Novel Dietary Proteins Selectively Affect Intestinal Health In Vitro after Clostridium difficile-Secreted Toxin A Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Caco-2 Cell Culture
2.3. TranswellTM Model (TW)
2.4. Bioengineered Intestinal Tubules
2.5. Static In Vitro Digestion
2.6. Toxin A and Potential Dietary Proteins
2.7. Transepithelial Electrical Resistance
2.8. Inulin-FITC Leakage Assay
2.9. Immunofluorescent Staining and Zonula Occludens-1 Quantification
2.10. Cell Viability
2.11. Alkaline Phosphatase Activity Assay
2.12. IL-6 and IL-8 Secretion
2.13. Nitric Oxide Content
2.14. Statistical Analysis
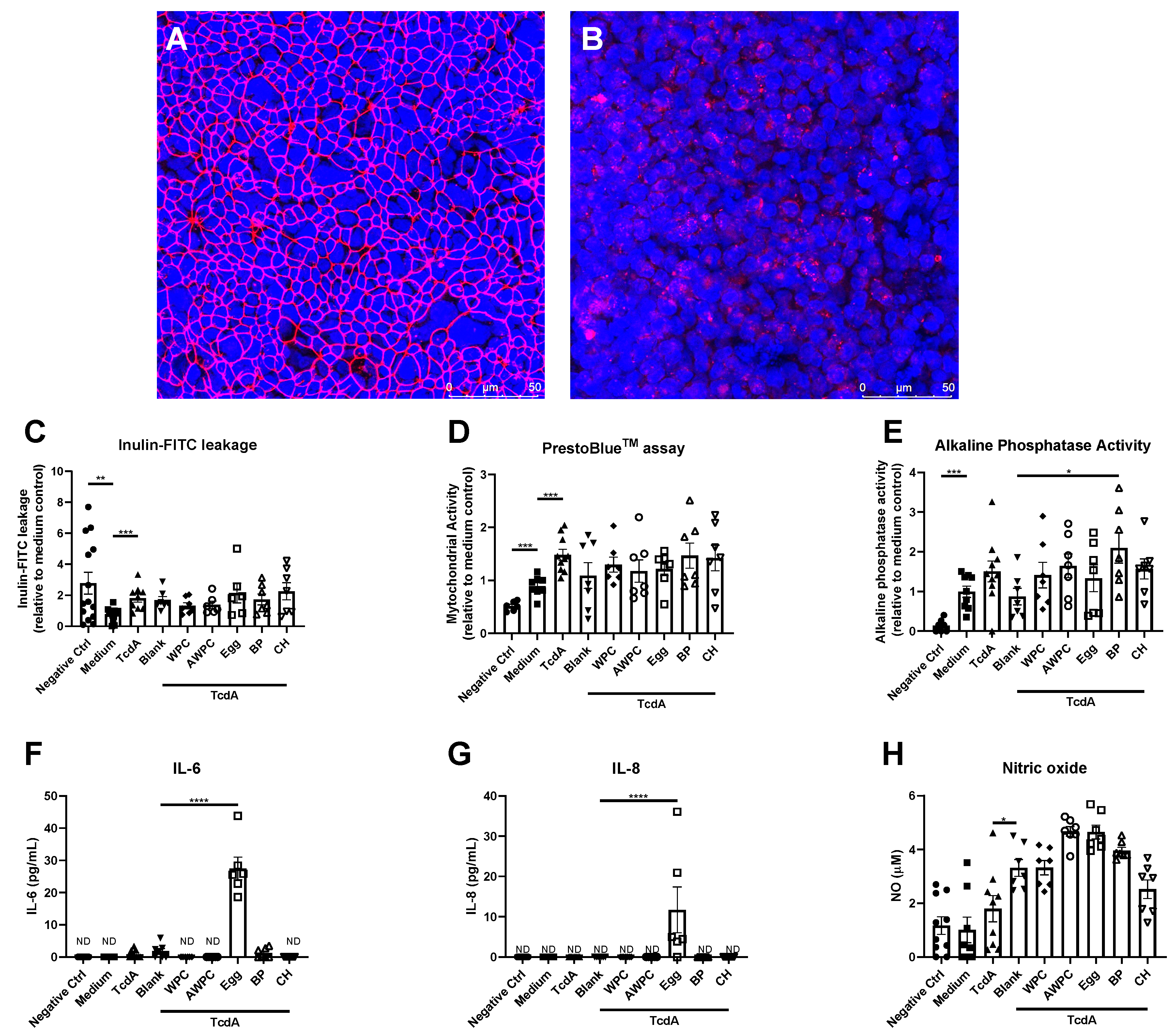
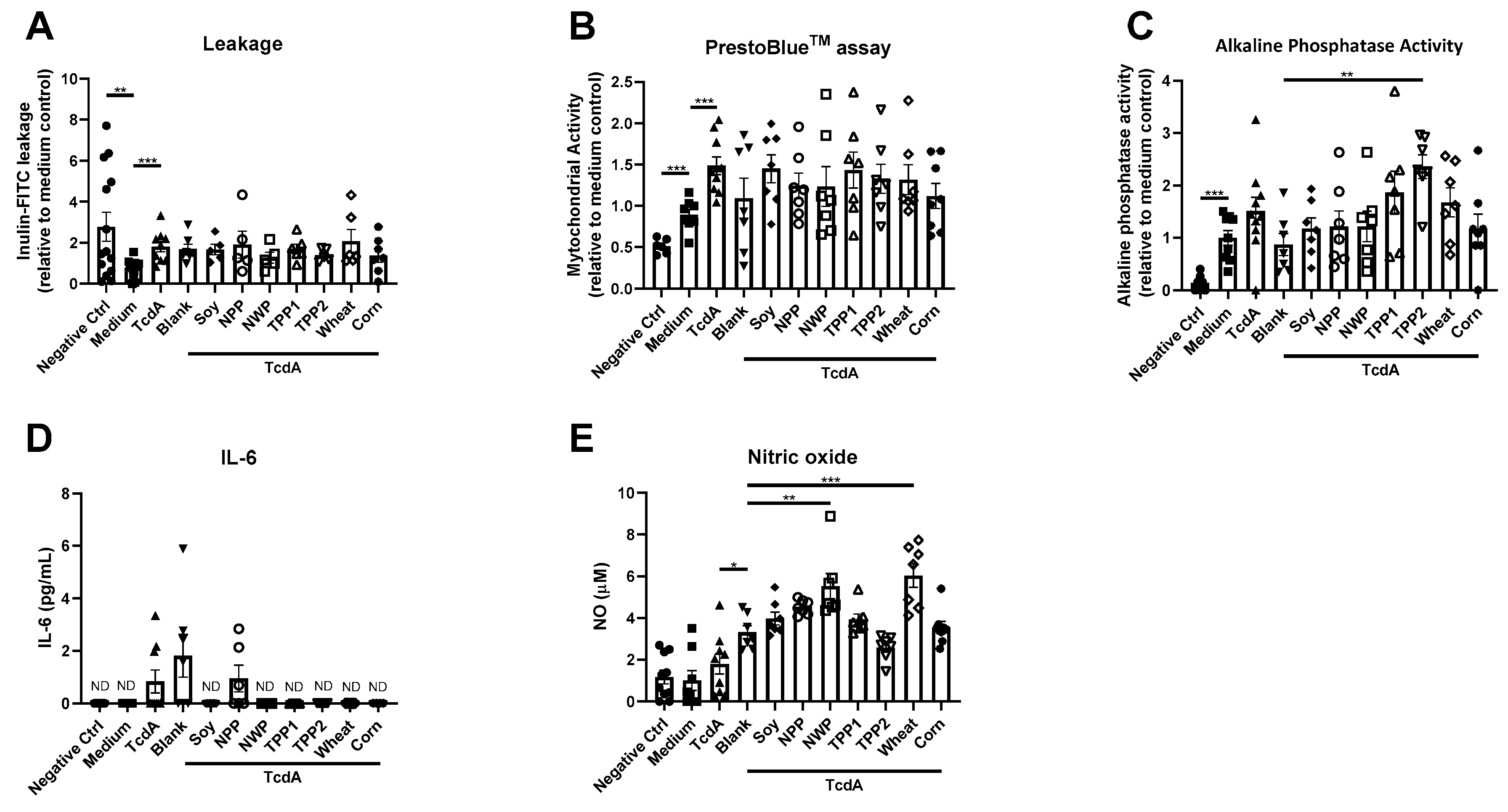
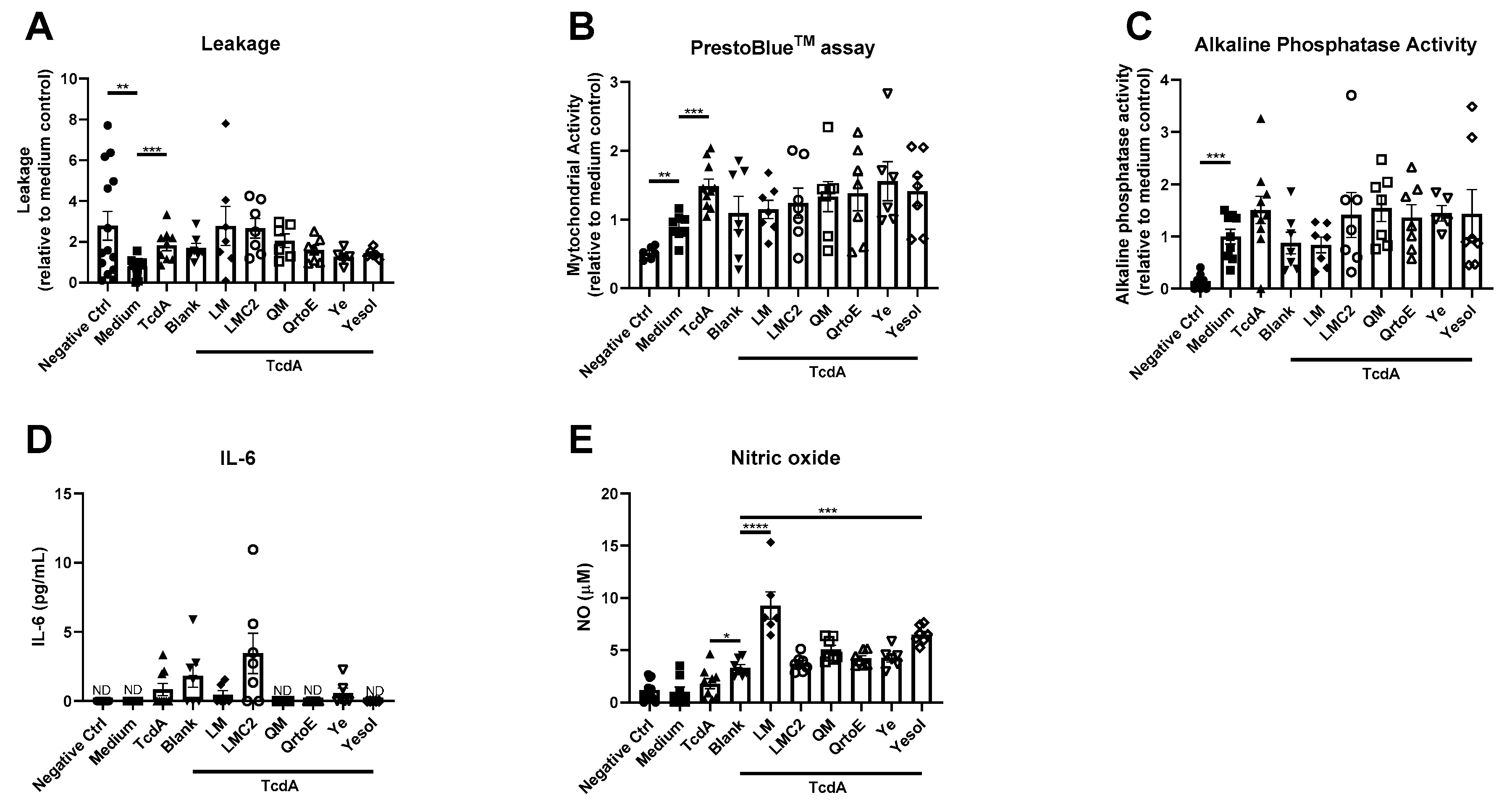
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sattar, S.B.A.; Singh, S. Bacterial Gastroenteritis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, CA, USA, 2019. [Google Scholar]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care–associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Deneve, C.; Janoir, C.; Poilane, I.; Fantinato, C.; Collignon, A. New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents 2009, 33, S24–S28. [Google Scholar] [CrossRef]
- Ofosu, A. Clostridium difficile infection: A review of current and emerging therapies. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2016, 29, 147–154. [Google Scholar] [CrossRef]
- Bouza, E.; Munoz, P.; Alonso, R. Clinical manifestations, treatment and control of infections caused by Clostridium difficile. Clin. Microbiol. Infect. 2005, 11, 57–64. [Google Scholar] [CrossRef]
- Nelson, R.L.; Suda, K.J.; Evans, C.T. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Alyousef, A.A. Clostridium difficile: Epidemiology, Pathogenicity, and an Update on the Limitations of and Challenges in Its Diagnosis. J. AOAC Int. 2018, 101, 1119–1126. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Jochems, P.; Garssen, J.; van Keulen, A.; Masereeuw, R.; Jeurink, P. Evaluating human intestinal cell lines for studying dietary protein absorption. Nutrients 2018, 10, 322. [Google Scholar] [CrossRef]
