Postgrad. Med. 2019, 131, 390–396. [Google Scholar] [CrossRef]
- Brinton, E.A.; Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.P.; Braeckman, R.A.; Soni, P.N. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: The ANCHOR study. Cardiovasc. Diabetol. 2013, 12, 1–100. [Google Scholar] [CrossRef]
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Barden, A.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atheroscler. 2003, 166, 85–93. [Google Scholar] [CrossRef]
- Kastelein, J.J.P.; Maki, K.C.; Susekov, A.; Ezhov, M.; Nordestgaard, B.G.; Machielse, B.N.; Kling, D.; Davidson, M.H. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The Epanova for lowering very high triglycerides (EVOLVE) trial. J. Clin. Lipidol. 2014, 8, 94–106. [Google Scholar] [CrossRef]
- Mosca, L.; Ballantyne, C.M.; Bays, H.E.; Guyton, J.R.; Philip, S.; Doyle, R.T.; Juliano, R.A. Usefulness of icosapent ethyl (eicosapentaenoic acid ethyl ester) in women to lower triglyceride levels (results from the marine and anchor trials). Am. J. Cardiol. 2017, 119, 397–403. [Google Scholar] [CrossRef]
- Krantz, M.J.; Havranek, E.P.; Pereira, R.I.; Beaty, B.; Mehler, P.S.; Long, C.S. Effects of omega-3 fatty acids on arterial stiffness in patients with hypertension: A randomized pilot study. J. Negat. Results Biomed. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Wooten, J.S.; Nambi, P.; Gillard, B.K.; Pownall, H.J.; Coraza, I.; Scott, L.W.; Nambi, V.; Ballantyne, C.M.; Balasubramanyam, A. intensive lifestyle modification reduces Lp-PLA2 in dyslipidemic HIV/HAART patients. Med. Sci. Sports Exerc. 2013, 45, 1043–1050. [Google Scholar] [CrossRef]
- Kim, M.; Jeung, S.R.; Jeong, T.-S.; Lee, S.-H.; Lee, J.H. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J. Lipid Res. 2014, 55, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Burke, V.; Mori, T.A.; Vandongen, R.; Beilin, L.J. Effects of garlic extract on platelet aggregation: A randomized placebo-controlled double-blind study. Clin. Exp. Pharmacol. Physiol. 1995, 22, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, I.B.; Gleason, J.A.; Sever, S.; Gedik, R.; Asztalos, B.F.; Horvath, K.V.; Dansinger, M.L.; Lamon-Fava, S.; Schaefer, E.J. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism 2016, 65, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Bays, H.E.; Dicklin, M.R.; Johnson, S.L.; Shabbout, M. Effects of prescription omega-3-acid ethyl esters, coadministered with atorvastatin, on circulating levels of lipoprotein particles, apolipoprotein CIII, and lipoprotein-associated phospholipase A2 mass in men and women with mixed dyslipidemia. J. Clin. Lipidol. 2011, 5, 483–492. [Google Scholar] [CrossRef]
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic acid ethyl ester (amr101) therapy in patients with very high triglyceride levels (from the multi-center, placebo-controlled, randomized, double-blind, 12-week study with an open-label extension [marine] trial). Am. J. Cardiol. 2011, 108, 682–690. [Google Scholar] [CrossRef]
- Dunbar, R.L.; Nicholls, S.J.; Maki, K.C.; Roth, E.M.; Orloff, D.G.; Curcio, D.; Johnson, J.; Kling, D.; Davidson, M.H. Effects of omega-3 carboxylic acids on lipoprotein particles and other cardiovascular risk markers in high-risk statin-treated patients with residual hypertriglyceridemia: A randomized, controlled, double-blind trial. Lipids Health Dis. 2015, 14, 1–10. [Google Scholar] [CrossRef][Green Version]
