Vitamin Status and Diet in Elderly with Low and High Socioeconomic Status: The Lifelines-MINUTHE Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Measurements
2.3. Definition of Vitamin Status
2.4. Dietary Assessment
2.5. Statistical Analyses
3. Results
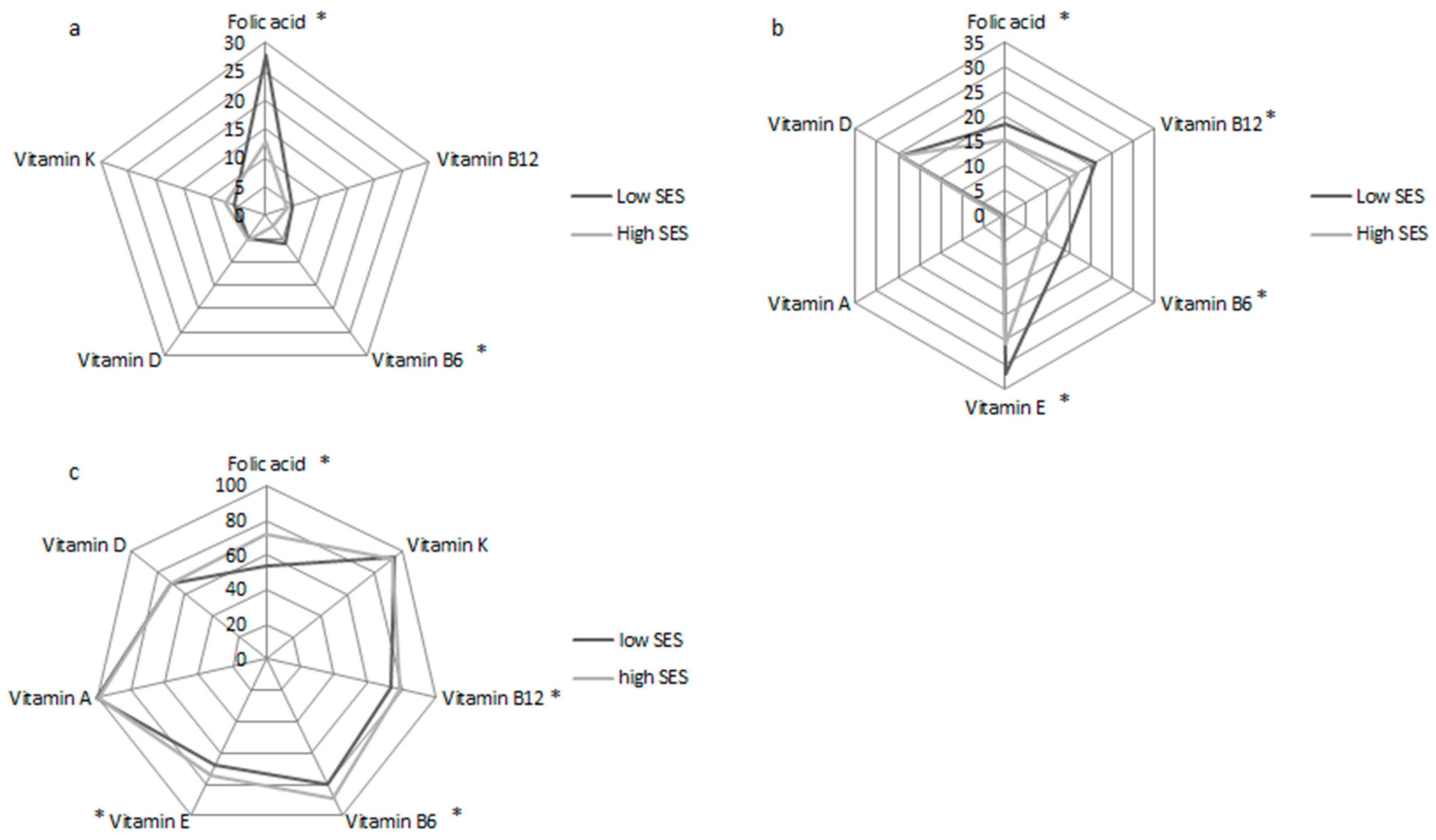
3.1. Participant Characteristics, Vitamin Status, and Diet
3.2. Associations between SES and Vitamin Status
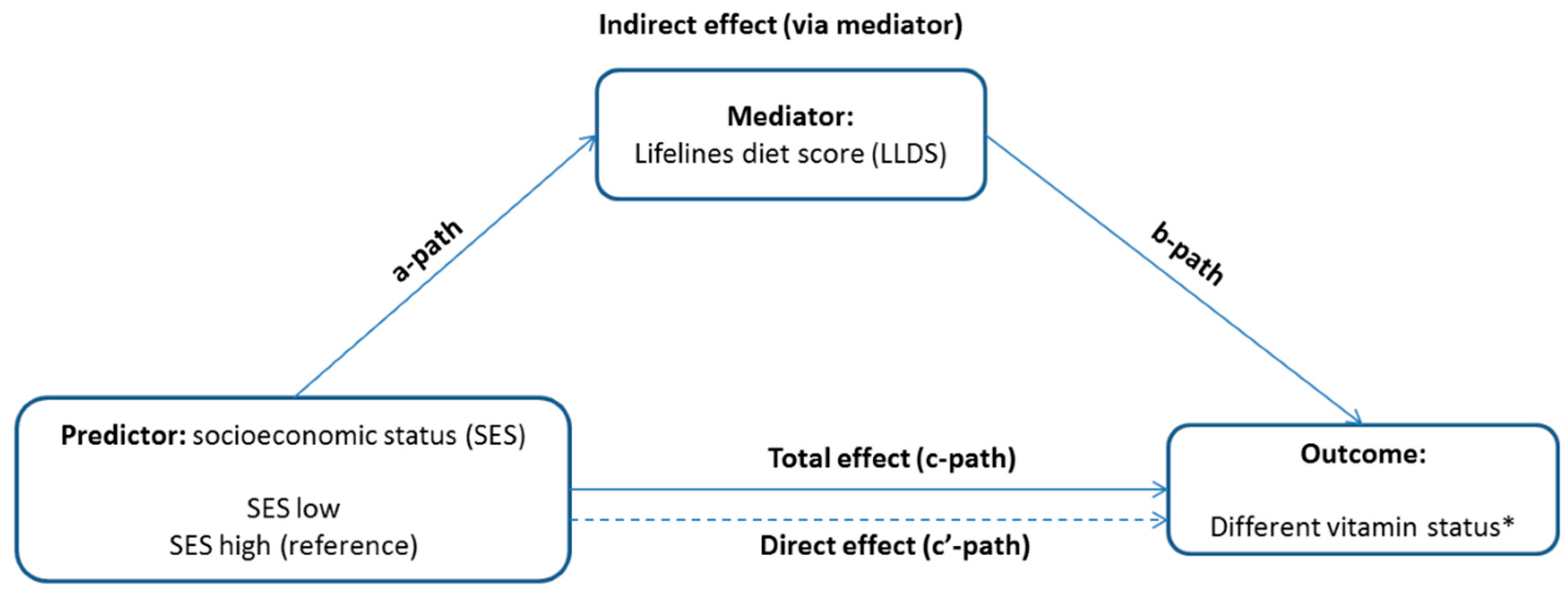
3.3. The Mediating role of the LLDS in the Associations between SES and Vitamin Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stringhini, S.; Carmeli, C.; Jokela, M.; Avendaño, M.; Muennig, P.; Guida, F.; Ricceri, F.; d’Errico, A.; Barros, H.; Bochud, M.; et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1·7 million men and women. Lancet 2017, 389, 1229–1237. [Google Scholar] [CrossRef]
- Hosseinpoor, A.R.; Bergen, N.; Mendis, S.; Harper, S.; Verdes, E.; Kunst, A.; Chatterji, S. Socioeconomic inequality in the prevalence of noncommunicable diseases in low—and middle-income countries: Results from the World Health Survey. BMC Public Health 2012, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Mackenbach, J.P.; Stirbu, I.; Roskam, A.-J.R.; Schaap, M.M.; Menvielle, G.; Leinsalu, M.; Kunst, A.E. Socioeconomic Inequalities in Health in 22 European Countries. N. Engl. J. Med. 2008, 358, 2468–2481. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan. for the Prevention and Control of Noncommunicable Diseases (2013–2020); WHO Document Production Services: Geneva, Switzerland, 2013. [Google Scholar]
- Bull, E.R.; Dombrowski, S.U.; McCleary, N.; Johnstone, M. Are interventions for low-income groups effective in changing healthy eating, physical activity and smoking behaviours? A systematic review and meta-analysis. BMJ Open 2014, 4, e006046. [Google Scholar] [CrossRef]
- Allen, L.; Williams, J.; Townsend, N.; Mikkelsen, B.; Roberts, N.; Foster, C.; Wickramasinghe, K. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: A systematic review. Lancet Glob. Health 2017, 5, e277–e289. [Google Scholar] [CrossRef]
- Stringhini, S.; Sabia, S.; Shipley, M.; Brunner, E.; Nabi, H.; Kivimaki, M.; Singh-Manoux, A. Association of Socioeconomic Position With Health Behaviors and Mortality. JAMA J. Am. Med. Assoc. 2010, 303, 1159–1166. [Google Scholar] [CrossRef]