- Jochems, P.G.; Keusters, W.R.; America, A.H.; Rietveld, P.C.; Bastiaan-Net, S.; Ariëns, R.M.; Tomassen, M.M.; Lewis, F.; Li, Y.; Westphal, K.G. A combined microphysiological-computational omics approach in dietary protein evaluation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Martínez-Augustin, O.; Rivero-Gutiérrez, B.; Mascaraque, C.; Sánchez de Medina, F. Food derived bioactive peptides and intestinal barrier function. Int. J. Mol. Sci. 2014, 15, 22857–22873. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Jochems, P.G.; van Bergenhenegouwen, J.; van Genderen, A.M.; Eis, S.T.; Versprille, L.J.W.; Wichers, H.J.; Jeurink, P.V.; Garssen, J.; Masereeuw, R. Development and validation of bioengineered intestinal tubules for translational research aimed at safety and efficacy testing of drugs and nutrients. Toxicol. Vitr. 2019, 60, 1–11. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- Chandrasekaran, R.; Lacy, D.B. The role of toxins in Clostridium difficile infection. FEMS Microbiol. Rev. 2017, 41, 723–750. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Gerhard, R.; Nottrott, S.; Schoentaube, J.; Tatge, H.; Olling, A.; Just, I. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J. Med Microbiol. 2008, 57, 765–770. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.S.; Jung, H.C.; Oh, Y.k.; Song, I.S.; Kim, C.Y. Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in response to Clostridium difficile toxin A. Microbiol. Immunol. 2002, 46, 333–342. [Google Scholar] [CrossRef]
- Taylor, R.C.; Webb Robertson, B.-J.M.; Markillie, L.M.; Serres, M.H.; Linggi, B.E.; Aldrich, J.T.; Hill, E.A.; Romine, M.F.; Lipton, M.S.; Wiley, H.S. Changes in translational efficiency is a dominant regulatory mechanism in the environmental response of bacteria. Integr. Biol. 2013, 5, 1393–1406. [Google Scholar] [CrossRef]