- Hedengran, A.; Szecsi, P.B.; Dyerberg, J.; Harris, W.S.; Stender, S. N-3 PUFA Esterified to glycerol or as ethyl esters reduce non-fasting plasma triacylglycerol in subjects with hypertriglyceridemia: A randomized trial. Lipids 2014, 50, 165–175. [Google Scholar] [CrossRef]
- Mayer, K.; Fegbeutel, C.; Hattar, K.; Sibelius, U.; Krämer, H.-J.; Heuer, K.-U.; Temmesfeld-Wollbrück, B.; Gokorsch, S.; Grimminger, F.; Seeger, W. ω-3 vs. ω-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: Impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003, 29, 1472–1481. [Google Scholar] [CrossRef]
- Kerely, C.P.; Hutchinson, K.; Bramham, J.; McGowan, A.; Faul, J.; Cormican, L. Vitamin D Improves selected metabolic parameters but not neuropsychological or quality of life indices in osa: A Pilot Study. J. Clin. Sleep Med. 2017, 13, 19–26. [Google Scholar] [CrossRef]
- Mori, T.A.; Vandongen, R.; Mahanian, F.; Douglas, A. Plasma lipid levels and platelet and neutrophil function in patients with vascular disease following fish oil and olive oil supplementation. Metabolism 1992, 41, 1059–1067. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.; Stein, E.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Efficacy and safety of eicosapentaenoic acid ethyl ester (amr101) therapy in statin-treated patients with persistent high triglycerides (from the anchor study). Am. J. Cardiol. 2012, 110, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Ballantyne, C.M.; Bays, H.E.; Granowitz, C.; Doyle, R.T.; Juliano, R.A.; Philip, S. Effects of icosapent ethyl (eicosapentaenoic acid ethyl ester) on atherogenic lipid/lipoprotein, apolipoprotein, and inflammatory parameters in patients with elevated high-sensitivity c-reactive protein (from the anchor study). Am. J. Cardiol. 2019, 124, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; van den Berg, R.; Kok, F.J.; Helander, A.; Vermunt, S.H.F.; Hendriks, H.F. Moderate alcohol consumption and lipoprotein-associated phospholipase A2 activity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 539–544. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Galeone, C.; Negri, E.; La Vecchia, C. Trends in adherence to the Mediterranean diet in an Italian population between 1991 and 2006. Eur. J. Clin. Nutr. 2010, 64, 1052–1056. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; de Carvalho Padilha, P.; Mantilla-Escalante, D.C.; Ulloa, N.; Brun, P.; Acevedo-Correa, D.; Arrantes Ferreira Peres, W.; Martorell, M.; Aires, M.T.; de Oliveira Cardoso, L.; et al. Covid-19 Confinement and Changes of Adolescent’s Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020, 12, 1807. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Molina-Montes, E.; Verardo, V.; Artacho, R.; García-Villanova, B.; Guerra-Hernández, E.J.; Ruiz-López, M.-D. Changes in dietary behaviours during the COVID-19 Outbreak Confinement in the Spanish COVIDiet Study. Nutrients 2020, 12, 1730. [Google Scholar] [CrossRef]
- PRESS RELEASE—The Role of Nutrition in the COVID-19 Era. Available online: https://www.hda.gr/deltio-typoy-o-rolos-tis-diatrofis-stin-epochi-tis-covid-19-prolipsi-antimetopisi-kai-o-paragontas-tis-pachusarkias/ (accessed on 27 January 2021).
| Nutrient/Food | Intervention | Volunteers | Age | Health Status | PAF Induced Platelet Aggregation | PAF Levels | PAF Biosynthetic Enzymes | PAF Catabolic Enzymes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Vitamin D | 15 weeks | n = 10 n = 9 (control) | 56 ± 10 52 ± 13 | Healthy | ↓ | [223] | |||
| Fish oils, omega-3 | |||||||||
| Fish oil Olive oil | 10 weeks | n = 15 (fish oil) n = 15 (olive oil) | 61.9 ± 1.2 | Peripheral vascular disease | ↓ In the fish oil group ↑ in the olive oil group | no changes (measured in neutrophils) | [224] | ||
