24-h Urine Collection: A Relevant Tool in CKD Nutrition Evaluation
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethics Statement
2.2. Study Concept
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Wen, C.P.; Cheng, T.Y.D.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Hostetter, T.H. Chronic Kidney Disease Predicts Cardiovascular Disease. N. Engl. J. Med. 2004, 351, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.L.; Shen, C.; Landsittel, D.P.; Grantham, J.J.; Cook, L.T.; Torres, V.E.; Chapman, A.B.; Bae, K.T.; Mrug, M.; Harris, P.C.; et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP). Long-term trajectory of kidney function in autosomal dominant polycystic kidney disease. Kidney Int. 2019, 95, 1253–1261. [Google Scholar] [CrossRef]
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Diseases 2019, 74, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.C.; Adler, S.; Burkart, J.M.; Greene, T.; Hebert, L.A.; Hunsicker, L.G.; King, A.J.; Klahr, S.; Massry, S.G.; Seifter, J.L. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann. Intern. Med. 1995, 123, 754–762. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef]
- Appel, L.J.; Champagne, C.M.; Harsha, D.W.; Cooper, L.S.; Obarzanek, E.; Elmer, P.J.; Stevens, V.J.; Vollmer, W.M.; Lin, P.H.; Svetkey, L.P.; et al. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA 2003, 289, 2083–2093. [Google Scholar] [CrossRef]
- Mallamaci, F.; Leonardis, D.; Bellizzi, V.; Zoccali, C. Does high salt intake cause hyperfiltration in patients with essential hypertension? J. Hum. Hypertens. 1996, 10, 157–161. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients 2019, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Centron, P.; Barrows, I.; Dwivedi, R.; Raj, D.S. Gut Microbiota and Cardiovascular Uremic Toxicities. Toxins 2018, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, T.W.; Pawlak, K.; Karbowska, M.; Myśliwiec, M.; Pawlak, D. Indoxyl sulfate—The uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017, 18, 35. [Google Scholar] [CrossRef]