- He, D.; Hagen, S.; Pothoulakis, C.; Chen, M.; Medina, N.; Warny, M.; LaMont, J. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 2000, 119, 139–150. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Sougioultzis, S.; Hagen, S.; Liu, J.; Keates, S.; Keates, A.C.; Pothoulakis, C.; LaMont, J.T. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 2002, 122, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Gravel, S.-P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Stierum, R.; Gaspari, M.; Dommels, Y.; Ouatas, T.; Pluk, H.; Jespersen, S.; Vogels, J.; Verhoeckx, K.; Groten, J.; van Ommen, B. Proteome analysis reveals novel proteins associated with proliferation and differentiation of the colorectal cancer cell line Caco-2. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2003, 1650, 73–91. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Grefte, S.; de Boer, V.C.; Zeper, L.; van Dartel, D.A.; van der Stelt, I.; Bekkenkamp-Grovenstein, M.; van Norren, K.; Wichers, H.J.; Keijer, J. Mitochondrial ATP depletion disrupts Caco-2 monolayer integrity and internalizes claudin 7. Front. Physiol. 2017, 8, 794. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.; de Boer, V.C. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. [Google Scholar] [CrossRef]
- Narisawa, S.; Huang, L.; Iwasaki, A.; Hasegawa, H.; Alpers, D.H.; Millán, J.L. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol. Cell. Biol. 2003, 23, 7525–7530. [Google Scholar] [CrossRef]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef]
- Ferrier, L.; Bérard, F.; Debrauwer, L.; Chabo, C.; Langella, P.; Buéno, L.; Fioramonti, J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am. J. Pathol. 2006, 168, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010, 68, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. WJG 2014, 20, 15650–15656. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef]
- de Medina, F.S.; Martínez-Augustin, O.; González, R.; Ballester, I.; Nieto, A.; Gálvez, J.; Zarzuelo, A. Induction of alkaline phosphatase in the inflamed intestine: A novel pharmacological target for inflammatory bowel disease. Biochem. Pharmacol. 2004, 68, 2317–2326. [Google Scholar] [CrossRef]