| Fish Fish oil (2 doses) Fish + fish oil placebo | 12 weeks | n = 120 (for all groups) | 30–60 | ↓ (not in the control group) | [204] | ||||
| EPA + DHA omega-6 | acute | n = 20 | Psoriasis | ↓ in n-3 Group ↑ in the n-6 group | [205] | ||||
| omega-3 +atorvastatin placebo + atorvastatin | 8 weeks | n =123 n = 122 | 56.1 ± 10.2 | Hypertriglyceridemia | ↓ (n-3 + atorvastatin vs. placebo + atorvastatin | [218] | |||
| EPA (2 doses) * | 12 weeks | n = 702 (for all groups) | 61 ± 10 | Hypertriglyceridemia | ↓ | [225] | |||
| EPA * | 12 weeks | n = 126 n = 120 | 60.2 ± 9.7 61.0 ± 9.9 | Hypertriglyceridemia, high CRP | ↓ | [226] | |||
| EPA * | 12 weeks | n = 19 (4 g) n = 30 (2 g) n = 36 (control) | 68.2 ± 7.2 67.9 ± 8.3 68.0 ± 8.4 | Hypertriglyceridemia, and chronic kidney disease | ↓ | [208] | |||
| EPA * (2 doses) | 12 weeks | 171 (2 g)_ 165 (4 g) 165(control) | Not reported | Hypertriglyceridemia, diabetes mellitus-2 and statin therapy | ↓ (high dose) | [209] | |||
| EPA or DHA | 6 weeks | n = 59 (for all groups, men) | 61.2 ± 51.2 | Hypertention and type 2 diabetes | no changes | [210] | |||
| omega-3 | 30 days | n = 54 | 30–80 | angina | ↓ | [109] | |||
| EPA+ DHA 0/0.85/ 3.4 g/day | 8 weeks | n = 25 (crossover) | 44.3 ± 9.8 | Hypertriglyceridemia | ↓ | [110] | |||
| EPA (2 g, 4 g) (control) | n = 77 (4 g) n = 76 (2 g) n = 76 (control) | 52.9 ± 9.34 | Hypertriglyceridemia | ↓ | [219] | ||||
| omega-3 (2 g, 4 g) control | 6 weeks | n = 209 (2 g) n= 207 (4 g) n = 211 (control | 60.8 ± 9.6 | Statin-treated patients with residual hypertriglyceridemia | ↓ | [220] | |||
| omega-3 esterified to glycerol or as ethyl esters | 8 weeks | n = 120 | 62.4 ± 10.0 | Hypertriglyceridemia | ↓ With ethyl esters of n-3 | [221] | |||
| omega-6 or omega -3 (parenteral nutrition) | 10 days | n = 10 patients n = 8 healthy control | 53.7 ± 13.8 | Sepsis | ↑in the n-3 group (baseline levels were suppressed) | [222] | |||
| omega-3 2 g, 3, 4 g | 12 weeks | n = 100 (2 g) n = 101 (3 g) n - = 99 (4 g) n = 99 (control) | 51.1 ± 9.8 51.2 ± 8.8 52.9 ± 10.9 50.8 ± 10.6 | Hypertriglyceridemia | ↓ | [211] | |||
| EPA (2 g, 4 g) control | 12 weeks | n = 215 (women) | ~60 ± 10 | Hypertriglyceridemia | ↓ | [212] | |||
| omega-3 | 3 months | n = 27 n = 35 (control) | 62.3 ± 9.7 60.2 ± 10.8 | Hypertension | no change | [213] | |||
| a-linolenic acid EPA+DHA | 8 weeks | n = 20 ALA n = 20 EPA + DHA n - = 19 (control) | 63.4 ± 8.2 62.1 ± 7.7 58.6 ± 6.3 | Healthy | no change | ||||
| omega-3 (2 g, 6.6 g) control (olive oil) | 12 weeks | n = 20 (2 g) n = 20 (6.6 g) n = 20 (control) | 36.5 ± 11 37.0 ± 10 37.9 ± 10 | Healthy | no change | [108] | |||
| olive oil (control) EPA 600 mg/day EPA 1800 mg/day, DHA 600 mg/day | 6 weeks | n = 26 (control) n = 27 (600 mg EPA) n = 26 (1800 mg EPA) n = 28 (600 mg DHA) | 52.2 ± 10.4 52.8 ± 11.6 52.2 ± 11.6 52.3 ± 12.6 | Healthy | ↓ high dose EPA | [217] | |||
| Mediterranean diet | |||||||||
| fast-food Mediterranean-type diet | 4 weeks | n = 22 healthy n = 22 type 2 diabetes n = 22 control | 56 ± 15 | Healthy and with type 2 diabetes | ↓ (not in the control group) | [154] | |||
| traditional Greek Mediterranean-type meals | 28 days | n = 22 healthy n = 24 type 2 diabetes n = 22 type 2 diabetes -control | 53 ± 12 | Healthy and with type 2 diabetes | ↓ (not in the control group) | [153] | |||
| Diet and exercise | |||||||||
| Diet and exercise | 24 weeks | n = 22 | 44.0 ± 1.3 | HIV | ↓ | [214] | |||