- Barsotti, G.; Cupisti, A.; Morelli, E.; Meola, M.; Cozza, V.; Barsotti, M.; Giovannetti, S. Secondary Hyperparathyroidism in Severe Chronic Renal Failure Is Corrected by Very-Low Dietary Phosphate Intake and Calcium Carbonate Supplementation. Nephron 1998, 79, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Bentley, B.; Lennie, T.A.; Biddle, M.; Chung, M.L.; Moser, D.K. Demonstration of psychometric soundness of the Dietary Sodium Restriction Questionnaire in patients with heart failure. Heart Lung 2009, 38, 121–128. [Google Scholar] [CrossRef]
- d’Almeida, K.S.M.; Souza, G.C.; Rabelo, E.R. Cross-cultural adaptation into Brazilian portuguese of the Dietary Sodium Restriction Questionnaire (DSRQ). Arq. Bras. Cardiol. 2012, 98, 70–75. [Google Scholar] [CrossRef][Green Version]
- Cohen, S.J.; Weinberger, M.H.; Fineberg, N.S.; Miller, J.Z.; Grim, C.E.; Luft, F.C. The effect of a household partner and home urine monitoring on adherence to a sodium restricted diet. Soc. Sci. Med. 1991, 32, 1057–1061. [Google Scholar] [CrossRef]
- Shaw, A.B.; Risdon, P.; Lewis-Jackson, J.D. Protein creatinine index and Albustix in assessment of proteinuria. Br. Med. J. 1983, 287, 929–932. [Google Scholar] [CrossRef][Green Version]
- Mitchell, S.C.M.; Sheldon, T.A.; Shaw, A.B. Quantification of Proteinuria: A Re-evaluation of the Protein/Creatinine Ratio for Elderly Subjects. Age Ageing 1993, 22, 443–449. [Google Scholar] [CrossRef]
- Tynkevich, E.; Flamant, M.; Haymann, J.P.; Metzger, M.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Houillier, P.; Froissart, M.; Stengel, B. Decrease in urinary creatinine excretion in early stage chronic kidney disease. PLoS ONE 2014, 9, 111949. [Google Scholar] [CrossRef] [PubMed]