- Gibson, P.R.; Nov, R.; Fielding, M.; Mcintyre, A.; Finch, C.F.; Rosella, O.; Mariadason, J.M.; Barkla, D.H.; Young, G.P. Relationship of hydrolase activities to epithelial cell turnover in distal colonic mucosa of normal rats. J. Gastroenterol. Hepatol. 1999, 14, 866–872. [Google Scholar] [CrossRef]
- Fishman, W.H.; Ghosh, N.K. Influence of reagents reacting with metal, thiol and amino sites of catalytic activity and l-phenylalanine inhibition of rat intestinal alkaline phosphatase. Biochem. J. 1967, 105, 1163–1170. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Brabcová, A.; Zdráhal, Z.; Horáčková, V. Amino acid composition and nutritional value of four cultivated South American potato species. J. Food Compos. Anal. 2015, 40, 78–85. [Google Scholar] [CrossRef]
- Luecke, R.W.; Olman, M.E.; Baltzer, B.V. Zinc deficiency in the rat: Effect on serum and intestinal alkaline phosphatase activities. J. Nutr. 1968, 94, 344–350. [Google Scholar] [CrossRef]
- Jagoš, P.; Dvořák, V.; Bouda, J. Levels of Mineral in the Blood Plasma of Cows and their Calves fed from Buckets. Acta Vet. Brno 1981, 50, 33–41. [Google Scholar] [CrossRef][Green Version]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Kozol, R.A. Neutrophil recruitment to the gastrointestinal tract. J. Surg. Res. 1992, 53, 310–315. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–195. [Google Scholar] [CrossRef]
- Andersen, C.J. Bioactive egg components and inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef]
- Tannock, L.R.; O’Brien, K.D.; Knopp, R.H.; Retzlaff, B.; Fish, B.; Wener, M.H.; Kahn, S.E.; Chait, A. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation 2005, 111, 3058–3062. [Google Scholar] [CrossRef]
- Ratliff, J.C.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr. Metab. 2008, 5, 6. [Google Scholar] [CrossRef]