- De Irala-Estevez, J.; Groth, M.; Johansson, L.; Oltersdorf, U.; Prättälä, R.; Martínez-González, M.A. A systematic review of socio-economic differences in food habits in Europe: Consumption of fruit and vegetables. Eur. J. Clin. Nutr. 2000, 54, 706–714. [Google Scholar] [CrossRef]
- Novaković, R.; Cavelaars, A.; Geelen, A.; Nikolić, M.; Altaba, I.I.; Vinas, B.R.; Ngo, J.; Golsorkhi, M.; Warthon Medina, M.; Brzozowska, A.; et al. Socio-economic determinants of micronutrient intake and status in Europe: A systematic review. Public Health Nutr. 2014, 17, 1031–1045. [Google Scholar] [CrossRef]
- Giskes, K.; Avendaňo, M.; Brug, J.; Kunst, A.E. A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes. Rev. 2010, 11, 413–429. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Mavrogianni, C.; Bos, R.; Singh-Povel, C. Micronutrient intakes among children and adults in Greece: The role of age, sex and socio-economic status. Nutrients 2014, 6, 4073–4092. [Google Scholar] [CrossRef]
- Cembranel, F.; Wagner, K.J.P.; González-Chica, D.A.; d’Orsi, E. Education and Income Levels are Associated With Energy and Micronutrients Intake: Results of a Study With Adults in a Capital City in Southern Brazil. Int. J. Vitam. Nutr. Res. 2019, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Du, Y.; Hong, W.; Tang, W.; Li, H.; Chen, M.; Zheng, S. Factors associated to serum 25-hydroxyvitamin D levels among older adult populations in urban and suburban communities in Shanghai, China. BMC Geriatr. 2017, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vega, M.F.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Vitamin D deficiency in older adults and its associated factors: A cross-sectional analysis of the Mexican Health and Aging Study. Arch. Osteoporos. 2017, 12, 8. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Akobundu, U.; Bailey, R.L.; Shlisky, J.; Beaudreault, A.R.; Bergeron, G.; Blancato, R.B.; Blumberg, J.B.; Bourassa, M.W.; Gomes, F.; et al. Hidden Hunger: Solutions for America’s Aging Populations. Nutrients 2018, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Von Arnim, C.A.F. Nutrition Security in Older Adults: Status Quo and Future Development. In Sustainable Nutrition in a Changing World; Hans Konrad Biesalski, A.D., Dwyer, J.T., Strain, J.J., Weber, P., Eggersdorfer, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 61–73. [Google Scholar]