- Calanchini, M.; Tadman, M.; Krogh, J.; Fabbri, A.; Grossman, A.; Shine, B. Measurement of urinary 5-HIAA: Correlation between spot versus 24-h urine collection. Endocr. Connect 2019, 8, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C.; Sloan, R.S.; Fineberg, N.S.; Free, A.H. The utility of overnight urine collections in assessing compliance with a low sodium intake diet. JAMA 1983, 249, 1764–1768. [Google Scholar] [CrossRef]
- Price, C.P.; Newall, R.G.; Boyd, J.C. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: A systematic review. Clin. Chem. 2005, 51, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Gaspari, F.; Perna, A.; Remuzzi, G. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ 1998, 316, 504–509. [Google Scholar] [CrossRef]
- Guy, M.; Borzomato, J.K.; Newall, R.G.; Kalra, P.A.; Price, C.P. Protein and albumin-to-creatinine ratios in random urines accurately predict 24 h protein and albumin loss in patients with kidney disease. Ann. Clin. Biochem. 2009, 46, 468–476. [Google Scholar] [CrossRef]
- Sampaio, E.; Delfino, V.D.A. Assessing albuminuria in spot morning samples from diabetic patients. Arq. Bras. Endocrinol. Metabol. 2008, 52, 1482–1488. [Google Scholar] [CrossRef][Green Version]
- Wilson, T.; Garcia-Perez, I.; Posma, J.M.; Lloyd, A.J.; Chambers, E.S.; Tailliart, K.; Zubair, H.; Beckmann, M.; Mathers, J.C.; Holmes, E.; et al. Spot and Cumulative Urine Samples Are Suitable Replacements for 24-Hour Urine Collections for Objective Measures of Dietary Exposure in Adults Using Metabolite Biomarkers. J. Nutr. 2019, 149, 1692–1700. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Li, K.; Peng, X. Assessing whether a spot urine specimen can predict 24-h urinary sodium excretion accurately: A validation study. J. Hypertens. 2019, 37, 99–108. [Google Scholar] [CrossRef]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I.; et al. Agreement Between 24-Hour Salt Ingestion and Sodium Excretion in a Controlled Environment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef]