- Andersen, C.J.; Blesso, C.N.; Lee, J.; Fernandez, M.L. Egg Intake Increases Peripheral Blood Mononuclear Cell Expression of Atp-Binding Cassette Transporter A1 in Parallel with Toll-Like Receptor 4 as a Potential Mechanism to Reduce Cellular Inflammation in Metabolic Syndrome; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 2013. [Google Scholar]
- Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients 2015, 7, 3449–3463. [Google Scholar] [CrossRef]
- Melo Filho, A.; Souza, M.; Lyerly, D.; Cunha, F.; Lima, A.; Ribeiro, R. Role of tumor necrosis factor and nitric oxide in the cytotoxic effects of Clostridium difficile toxin A and toxin B on macrophages. Toxicon 1997, 35, 743–752. [Google Scholar] [CrossRef]
- Sobko, T.; Reinders, C.; Jansson, E.; Norin, E.; Midtvedt, T.; Lundberg, J. Gastrointestinal bacteria generate nitric oxide from nitrate and nitrite. Nitric Oxide 2005, 13, 272–278. [Google Scholar] [CrossRef]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology 2017, 152, 515–532.e512. [Google Scholar] [CrossRef] [PubMed]
| Full Name | Protein Source | Abbreviation | Protein Origin | Carbohydrates (%) | Fat (%) | Protein (%) | Kcal/100 g | Distributor |
|---|---|---|---|---|---|---|---|---|
| Whey protein concentrate | Whey | WPC | Animal | 17 | 0.4 | 74 | N/A | Danone Nutricia Research |
| Acidic whey protein concentrate | Whey | AWPC | Animal | 7 | 8 | 82 | N/A | Danone Nutricia Research |
| Egg | Egg white | Egg | Animal | 4.5 | 0.3 | 82.2 | 373 | Bulk Powders |
| Soy | Soya Bean | Soy | Plant | 1 | 3.3 | 91 | 378 | Bulk Powders |
| NPP | Pea | NPP | Plant | 3 | 6 | 80 | 395 | Roquette |
| NWP | Wheat | NWP | Plant | 4 | 7 | 82 | 409 | Roquette |
| Bovine plasma | Blood protein | BP | Animal | 0 | 2 | 72 | 298 | Darling Ingredients |
| Collagen hydrolysate | Blood protein | CH | Animal | 0 | 0 | 91.8 | 360 | Darling Ingredients |
| Lesser mealworm | Insect | LM | Alternative | 11 | 30.9 | 58 | N/A | Proti-Farm |
| Lesser mealworm concentrate 2 | Insect | LMC2 | Alternative | N/A | N/A | N/A | N/A | Proti-Farm |