- Stolk, R.P.; Rosmalen, J.G.; Postma, D.S.; de Boer, R.A.; Navis, G.; Slaets, J.P.; Wolffenbuttel, B.H. Universal risk factors for multifactorial diseases. Eur. J. Epidemiol. 2008, 23, 67–74. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.; Dotinga, A.; Vonk, J.M.; van Dijk, F.; van Zon, S.K.R.; Wijmenga, C.; Wolffenbuttel, B.H.R.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2014, 44, 1172–1180. [Google Scholar] [CrossRef]
- Klijs, B.; Scholtens, S.; Mandemakers, J.J.; Snieder, H.; Stolk, R.P.; Smidt, N. Representativeness of the LifeLines Cohort Study. PLoS ONE 2015, 10, e0137203. [Google Scholar] [CrossRef]
- Vart, P.; Gansevoort, R.T.; Coresh, J.; Reijneveld, S.A.; Bültmann, U. Socioeconomic measures and CKD in the United States and The Netherlands. Clin. J. Am. Soc. Nephrol. 2013, 8, 1685–1693. [Google Scholar] [CrossRef]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Smith, G.D. Indicators of socioeconomic position (part 1). J. Epidemiol. Commun. Health 2006, 60, 7–12. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO consultation. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Comstock, G.W.; Alberg, A.J.; Helzlsouer, K.J. Reported effects of long-term freezer storage on concentrations of retinol, beta-carotene, and alpha-tocopherol in serum or plasma summarized. Clin. Chem. 1993, 39, 1075–1078. [Google Scholar] [CrossRef]
- Jansen, E.H.J.M.; Beekhof, P.K. Stability of Folate and Vitamin B12 in Human Serum after Long-Term Storage: A Follow-Up after 13 Years. J. Nutr. Metab. 2018, 2018, 9834181. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Quasim, T.; McMillan, D.C.; Kinsella, J.; Williamson, C.; O’Reilly, D.S. Optimisation and validation of a sensitive high-performance liquid chromatography assay for routine measurement of pyridoxal 5-phosphate in human plasma and red cells using pre-column semicarbazide derivatisation. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2003, 792, 333–343. [Google Scholar] [CrossRef]
- De Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewé, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Trenson, S.; Verhamme, P.; Vermeer, C.; Staessen, J.A. Vitamin K–Dependent Matrix Gla Protein as Multifaceted Protector of Vascular and Tissue Integrity. Hypertension 2019, 73, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015, 53, 1149–1159. [Google Scholar] [CrossRef]
- Leklem, J.E. Vitamin B-6: A Status Report. J. Nutr. 1990, 120, 1503–1507. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998.