- Land, M.A.; Webster, J.; Christoforou, A.; Praveen, D.; Jeffery, P.; Chalmers, J.; Smith, W.; Woodward, M.; Barzi, F.; Nowson, C.; et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open 2014, 4, e003720. [Google Scholar] [CrossRef] [PubMed]
- Essig, M.; Escoubet, B.; de Zuttere, D.; Blanchet, F.; Arnoult, F.; Dupuis, E.; Michel, C.; Mignon, F.; Mentre, F.; Clerici, C.; et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol. Dial Transplant. 2008, 23, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Faucon, A.L.; Flamant, M.; Metzger, M.; Boffa, J.J.; Haymann, J.P.; Houillier, P.; Thervet, E.; Vrtovsnik, F.; Stengel, B.; Geri, G.; et al. Extracellular fluid volume is associated with incident end-stage kidney disease and mortality in patients with chronic kidney disease. Kidney Int. 2019, 96, 1020–1029. [Google Scholar] [CrossRef]
- El-Damanawi, R.; Lee, M.; Harris, T.; Mader, L.B.; Bond, S.; Pavey, H.; Sandford, R.N.; Wilkinson, I.B.; Burrows, A.; Woznowski, P.; et al. Randomised controlled trial of high versus ad libitum water intake in patients with autosomal dominant polycystic kidney disease: Rationale and design of the DRINK feasibility trial. BMJ Open 2018, 8, e022859. [Google Scholar] [CrossRef]
- Amro, O.W.; Paulus, J.K.; Noubary, F.; Perrone, R.D. Low-Osmolar Diet and Adjusted Water Intake for Vasopressin Reduction in Autosomal Dominant Polycystic Kidney Disease: A Pilot Randomized Controlled Trial. Am. J. Kidney Diseases 2016, 68, 882–891. [Google Scholar] [CrossRef]
- Siener, R. Dietary Treatment of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2018, 10, 512. [Google Scholar] [CrossRef]
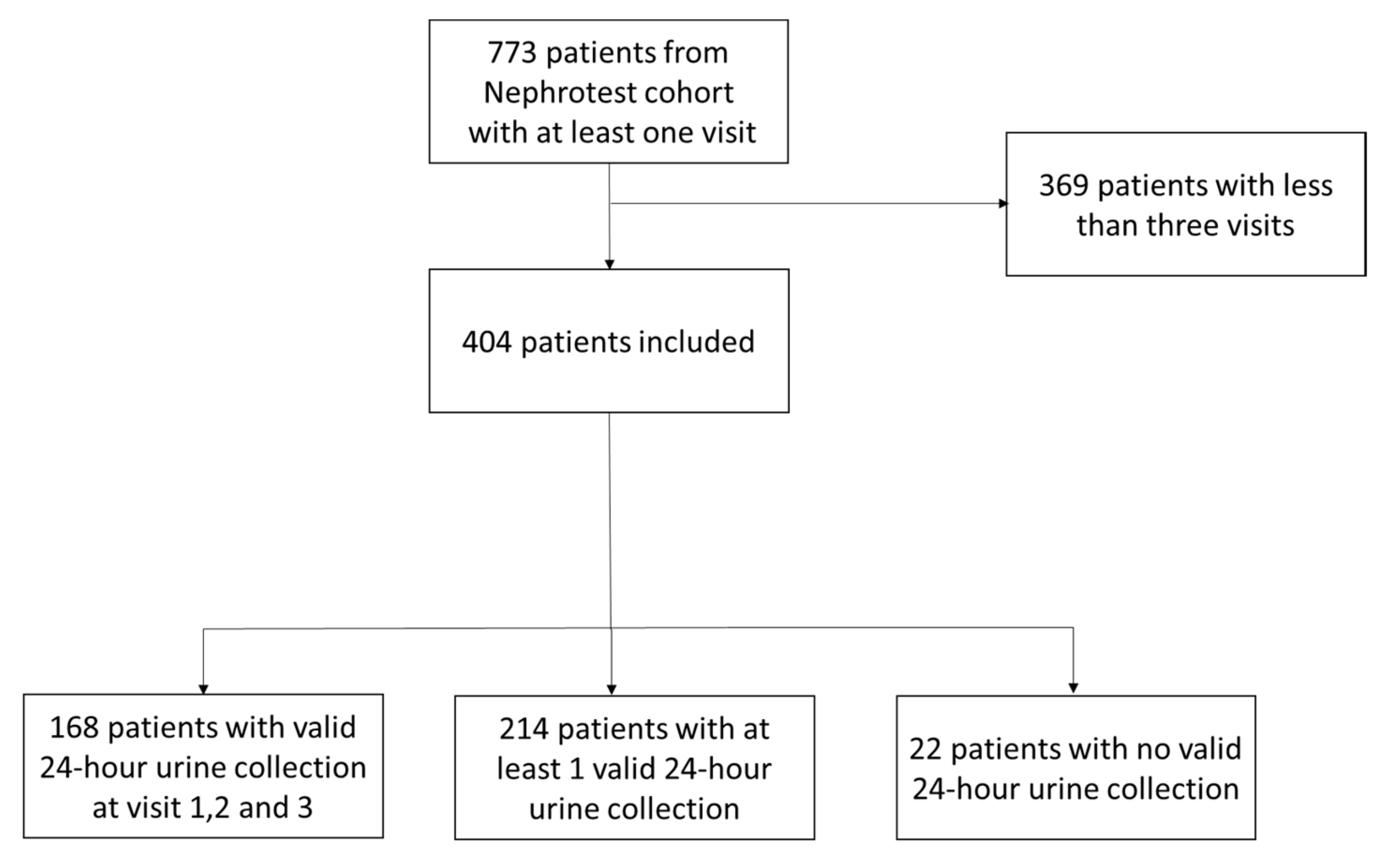
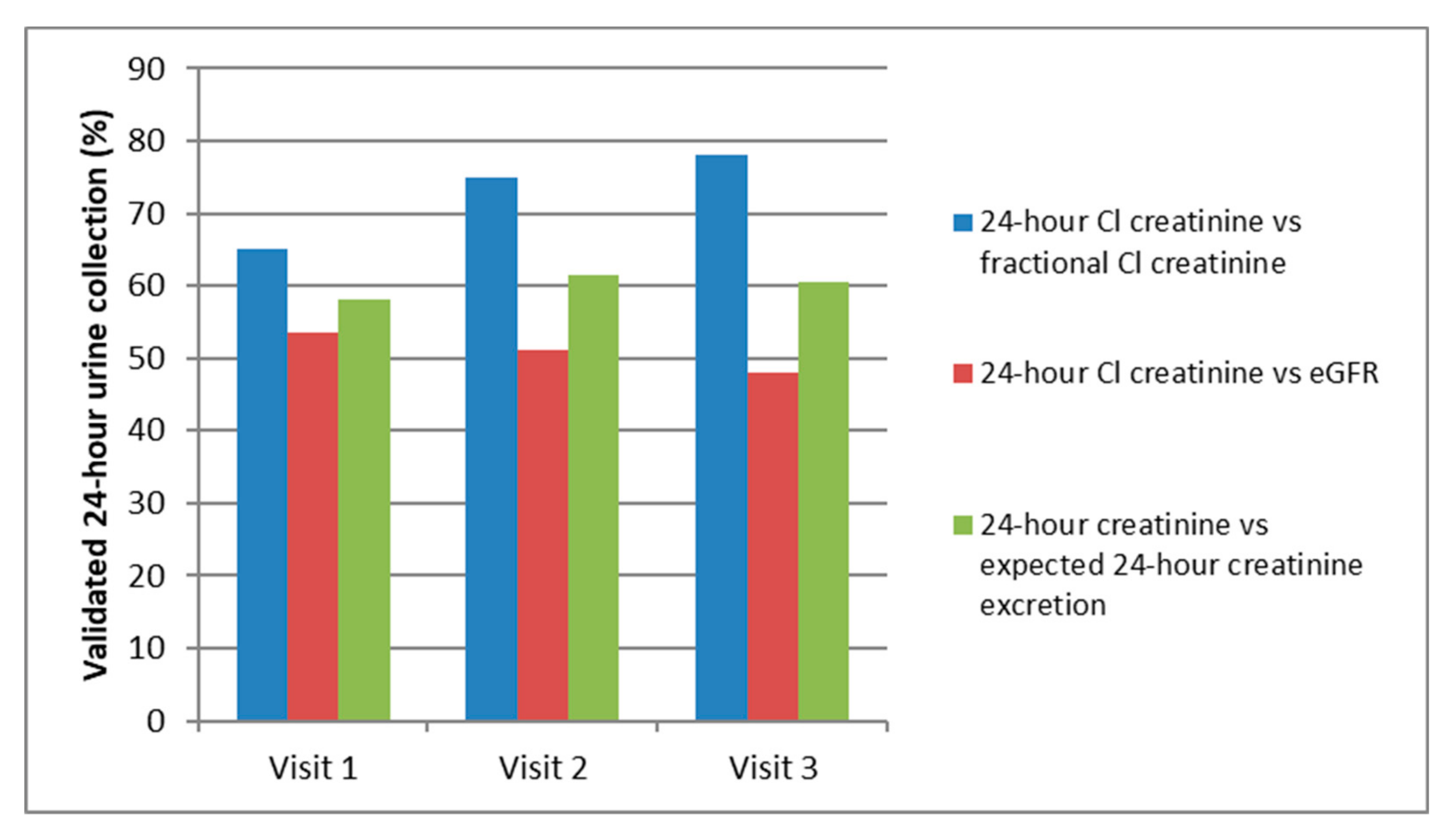
| Biological Data | Patients with 3 Valid 24-h Urine Collections | Patients with No Valid 24-h Urine Collections | p-Value |
