Pharmacokinetics of Nitrate and Nitrite Following Beetroot Juice Drink Consumption
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Study Design and Procedures
2.3. Intervention
2.4. Blood Measurements
2.5. HPLC Analysis
2.6. Cardiovascular Measurements
2.7. Statistical Analysis
3. Results
3.1. Participants
3.1.1. Plasma NO2−
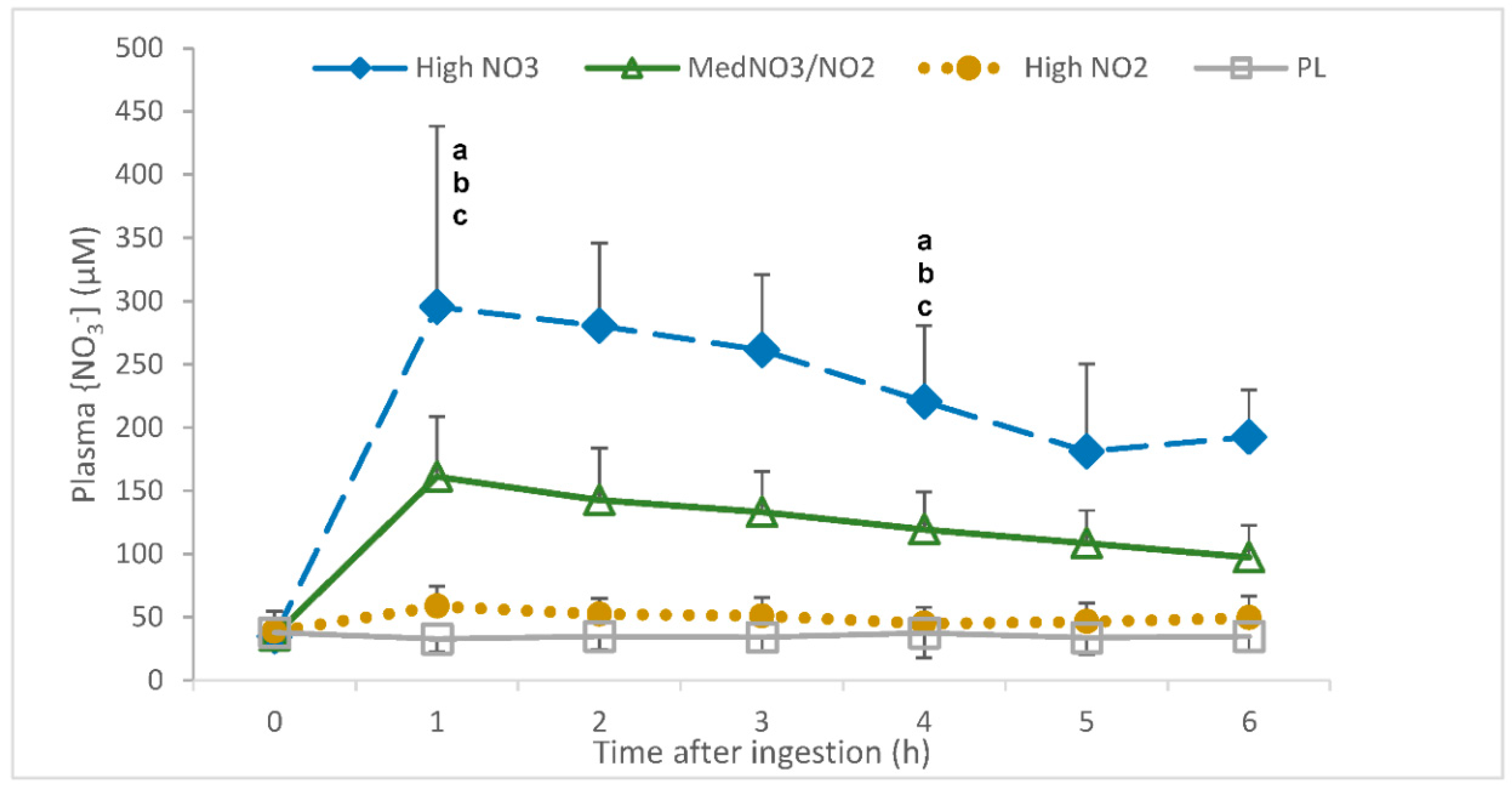
3.1.2. Plasma NO3−
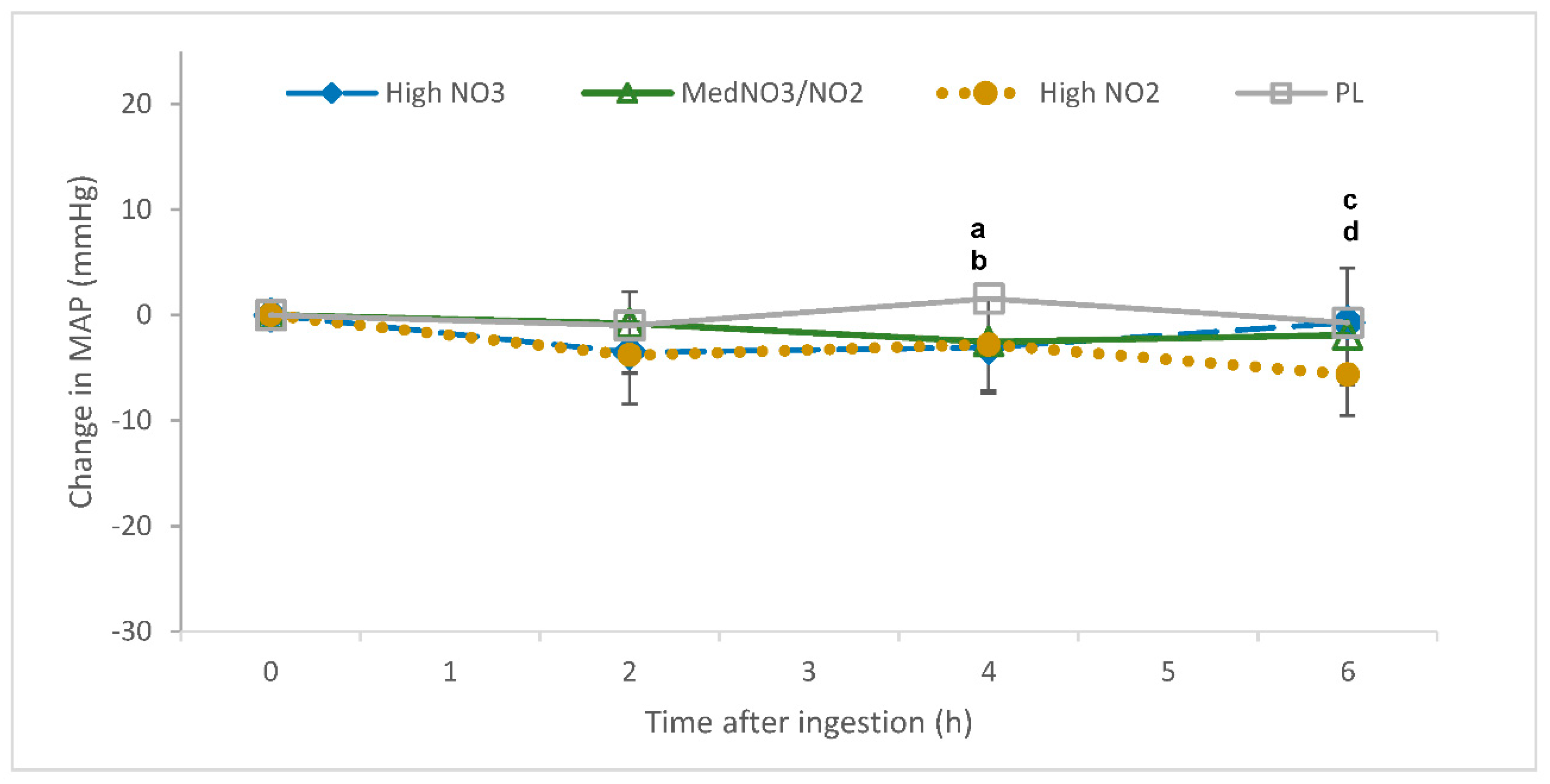
3.2. Blood Pressure (BP)
3.3. Heart Rate
3.4. USCOM Measures
3.5. Correlations
4. Discussion
4.1. Plasma [NO2−] and [NO3−]
4.2. Blood Pressure
4.2.1. Systolic and Diastolic Blood Pressure
4.2.2. Cardiovascular Functions
4.2.3. Mean Arterial Pressure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heart Foundation NZ. General Health Statistics in New Zealand. Available online: https://www.heartfoundation.org.nz/statistics-2 (accessed on 1 December 2017).