- Peter, S.; Friedel, A.; Roos, F.F.; Wyss, A.; Eggersdorfer, M.; Hoffmann, K.; Weber, P. A Systematic Review of Global Alpha-Tocopherol Status as Assessed by Nutritional Intake Levels and Blood Serum Concentrations. Int. J. Vitam. Nutr. Res. 2015, 85, 261–281. [Google Scholar] [CrossRef]
- World Health Organization. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Molag, M.L.; de Vries, J.H.; Duif, N.; Ocké, M.C.; Dagnelie, P.C.; Goldbohm, R.A.; van’t Veer, P. Selecting informative food items for compiling food-frequency questionnaires: Comparison of procedures. Br. J.Nutr. 2010, 104, 446–456. [Google Scholar] [CrossRef]
- Siebelink, E.; Geelen, A.; de Vries, J.H. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef]
- RIVM. Dutch Food Composition Table (NEVO). 2011. Available online: https://www.rivm.nl/nieuws/nieuwe-nevo-tabel-2011-beschikbaar. (accessed on 12 April 2012).
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Spaaij, C.J.K.; de Goede, J.; Weggemans, R.M. The 2015 Dutch food-based dietary guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Vinke, P.C.; Corpeleijn, E.; Dekker, L.H.; Jacobs, D.R.; Navis, G.; Kromhout, D. Development of the food-based Lifelines Diet. Score (LLDS) and its application in 129,369 Lifelines participants. Eur. J. Clin. Nutr. 2018, 72, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Stahre, M.; Roeber, J.; Kanny, D.; Brewer, R.D.; Zhang, X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic Dis. 2014, 11, E109. [Google Scholar] [CrossRef]
- Rosseel, Y. Lavaan: An. R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Sanchez, H.; Albala, C.; Lera, L.; Dangour, A.D.; Uauy, R. Effectiveness of the National Program of Complementary Feeding for older adults in Chile on vitamin B12 status in older adults; secondary outcome analysis from the CENEX Study (ISRCTN48153354). Nutr. J. 2013, 12, 124. [Google Scholar] [CrossRef]
- Iglesia, I.; Huybrechts, I.; Gonzalez-Gross, M.; Mouratidou, T.; Santabarbara, J.; Chajès, V.; Park, J.Y.; Bel-Serrat, S.; Cuenca-Garsia, M.; Castillo, M.; et al. Folate and vitamin B12 concentrations are associated with plasma DHA and EPA fatty acids in European adolescents: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br. J. Nutr. 2017, 117, 124–133. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; Ghada El-Hajj, F.; Bouillon, R. Current vitamin D status in European and Middle East. countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.J.; O’Halloran, A.M.; Carey, D.; O’Connor, D.; Kenny, R.A.; Molloy, A.M. Voluntary fortification is ineffective to maintain the vitamin B12 and folate status of older Irish adults: Evidence from the Irish Longitudinal Study on Ageing (TILDA). Br. J. Nutr. 2018, 120, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Quay, T.A.; Devlin, A.M.; Lamers, Y. Prevalence and Predictors of Low Vitamin B6 Status in Healthy Young Adult Women in Metro Vancouver. Nutrients 2016, 8, 538. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Kiely, M.; Lamberg-Allardt, C.; Durazo-Arvizu, R.A.; Sempos, C.T.; Koskinen, S.; Lundqvist, A.; Sundvall, J.; et al. Standardizing serum 25-hydroxyvitamin D data from four Nordic population samples using the Vitamin D Standardization Program. protocols: Shedding new light on vitamin D status in Nordic individuals. Scand. J. Clin. Lab. Invest. 2015, 75, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.; Luiking, Y.C.; de Groot, L.C. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, L.; Man, Q.; Wang, J.; Zhao, W.; Zhang, J. Dietary Micronutrients Intake Status among Chinese Elderly People Living at Home: Data from CNNHS 2010-2012. Nutrients 2019, 11, 1787. [Google Scholar] [CrossRef]