| substitution of whole grains and legumes for refined rice | 12 weeks | n = 50 (whole grain) n = 49 (control) | 56.3 ± 1.2 55.4 ± 1.5 | Impaired fasting glucose, impaired glucose tolerance or newly diagnosed T2D | ↓ | [215] | |||
| Plants and plant extracts | |||||||||
| wild plant meals, namely, Reichardia picroides, Cynara cardunculus, Urospermum picroides and Chrysanthemum coronarium, and a control meal, which contained no wild plant | acute | n = 24 | 58.6 ± 11.3 | Metabolic syndrome | ↓ with the Urospermum picroides meal | [191] | |||
| plant extract supplement | 8 weeks | n = 30 (supplement) n = 28 (control) | 34.9 ± 5.8 (supplement) 32.9 ± 5.6 (control group) | Healthy | ↓ | no change | ↑ | [206] | |
| ginkgolide mixture | acute | n = 6 | 25–35 | Healthy | ↓ | [207] | |||
| Garlic extract | 5 days | n = 14 | 20–55 | Healthy | no change | [216] | |||
| Alcohol and wine | |||||||||
| Wine (Robola, Cabernet Sauvignon) | acute | n = 12 | 31.3 ± 4.3y | Healthy | ↓lyso-PAf-AT ↓ PAF-CPT | no changes | [198] | ||
| Wine (Robola, Cabernet Sauvignon) | acute | n = 10 | 31.3 ± 4.3 | Healthy | ↓ | [197] | |||
| Beer or alcohol-free | 3 weeks | n = 11 lean n = 9 overweight | 19 ± 2 21 ± 2 | Healthy | no changes | [227] | |||
| Others | |||||||||
| Yogurt with bioactive ingredients from olive-oil by-products | 8 weeks | n = 92 | 35–65 | Healthy | ↓ | [162] |
| Nutrient-Food | Quantity | Duration | Volunteers | Main Outcomes | Registration at www.clinicaltrials.gov |
|---|---|---|---|---|---|
| Vitamin C | 10 g | 400 | NCT04584437 | ||
| Vitamin C | 10 g intravenously | 72 hours | 500 | In-hospital mortality, length of stay, virus load | NCT04323514 |
| Vitamin C and melatonin | 1 g vitamin C 10 mg melatonin | 14 days | 150 | Symptom severity | NCT04530539 |
| Vitamin C and zinc | 8 g vitamin C or 50 mg zinc or 8 g vitamin C + 50 mg zinc | 28 days | 520 | Symptom duration | NCT04342728 |
| Vitamin C, vitamin D, zinc | Not reported | 12 weeks | 600 | Rate of recover, symptoms, | NCT04334512 |
| Vitamin C, vitamin D, zinc, B12 | Vitamin C 28 g intravenously zinc Citrate 30 mg Vitamin D3 5000 IU daily Vitamin B12 500 ug | 7–14 days | 200 | Symptoms, length of stay | NCT04395768 |
| Vitamin C, vitamin D, zinc | Not reported | 14 weeks | 600 medical workers | Prevention of COVID-19 symptoms | NCT04335084 |
| Vitamin D | 9600 IU/day on days 1 and 2, and 3200 IU/day on days 3 through 28 | 28 days | 2700 participants with newly diagnosed COVID-19 | Hospitalization or death in index cases, self-reported disease severity in index cases time to hospitalization or death in index cases, ICU admission/ventilation support in index cases, SARS-CoV-2 infection in close household contacts, self-reported disease severity in close household contacts | NCT04536298 |
| Vitamin D | 50,000 IU/week | 8 weeks | 100 | Cytokine levels | NCT04476745 |
| Vitamin D | 200,000 IU on admission | 240 | Length of hospitalization, Number of cases admitted to Intensive Care Unit, Length of use of mechanic ventilator inflammatory markers, vitamin D, | NCT04449718 | |
| Vitamin D | 10,000 IU bolus dose followed by 10,000 IU once a week | 16 weeks | 2414 health care workers | Distribution of disease severity, disease severity | NCT04483635 |
| Vitamin D | 800 IU 3200 IU | 6 m | 6200 individuals with 25-hydroxyvitamin D level <75 nmol/L | Acute respiratory infection, COVID-19 diagnosis | NCT04579640 |