- Deb, S.; Dasgupta, A. A Study on Risk Factors of Cardiovascular Diseases in an Urban Health Center of Kolkata. Indian J. Community Med. Off. Publ. Indian Assoc. Prev. Soc. Med. 2008, 33, 271. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, E.T.; Fabsitz, R.R.; Devereux, R.; Best, L.; Welty, T.K.; Howard, B.V. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: The strong heart study. Hypertension 2006, 47, 403–409. [Google Scholar] [CrossRef]
- Borgi, L.; Muraki, I.; Satija, A.; Willett, W.C.; Rimm, E.B.; Forman, J.P. Fruit and Vegetable Consumption and the Incidence of Hypertension in Three Prospective Cohort Studies. Hypertension 2016, 67, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Challa, H.J.; Uppaluri, K.R. Dash Diet (Dietary Approaches to Stop Hypertension); StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Dauchet, L.; Kesse-Guyot, E.; Czernichow, S.; Bertrais, S.; Estaquio, C.; Péneau, S.; Vergnaud, A.-C.; Chat-Yung, S.; Castetbon, K.; Deschamps, V. Dietary patterns and blood pressure change over 5-Y follow-up in the Su. Vi. max cohort. Am. J. Clin. Nutr. 2007, 5, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The women’s health study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The physicians’ health study ii randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Waters, D.D.; Alderman, E.L.; Hsia, J.; Howard, B.V.; Cobb, F.R.; Rogers, W.J.; Ouyang, P.; Thompson, P.; Tardif, J.C.; Higginson, L. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA 2002, 288, 2432–2440. [Google Scholar] [CrossRef]
- Remington, J.; Winters, K. Effectiveness of dietary inorganic nitrate in lowering blood pressure in hypertensive adults: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 2445–2452. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Ascherio, A.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Hennekens, C.H.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999, 282, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Eliot, K.; Heuertz, R.; Weiss, E. Whole beetroot consumption acutely improves running performance. J. Acad. Nutr. Diet. 2012, 112, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Habermeyer, M.; Roth, A.; Guth, S.; Diel, P.; Engel, K.H.; Epe, B.; Fürst, P.; Heinz, V.; Humpf, H.U.; Joost, H.G. Nitrate and nitrite in the diet: How to assess their benefit and risk for human health. Mol. Nutr. Food Res. 2015, 59, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Shallenberger, R.; Downing, D.; Stoewsand, G.; Peck, N. Nitrate and nitrite nitrogen in fresh, stored and processed table beets and spinach from different levels of field nitrogen fertilisation. J. Sci. Food Agric. 1971, 2, 90–92. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as Found in Green Leafy Vegetables and Beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef]
- Carlström, M.; Lundberg, J.O.; Weitzberg, E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Actual Physiol. 2018, 224, e13080. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Bailey, S.J.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. Eur. J. Sport Sci. 2012, 12, 309–320. [Google Scholar] [CrossRef]
- Bian, K.; Doursout, M.F.; Murad, F. Vascular system: Role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. 2008, 10, 304–310. [Google Scholar] [CrossRef]
- Contreras, F.; Rivera, M.; Vasquez, J.; De la Parte, M.; Velasco, M. Endothelial dysfunction in arterial hypertension. J. Hum. Hypertens. 2000, 14, S20. [Google Scholar] [CrossRef] [PubMed]
- d’El-Rei, J.; Cunha, A.R.; Trindade, M.; Neves, M.F. Beneficial effects of dietary nitrate on endothelial function and blood pressure levels. Int. J. Hypertens. 2016, 2016, 6791519. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Shinde, S.; Moodley, Y.; Lundberg, J.O.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 368–375. [Google Scholar] [CrossRef]