- De Walle, H.E.; de Jong-van den Berg, L.T. Growing gap in folic acid intake with respect to level of education in the Netherlands. Commun. Genet. 2007, 10, 93–96. [Google Scholar] [CrossRef]
- Weaver, S.P.; Passmore, C.; Collins, B.; Fung, E. Vitamin D, Sunlight Exposure, and Bone Density in Elderly African American Females of Low Socioeconomic Status. Fam. Med. 2010, 42, 47–51. [Google Scholar]
- Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; N, Estimation of the Period Covered by Vitamin B12 Stores; National Academies Press (US): Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- Froese, D.S.; Gravel, R.A. Genetic disorders of vitamin B12 metabolism: Eight complementation groups—eight genes. Expert Rev. Mol. Med. 2010, 12, e37. [Google Scholar] [CrossRef]
- Sun, A.L.; Ni, Y.H.; Li, X.B.; Zhuang, X.H.; Liu, Y.T.; Liu, X.H.; Chen, S.H. Urinary methylmalonic acid as an indicator of early vitamin B12 deficiency and its role in polyneuropathy in type 2 diabetes. J. Diabetes. Res. 2014, 2014, 921616. [Google Scholar] [CrossRef]
- Young, M.F.; Guo, J.; Williams, A.; Whitfield, K.C.; Nasrin, S.; Kancherla, V.; Suchdev, P.; Crider, K.S.; Pfeiffer, C.M.; Serdula, M. Interpretation of vitamin B-12 and folate concentrations in population-based surveys does not require adjustment for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2020, 111, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Lucock, M.; Scarlett, C.J.; Veysey, M.; Beckett, E.L. Folate and Inflammation—links between folate and features of inflammatory conditions. J. Nutr. Intermed. Metabol. 2019, 18, 100104. [Google Scholar] [CrossRef]
- Beenackers, M.A.; Kamphuis, C.B.; Giskes, K.; Brug, J.; Kunst, A.E.; Burdorf, A.; Van Lenthe, F.J. Socioeconomic inequalities in occupational, leisure-time, and transport related physical activity among European adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I. Public Health Aspects in the Prevention and Control. of Vitamin Deficiencies. Curr. Dev. Nutr. 2019, 3, nzz075. [Google Scholar] [CrossRef]
- Gödecke, T.; Stein, A.J.; Qaim, M. The global burden of chronic and hidden hunger: Trends and determinants. Glob. Food Secur. 2018, 17, 21–29. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); Population Division (2019); United Nations, Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
| Vitamins * | Vitamin status | ||
|---|---|---|---|
| Deficiency | Insufficiency | Sufficiency | |
| Folic acid, nmol/L [26] | <10.2 | 10.2–13.6 | >13.6 |
| Vitamin K, pmol/L [27,28] | - | >500 | ≤500 |
| Vitamin B12, pmol/L [26,29] | <150 | 150–220 | >200 |
| Vitamin B6, nmol/L [30,31] | <20 | 20–30 | >30 |
| Vitamin E, μmol/L [32] | - | <30 | ≥30 |
| Vitamin A, μmol/L [33] | - | <0.7 | ≥0.7 |
| Vitamin D, nmol/L [34] | <30 | 30–50 | >50 |
| Characteristics | Total | Low SES | High SES | p-Value |
|---|---|---|---|---|
| N = 1605 | N = 803 | N = 802 | ||
| Demographics | ||||
| Age, years | 65 (62–69) | 66 (63–70) | 64 (62–67) | <0.001 |
| Male gender, n (%) | 802 (50) | 400 (49.8) | 402 (50.1) | 0.9 |
| BMI, kg/m2 | 27.0 ± 4.1 | 28.2 ± 4.2 | 25.7 ± 3.6 | <0.001 |
| Normal, n (%) | 531 (33.1) | 161 (20.1) | 370 (46.1) | <0.001 |
| Overweight, n (%) | 749 (46.7) | 410 (51.2) | 339 (42.3) | |
| Obese, n (%) | 323 (20.1) | 230 (28.7) | 93 (11.6) | |