| TPP1 | Potato | TPP1 | Plant | 0 | 5 | 95 | 378 | Avebe |
| TPP2 | Potato | TPP2 | Plant | 2 | 0 | 90 | 374 | Avebe |
| Quorn mycoprotein | Fungi | QM | Alternative | 5.6 | 7.5 | 53 | 361 | Quorn Foods |
| Quorn ready-to-eat | Fungi/egg | QrtoE | Alternative | 1.7 | 2.6 | 13.8 | 417 | Quorn Foods |
| Wheat protein | Wheat | Wheat | Plant | 9 | 5 | 79 | 399 | Cargill |
| Corn protein | Corn | Corn | Plant | 4 | 0.4 | 84 | 370 | Cargill |
| Yeast | Yeast | YE | Alternative | 1.2 | 3.2 | 88.4 | N/A | Lesaffre |
| Yesol | Yeast | Yesol | Alternative | N/A | N/A | N/A | N/A | Lesaffre |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochems, P.G.M.; Garssen, J.; Rietveld, P.C.S.; Govers, C.; Tomassen, M.M.M.; Wichers, H.J.; van Bergenhenegouwen, J.; Masereeuw, R. Novel Dietary Proteins Selectively Affect Intestinal Health In Vitro after Clostridium difficile-Secreted Toxin A Exposure. Nutrients 2020, 12, 2782. https://doi.org/10.3390/nu12092782
Jochems PGM, Garssen J, Rietveld PCS, Govers C, Tomassen MMM, Wichers HJ, van Bergenhenegouwen J, Masereeuw R. Novel Dietary Proteins Selectively Affect Intestinal Health In Vitro after Clostridium difficile-Secreted Toxin A Exposure. Nutrients. 2020; 12(9):2782. https://doi.org/10.3390/nu12092782
Chicago/Turabian StyleJochems, Paulus G. M., Johan Garssen, Pascale C. S. Rietveld, Coen Govers, Monic M. M. Tomassen, Harry J. Wichers, Jeroen van Bergenhenegouwen, and Rosalinde Masereeuw. 2020. "Novel Dietary Proteins Selectively Affect Intestinal Health In Vitro after Clostridium difficile-Secreted Toxin A Exposure" Nutrients 12, no. 9: 2782. https://doi.org/10.3390/nu12092782
APA StyleJochems, P. G. M., Garssen, J., Rietveld, P. C. S., Govers, C., Tomassen, M. M. M., Wichers, H. J., van Bergenhenegouwen, J., & Masereeuw, R. (2020). Novel Dietary Proteins Selectively Affect Intestinal Health In Vitro after Clostridium difficile-Secreted Toxin A Exposure. Nutrients, 12(9), 2782. https://doi.org/10.3390/nu12092782