| Vitamin D | 10,000 IU/day (age 18–69 years) or 15,000 IU/day (age 70+) 2 w: if vitamin D <30 ng/mL, continue the dosage for 3 more weeks. If vitamin D: 30–49 ng/mL, continue at a dosage of 5000 IU/day. If vitamin D >50 ng/mL, stop supplementation. | 6 weeks | 41 | Vitamin D, severity of COVID-19 symptoms | NCT04407286 |
| Vitamin D | 6000 IU 6000 IU + 20,000 IU vitamin D3 daily for 3 days | 12 m | 140 | Vitamin D, Change in SARS-CoV-2 antibody titers, inflammatory markers | NCT04482673 |
| Vitamin D | 5000 IU) | 9 m | 2099 hospital workers | Respiratory tract infections | NCT04596657 |
| vitamin D and zinc | 2000 IU 30 mg | 2 m | 3140 | Survival rate | NCT04351490 |
| vitamin D and zinc | 180,000 international units (IU) 40 mg of zinc | 8 weeks | 700 | Time to recover, all-cause mortality, symptoms, levels of vitamins | NCT04641195 |
| Omega-3 | 300 mg of omega3-FA | 8 weeks | 100 | Serum ACE levels, serum ACE2 levels, lipid profile | NCT04658433 |
| Fish oil | wild salmon and fish oil complex 1 g, 300 mg omega-3 | 8 weeks | 100 | Cytokine levels, lipid profile, glucose levels | NCT04483271 |
| Fish oil | Cod liver oil: 5 mL (Contains: 10 ug of vitamin D, 1.2 g of long-chained n-3 polyunsaturated fatty acids (DHA 0.6 g and EPA 0.4 g), 250 ug vitamin A and 10 mg vitamin E). | 6 m | 80,000 | Number of participants diagnosed with serious Covid-19, self-reported airway infection, hospitalization, infections | NCT04609423 |
| Zinc, Quercetin, Bromelain and Vitamin C | zinc 50 mg vitamin C 1000 mg | 5–10 days | 60 | Time to hospital discharge serum zinc Time of negativization of COVID-PCR | NCT04468139 |
| Zinc, vitamin C | Zinc 220 mg vitamin C 1 g | 10 days | 50 | Symptoms reduction time frame, severity of symptoms | NCT04558424 |
| Zinc | high dose Zinc supplementation in combination with copper, vitamin C/E and beta-carotene vs. low dose zinc and multivitamin supplement | 3 m | 4500 | Hospitalization, Illness without hospitalization, mortality | NCT04551339 |
| Anti-inflammatory/antioxidant supplement | vitamin A (as β-carotene) 500 ug, Vitamin C 250 mg, vitamin E 90 mg, Selenium 15 ug, Zinc 7.5 mg. | 14 days | 40 | Nutritional risk, inflammatory indices, ferritin, anthropometry etc. | NCT04323228 |
| Quercetin | 500 mg | 30 days | 200 | Survival time, Length of stay in hospital, days of mechanical ventilation, blood exams etc. | NCT04578158 |
| Licorice | 250 mg standardized extract (25% Glycyrrhizin - 62.5 mg) | 10 days | 70 | Number of people recovering from COVID-19, mechanical support, hospital stay | NCT04487964 |
| Plant polyphenol | Plant polyphenol +Vitamin D3 100,000 IU on day 1 | 15 days | 200 | Hospitalization rates for COVID-19 | NCT04400890 |
| Herbal extract (Cretan IAMA) | 1 mL/day Thymbra 59 capitata (L.) Cav., Origanum dictamnus L., Salvia fruticosa Mill. in extra virgin olive oil | 2 weeks | 20 | Symptom resolution | NCT04705753 |
| Honey | 1 gm/kg/day | 14 days | 1000 | Rate of recovery, resolution of lung inflammation | NCT04323345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. https://doi.org/10.3390/nu13020462
Detopoulou P, Demopoulos CA, Antonopoulou S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients. 2021; 13(2):462. https://doi.org/10.3390/nu13020462
Chicago/Turabian StyleDetopoulou, Paraskevi, Constantinos A. Demopoulos, and Smaragdi Antonopoulou. 2021. "Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism" Nutrients 13, no. 2: 462. https://doi.org/10.3390/nu13020462
APA StyleDetopoulou, P., Demopoulos, C. A., & Antonopoulou, S. (2021). Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients, 13(2), 462. https://doi.org/10.3390/nu13020462