| Smoking | 0.3 | |||
| Never, n (%) | 547 (34.1) | 264 (33.1) | 283 (35.7) | |
| Former smoker, n (%) | 853 (53.1) | 429 (53.8) | 424 (53.5) | |
| Current smoker, n (%) | 191 (11.9) | 105 (13.2) | 86 (10.8) | |
| Waist/Hip ratio | 0.94 ± 0.10 | 0.96 ± 0.08 | 0.93 ± 0.11 | <0.001 |
| Systolic blood pressure, mmHg | 134 ± 18 | 137 ± 18 | 131 ± 17 | <0.001 |
| Diastolic blood pressure, mmHg | 75 ± 9 | 76 ± 9 | 75 ± 10 | 0.09 |
| Diet | ||||
| Lifelines diet score | 23.9 ± 6.2 | 22.5 ± 6.2 | 25.1 ± 6.0 | <0.001 |
| Total energy, Kcal/day | 1909.0 ± 518.0 | 1936.5 ± 548.3 | 1888.0 ± 493.0 | 0.09 |
| Total carbohydrate, g/day | 208.4 ± 62.1 | 216.5 ± 64.5 | 202.2 ± 59.5 | <0.001 |
| Total fat, g/day | 74.7 ± 26.3 | 76.4 ± 27.6 | 73.3 ± 25.1 | 0.03 |
| Total protein, g/day | 71.8 ± 9.7 | 72.1 ± 19.4 | 71.6 ± 17.5 | 0.7 |
| Total animal protein, g/day | 42.7 ± 13.4 | 42.4 ± 13.6 | 42.9 ± 13.2 | 0.6 |
| Total plant protein, g/day | 29.3 ± 9.7 | 29.7 ± 10.2 | 28.9 ± 9.2 | 0.1 |
| Percentage energy from: | ||||
| Carbohydrates | 43.8 ± 6.3 | 44.9 ± 5.9 | 42.9 ± 6.5 | <0.001 |
| Protein | 15.3 ± 2.2 | 15.1 ± 2.2 | 15.4 ± 2.2 | 0.01 |
| Fat | 34.8 ± 5.6 | 35.1 ± 5.6 | 34.6 ± 5.6 | 0.09 |
| Total alcohol, g/day | 6.4 (1.2-16.6) | 2.7 (0.006–9.2) | 8.9 (2.7–17.8) | <0.001 |
| Alcohol use per day | <0.001 | |||
| no drink, n (%) | 256 (16.0) | 165 (28.7) | 91 (12.0) | |
| ≤1 drink/day, n (%) | 572 (35.6) | 262 (45.6) | 310 (41.0) | |
| 1-2 drinks/day, n (%) | 323 (20.1) | 105 (18.3) | 218 (28.8) | |
| >2 drinks/day, n (%) | 181 (11.3) | 43 (7.5) | 138 (18.2) | |
| Heavy drinker, n (%) | 296 (18.4) | 73 (12.7) | 223 (29.5) | |
| Vitamin Supplementation use, n (%) | 236 (14.7) | 91 (11.3) | 145 (18.1) | 0.3 |
| Serum biomarkers | ||||
| Glucose, mmol/L | 5.2 (4.8-5.7) | 5.3 (4.9–5.9) | 5.1 (4.8–5.6) | <0.001 |
| HbA1C % | 5.9 ± 0.6 | 5.9 ± 0.6 | 5.8 ± 0.5 | <0.001 |
| Homocysteine, umol/L | 13.0 (11.0–15.0) | 14.0 (12.0–16.0) | 13 (11–15) | <0.001 |
| Folic acid, nmol/L | 16.6 (11.1–24.9) | 14.4 (9.7–22.7) | 18.4 (12.7–27.4) | <0.001 |
| Deficiency, n (%) | 313 (19.5) | 214 (27.8) | 99 (12.9) | <0.001 |
| Insufficiency, n (%) | 262 (16.3) | 144 (18.7) | 118 (15.4) | |
| Sufficiency, n (%) | 963 (60.0) | 412 (53.5) | 551 (71.7) | |
| Vitamin K, pmol/L | 209.0 (136.9–291.2) | 200.5 (135.7–287.5) | 213.9 (138.3–296.6) | 0.3 |
| Deficiency, n (%) | 105 (6.5) | 46 (5.8) | 59 (7.4) | 0.2 |
| Sufficiency, n (%) | 1487 (92.6) | 749 (94.2) | 738 (92.6) | |
| Vitamin B12, pmol/L | 290 (224.0–362.0) | 275 (218–347.3) | 303 (233.5–379.5) | <0.001 |
| deficiency, n (%) | 67 (4.2) | 37 (4.8) | 30 (3.9) | 0.07 |
| insufficiency, n (%) | 295 (18.4) | 162 (21.1) | 133 (17.2) | |
| Sufficiency, n (%) | 1177 (73.3) | 567 (74.0) | 610 (78.9) | |
| Vitamin B6, nmol/L | 53.9 (36.9–81.3) | 48.8 (34.0–72.9) | 60 (40.7–91.2) | <0.001 |
| deficiency, n (%) | 65 (4.0) | 48 (6.0) | 17 (2.1) | <0.001 |
| insufficiency, n (%) | 178 (11.1) | 108 (13.6) | 70 (8.8) | |
| Sufficiency, n (%) | 1350 (84.1) | 638 (80.4) | 712 (89.1) | |
| Vitamin E, umol/L | 33.4 (29.3–38.5) | 33.2 (28.4–38.1) | 33.6 (29.7–38.9) | 0.06 |
| Deficiency, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| Insufficiency, n (%) | 465 (29) | 256 (32.1) | 209 (26.2) | 0.01 |
| Sufficiency, n (%) | 1131 (70.5) | 542 (67.9) | 589 (73.8) | |
| Vitamin A, umol/L | 2.1 ± 0.5 | 2.1 ± 0.4 | 2.1 ± 0.5 | 0.09 |
| Deficiency, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| insufficiency, n (%) | 7 (0.4) | 3 (0.4) | 4 (0.5) | 0.7 |