- Floyd, C.N.; Lidder, S.; Hunt, J.; Omar, S.A.; McNeill, K.; Webb, A.J. Acute interaction between oral glucose (75 G as Lucozade) and inorganic nitrate: Decreased insulin clearance, but lack of blood pressure-lowering. Br. J. Clin. Pharmacol. 2019, 85, 1443–1453. [Google Scholar] [CrossRef]
- Brunton, T.L. On the use of nitrite of amyl in angina pectoris. Lancet 1867, 90, 97–98. [Google Scholar] [CrossRef]
- Reichert, E.T.; Mitchell, S.W. The physiological action of potassium nitrite. Am. J. Med. Sci. 1880, 156, 158–180. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Shelhamer, J.H.; Schechter, A.N.; Pease-Fye, M.E.; Waclawiw, M.A.; Panza, J.A.; Ognibene, F.P.; Cannon, R.O. Role of circulating nitrite and s-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc. Natl. Acad. Sci. USA 2000, 97, 11482–11487. [Google Scholar] [CrossRef] [PubMed]
- Hunault, C.C.; van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Meulenbelt, J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol. Lett. 2009, 190, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; Pinckaers, P.J.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults–3. J. Nutr. 2016, 146, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Ormesher, L.; Myers, J.E.; Chmiel, C.; Wareing, M.; Greenwood, S.L.; Tropea, T.; Lundberg, J.O.; Weitzberg, E.; Nihlen, C.; Sibley, C.P. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: A randomised, double-blind, placebo-controlled feasibility trial. Nitric Oxide 2018, 80, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef]
- DeVan, A.E.; Johnson, L.C.; Brooks, F.A.; Evans, T.D.; Justice, J.N.; Cruickshank-Quinn, C.; Reisdorph, N.; Bryan, N.S.; McQueen, M.B.; Santos-Parker, J.R. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J. Appl. Physiol. 2015, 120, 416–425. [Google Scholar] [CrossRef]
- Duranski, M.R.; Greer, J.J.; Dejam, A.; Jaganmohan, S.; Hogg, N.; Langston, W.; Patel, R.P.; Yet, S.-F.; Wang, X.; Kevil, C.G. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005, 115, 1232–1240. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Nagababu, E.; Barbiro-Michaely, E.; Ramasamy, S.; Pluta, R.M.; Mayevsky, A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: A role for red cell NO. Nitric Oxide 2007, 16, 448–456. [Google Scholar] [CrossRef]
- Sindler, A.L.; Fleenor, B.S.; Calvert, J.W.; Marshall, K.D.; Zigler, M.L.; Lefer, D.J.; Seals, D.R. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 2011, 10, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.T.; Clifton, P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutr. J. 2012, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X. Nitrite reduction to Nitric Oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498. [Google Scholar] [CrossRef] [PubMed]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Wong, M.; Jirangrat, W.; Teh, K.H.; Ali, A. Acute supplementation with Nitrate-rich beetroot juice causes a greater increase in plasma nitrite and reduction in blood pressure of older compared to younger adults. Nutrients 2019, 11, 1683. [Google Scholar] [CrossRef]
- Chou, S.-S.; Chung, J.-C.; Hwang, D.-F. A high performance liquid chromatography method for determining nitrate and nitrite levels in vegetables. J. Food Drug Anal. 2003, 11. [Google Scholar] [CrossRef]
- Cheng, C.F.; Tsang, C.W. Simultaneous determination of nitrite, nitrate and ascorbic acid in canned vegetable juices by reverse-phase ion-interaction HPLC. Food Addit. Contam. 1998, 15, 753–758. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef]
- Ogedegbe, G.; Pickering, T. Principles and Techniques of Blood Pressure Measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Chan, Y. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Clements, W.T.; Lee, S.-R.; Bloomer, R.J. Nitrate Ingestion: A review of the health and physical performance effects. Nutrients 2014, 6, 5224–5264. [Google Scholar] [CrossRef] [PubMed]