| Sufficiency, n (%) | 1562 (97.3) | 774 (99.6) | 788 (99.5) | |
| Vitamin D, nmol/L | 63.2 ± 22.1 | 62.9 ± 21.3 | 63.5 ± 22.8 | 0.6 |
| deficiency, n (%) | 76 (4.7) | 36 (4.8) | 40 (5.3) | 0.9 |
| insufficiency, n (%) | 369 (23.0) | 184 (24.6) | 185 (24.4) | |
| Sufficiency, n (%) | 1060 (66.0) | 528 (70.6) | 532 (70.3) | |
| Multivitamin status | ||||
| Single vitamin deficiency, n (%) | 460 (28.7) | 265 (33.0) | 195 (24.3) | <0.001 |
| Single vitamin insufficienciy, n (%) | 894 (55.7) | 460 (57.3) | 434 (54.1) | 0.006 |
| 2 deficiencies, n (%) | 89 (5.5) | 58 (7.2) | 31 (3.9) | 0.001 |
| 2 insufficiencies, n (%) | 397 (24.7) | 216 (26.9) | 181 (22.6) | 0.008 |
| >=3 deficiencies, n (%) | 18 (1.1) | 16 (2.0) | 2 (0.2) | 0.001 |
| >=3 insufficiencies, n (%) | 111 (6.9) | 65 (8.1) | 46 (5.7) | 0.03 |
| Folic Acid | Vitamin B12 | Vitamin B6 | Vitamin D | Vitamin E | Vitamin K | Single Vitamin | Multivitamin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | p | OR [95% CI] | p | OR [95% CI] | p | OR [95% CI] | p | OR [95% CI] | p | OR [95% CI] | p | OR [95% CI] | p | OR [95% CI] | p | |
| Model 1 b | ||||||||||||||||
| Deficiency | 2.89 [2.21–3.79] | <0.001 | 1.33 [0.81–2.18] | 0.3 | 3.15 [1.79–5.54] | <0.001 | 0.91 [0.57–1.45] | 0.7 | / | 0.77 [0.51–1.14] | 0.2 | 1.71 [1.36–2.14] | <0.001 | 2.06 [1.31–3.23] | 0.002 | |
| Insufficiency | 1.63 [1.24–2.15] | <0.001 | 1.31 [1.01–1.69] | 0.04 | 1.72 [1.25–2.37] | 0.001 | 1.00 [0.79–1.27] | 0.9 | 1.33 [1.07–1.65] | 0.01 | / | / | / | |||
| Sufficiency | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Model 2 | ||||||||||||||||
| Deficiency | 2.88 [2.18–3.80] | <0.001 | 1.30 [0.78–2.16] | 0.3 | 2.99 [1.68–5.32] | <0.001 | 0.85 [0.53–1.38] | 0.5 | / | 0.75 [0.50–1.13] | 0.2 | 1.65 [1.30–2.08] | <0.001 | 1.96 [1.24–3.12] | 0.004 | |
| Insufficiency | 1.69 [1.28–2.25] | <0.001 | 1.24 [0.95–1.62] | 0.1 | 1.60 [1.15–2.22] | 0.005 | 0.99 [0.77–1.26] | 0.9 | 1.33[1.06–1.66] | 0.01 | / | / | / | |||
| Sufficiency | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Model 3 | ||||||||||||||||
| Deficiency | 2.72 [2.03–3.65] | <0.001 | 1.61 [0.94–2.75] | 0.09 | 2.92 [1.59–5.36] | 0.001 | 0.72 [0.43–1.20] | 0.2 | / | 0.70 [0.45–1.08] | 0.1 | 1.57 [1.22–2.01] | <0.001 | 1.79 [1.10–2.93] | 0.02 | |
| Insufficiency | 1.62 [1.20–2.19] | 0.002 | 1.31 [0.99–1.73] | 0.06 | 1.37 [0.97–1.94] | 0.07 | 0.86 [0.66–1.11] | 0.2 | 1.30 [1.03–1.65] | 0.03 | / | / | / | |||
| Sufficiency | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Model 4 | ||||||||||||||||
| Deficiency | 2.46 [1.78–3.40] | <0.001 | 1.29 [0.70–2.36] | 0.4 | 1.63 [0.78–3.38] | 0.2 | 0.49 [0.26–0.93] | 0.03 | / | 0.74 [0.46–1.19] | 0.2 | 1.39 [1.06–1.84] | 0.02 | 1.28 [0.74–2.21] | 0.4 | |
| Insufficiency | 1.41 [1.01–1.97] | 0.05 | 1.16 [0.85–1.59] | 0.4 | 1.23 [0.84–1.81] | 0.3 | 0.82 [0.61–1.11] | 0.2 | 1.31 [1.00–1.71] | 0.05 | / | / | / | |||
| Sufficiency | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Model 5 | ||||||||||||||||
| Deficiency | 2.58 [1.87–3.57] | <0.001 | 1.32 [0.72–2.40] | 0.4 | 1.63 [0.79–3.38] | 0.2 | 0.48 [0.26–0.90] | 0.02 | / | 0.73 [0.45–1.16] | 0.2 | 1.40 [1.06–1.84] | 0.02 | 1.35 [0.78–2.34] | 0.3 | |
| Insufficiency | 1.49 [1.07–2.08] | 0.02 | 1.14 [0.83–1.57] | 0.4 | 1.34 [0.92–1.97] | 0.1 | 0.85 [0.64–1.15] | 0.3 | 1.28 [1.00–1.66] | 0.07 | / | / | / | |||
| Sufficiency | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Model 6 | ||||||||||||||||
| Deficiency | 2.55 [1.85–3.52] | <0.001 | 1.65 [0.58–4.74] | 0.3 | 1.76 [0.85–3.62] | 0.13 | 0.46 [0.25–0.86] | 0.02 | / | 0.76 [0.48–1.22] | 0.3 | 1.40 [1.06–1.84] | 0.02 | 1.25 [0.72–2.17] | 0.4 | |
| Insufficiency | 1.50 [1.08–2.09] | 0.02 | 1.20 [0.88–1.64] | 0.3 | 1.32 [0.91–1.95] | 0.1 | 0.84 [0.63–1.12] | 0.2 | 1.28 [0.98–1.67] | 0.07 | / | / | / | |||
| Sufficiency | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| Predictor: Low SES b Mediator: LLDS | a Path | b Path | Mediation Effect | Total Effect | Prop. Mediated c | ||||
|---|---|---|---|---|---|---|---|---|---|
| a (SE) | p | b (SE) | p | m (SE) | p | t (SE) | p | ||
| Single-vitamin deficiency | −2.26 (0.37) | <0.001 | −0.007 (0.007) | 0.3 | 0.017 (0.016) | 0.3 | 0.22 (0.09) | 0.01 | - |
| Multi-vitamin deficiency | −2.26 (0.40) | <0.001 | −0.019 (0.011) | 0.09 | 0.043 (0.027) | 0.1 | 0.16 (0.13) | 0.3 | - |
| Folic acid deficiency | −2.35 (0.35) | <0.001 | −0.022 (0.007) | 0.002 | 0.051 (0.018) | 0.004 | 0.51 (0.09) | <0.001 | 10.1% |
| Folic acid insufficiency | −2.35 (0.36) | <0.001 | −0.018 (0.007) | 0.01 | 0.043 (0.020) | 0.03 | 0.11 (0.091) | 0.2 | 39.1% |
| Vit B12 deficiency | −2.35 (0.36) | <0.001 | −0.006 (0.013) | 0.6 | 0.015 (0.030) | 0.6 | 0.12 (0.14) | 0.4 | - |
| Vit B12 insufficiency | −2.34 (0.35) | <0.001 | −0.018 (0.008) | 0.02 | 0.041 (0.019) | 0.03 | 0.11 (0.09) | 0.2 | 37.2% |
| Vit B6 deficiency | −2.39 (0.35) | <0.001 | −0.019 (0.013) | 0.2 | 0.045 (0.033) | 0.2 | 0.22 (0.18) | 0.2 | - |
| Vit B6 insufficiency | −2.39 (0.35) | <0.001 | −0.022 (0.008) | 0.006 | 0.052 (0.021) | 0.01 | 0.14 (0.10) | 0.1 | 37.1% |
| Vit D deficiency | −2.31 (0.36) | <0.001 | 0.009 (0.012) | 0.5 | −0.021 (0.030) | 0.5 | −0.32 (0.14) | 0.03 | - |
| Vit D insufficiency | −2.31 (0.36) | <0.001 | −0.011 (0.007) | 0.1 | 0.025 (0.017) | 0.1 | −0.07 (0.09) | 0.5 | - |
| Vit E insufficiency | −2.39 (0.36) | <0.001 | 0.004 (0.007) | 0.5 | −0.010 (0.016) | 0.5 | 0.15 (0.08) | 0.07 | - |
| Vit K deficiency | −2.36 (0.33) | <0.001 | −0.000 (0.009) | 0.9 | 0.000 (0.022) | 0.9 | −0.14 (0.12) | 0.2 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Minović, I.; Dekker, L.H.; Eggersdorfer, M.L.; van Zon, S.K.R.; Reijneveld, S.A.; Kootstra-Ros, J.E.; Kema, I.P.; Bakker, S.J.L.; Navis, G.J.; et al. Vitamin Status and Diet in Elderly with Low and High Socioeconomic Status: The Lifelines-MINUTHE Study. Nutrients 2020, 12, 2659. https://doi.org/10.3390/nu12092659
Zhu Y, Minović I, Dekker LH, Eggersdorfer ML, van Zon SKR, Reijneveld SA, Kootstra-Ros JE, Kema IP, Bakker SJL, Navis GJ, et al. Vitamin Status and Diet in Elderly with Low and High Socioeconomic Status: The Lifelines-MINUTHE Study. Nutrients. 2020; 12(9):2659. https://doi.org/10.3390/nu12092659
Chicago/Turabian StyleZhu, Yinjie, Isidor Minović, Louise H. Dekker, Manfred L. Eggersdorfer, Sander K.R. van Zon, Sijmen A. Reijneveld, Jenny E. Kootstra-Ros, Ido P. Kema, Stephan J.L. Bakker, Gerjan J. Navis, and et al. 2020. "Vitamin Status and Diet in Elderly with Low and High Socioeconomic Status: The Lifelines-MINUTHE Study" Nutrients 12, no. 9: 2659. https://doi.org/10.3390/nu12092659
APA StyleZhu, Y., Minović, I., Dekker, L. H., Eggersdorfer, M. L., van Zon, S. K. R., Reijneveld, S. A., Kootstra-Ros, J. E., Kema, I. P., Bakker, S. J. L., Navis, G. J., & Riphagen, I. J. (2020). Vitamin Status and Diet in Elderly with Low and High Socioeconomic Status: The Lifelines-MINUTHE Study. Nutrients, 12(9), 2659. https://doi.org/10.3390/nu12092659