- McIlvenna, L.C.; Monaghan, C.; Liddle, L.; Fernandez, B.O.; Feelisch, M.; Muggeridge, D.J.; Easton, C. Beetroot juice versus chard gel: A pharmacokinetic and pharmacodynamic comparison of nitrate bioavailability. Nitric Oxide 2017, 64, 61–67. [Google Scholar] [CrossRef]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Wong, M.; Jirangrat, W.; Teh, K.H.; Ali, A. Does acute supplementation with nitrate-rich beetroot juice benefit older adults more than younger adults. Multidiscip. Digit. Publ. Inst. Proc. 2019, 8, 26. [Google Scholar] [CrossRef]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The nitrate-independent blood pressure–lowering effect of beetroot juice: A systematic review and meta-analysis. Adv. Nutr. 2017, 8, 830–838. [Google Scholar] [CrossRef]
- Oggioni, C.; Jakovljevic, D.; Klonizakis, M.; Ashor, A.; Ruddock, A.; Ranchordas, M.; Williams, E.; Siervo, M. Dietary nitrate does not modify blood pressure and cardiac output at rest and during exercise in older adults: A randomised cross-over study. Int. J. Food Sci. Nutr. 2018, 69, 74–83. [Google Scholar] [CrossRef]
- Carlström, M.; Liu, M.; Yang, T.; Zollbrecht, C.; Huang, L.; Peleli, M.; Borniquel, S.; Kishikawa, H.; Hezel, M.; Persson, A.E.G. Cross-Talk between nitrate-nitrite-no and no synthase pathways in control of vascular no homeostasis. Antioxid. Redox Signal. 2015, 23, 295–306. [Google Scholar] [CrossRef]
- Lee, C.-W.; Li, D.; Channon, K.M.; Paterson, D.J. L-Arginine supplementation reduces cardiac noradrenergic neurotransmission in spontaneously hypertensive rats. J. Mol. Cell. Cardiol. 2009, 47, 149–155. [Google Scholar] [CrossRef][Green Version]
- White, D.W.; Raven, P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited. J. Physiol. 2014, 592, 2491–2500. [Google Scholar] [CrossRef]
- Thorin, E.; Thorin-Trescases, N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovasc. Res. 2009, 84, 24–32. [Google Scholar] [CrossRef]
- Zand, J.; Lanza, F.; Garg, H.K.; Bryan, N.S. All-Natural nitrite and nitrate containing dietary supplement promotes Nitric Oxide production and reduces triglycerides in humans. Nutr. Res. 2011, 31, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.T.; Levitt, E.L.; Hodges, G.J.; Wong, B.J. Short-term dietary nitrate supplementation augments cutaneous vasodilatation and reduces mean arterial pressure in healthy humans. Microvasc. Res. 2015, 98, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kemmner, S.; Lorenz, G.; Wobst, J.; Kessler, T.; Wen, M.; Günthner, R.; Stock, K.; Heemann, U.; Burkhardt, K.; Baumann, M. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: A pilot study. Nitric Oxide 2017, 64, 7–15. [Google Scholar] [CrossRef] [PubMed]



| Drink | Nitrate mg | Nitrite mg | Nitrate mmol | Nitrite mmol |
|---|---|---|---|---|
| High NO3−, low NO2− (HL) | 572 | 32 | 6.72 | 0.46 |
| Medium NO3−, medium NO2− (MM) | 280 | 237 | 3.29 | 3.43 |
| Low NO3−, medium NO2− (LM) | 43 | 262 | 0.51 | 3.79 |
| Low NO3−, low NO2− (PL) | 8 | 5.8 | 0.09 | 0.08 |
| Participant Characteristics | Total (n = 11) |
|---|---|
| Age (y) | 24 ± 5.7 |
| Height (cm) | 173 ± 8.9 |
| Body mass (kg) | 67.6 ± 13.3 |
| Plasma NO2− (μM) | 0.77 ± 0.086 * |
| Plasma NO3− (μM) | 36.74 ± 1.89 * |
| SBP (mmHg) | 111.36 ± 1.73 * |
| DBP (mmHg) | 70.83 ± 1.22 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubcik, E.M.; Rutherfurd-Markwick, K.; Chabert, M.; Wong, M.; Ali, A. Pharmacokinetics of Nitrate and Nitrite Following Beetroot Juice Drink Consumption. Nutrients 2021, 13, 281. https://doi.org/10.3390/nu13020281
Jakubcik EM, Rutherfurd-Markwick K, Chabert M, Wong M, Ali A. Pharmacokinetics of Nitrate and Nitrite Following Beetroot Juice Drink Consumption. Nutrients. 2021; 13(2):281. https://doi.org/10.3390/nu13020281
Chicago/Turabian StyleJakubcik, Emily Margaret, Kay Rutherfurd-Markwick, Marsanne Chabert, Marie Wong, and Ajmol Ali. 2021. "Pharmacokinetics of Nitrate and Nitrite Following Beetroot Juice Drink Consumption" Nutrients 13, no. 2: 281. https://doi.org/10.3390/nu13020281
APA StyleJakubcik, E. M., Rutherfurd-Markwick, K., Chabert, M., Wong, M., & Ali, A. (2021). Pharmacokinetics of Nitrate and Nitrite Following Beetroot Juice Drink Consumption. Nutrients, 13(2), 281. https://doi.org/10.3390/nu13020281