|---|---|---|---|
| n = | 168 | 22 | |
| Blood | |||
| mGFR (mL/min/1.73 m2) | 43.7(31.3–56.4) | 40.3 (19.5–53.8) | 0.2 |
| Creatinine Clearance (mL/min) | 56.9(45.2–70) | 62 (37.7–82.2) | 0.84 |
| Serum creatinine (µmol/L) | 134 (107.3–172) | 126.6 (95.5–190.8) | 0.98 |
| Natremia (mmol/L) | 141 (139–142) | 141 (140–142) | 0.37 |
| Serum potassium (mmol/L) | 4.2 (4.2–4.5) | 4.1 (4–4.3) | 0.17 |
| Hemoglobin (g/dL) | 12.8 (11.8–14) | 12.4 (11.6–13.9) | 0.59 |
| Ionized calcium (mmol/L) | 1.19 (1.16–1.21) | 1.19 (1.15–1.22) | 0.84 |
| PTH (pg/mL) | 43 (30–71.5) | 45 (34–96) | 0.24 |
| Urine | |||
| Diuresis (mL/Day) | 2175 (1716–2700) | 1657 (1350–2207) | 0.003 |
| Proteinuria (mg/mmol creatinine) | 19.1 (3–88.6) | 17.1 (5–73) | 0.42 |
| ACR (mg/mmol creatinine) | 5.1 (1.7–38.4) | 10.3 (2.9–40.1) | 0.37 |
| Creatinine (mmol/day) | 13.1 (9.5–17.3) | 8.2 (5.7–13.3) | 0.008 |
| Creatinine (mmol/kg/day) | 0.17 (0.13–0.23) | 0.10 (0.07–0.18) | 0.004 |
| Parameters | Visit 1 | Visit 2 | Visit 3 | p-Value |
|---|---|---|---|---|
| Blood pressure > 130/80 mmHg (%) | 54.5 | 57.6 | 62.6 | 0.12 |
| Weight (kg) | 70 (63–85) | 74 (64–84) | 75 (65–85) | 0.07 |
| Blood | ||||
| mGFR mL/min/1.73 m2) | 43.7 (31.3–56.4) | 41.2 (29.1–55.5) | 38.5 (25–52.2) | <0.001 |
| Creatinine Clearance (mL/min) | 56.9 (45.2–70) | 55.9 (40.6–72.6) | 51.6 (36.2–68.9) | <0.001 |
| Serum creatinine (µmol/L) | 134 (107.3–172) | 137.5 (106.5–178.5) | 136.8 (106.4–190.3) | <0.001 |
| Haemoglobin (g/dL) | 12.8 | 12.7 | 12.6 | 0.10 |
| Serum potassium (mmol/L) | 4.2 (4.2–4.5) | 4.2 (3.9–4.6) | 4.2 (4–4.6) | 0.24 |
| Bicarbonate (mmol/L) | 26.3 (24.3–28) | 26.5 (24.9–28.5) | 26.3 [24.5–28.1) | 0.37 |
| Phosphorus(mmol/L) | 1.03 | 1.03 | 1.06 | 0.18 |
| Ionized calcium (mmol/L) | 1.19 (1.16–1.21) | 1.19 (1.16–1.22) | 1.19 (1.16–1.22) | 0.06 |
| PTH (pg/mL) | 43 (30–71.5) | 48 (2.3–80.8) | 50 (36.5–85.3) | <0.001 |
| Secondary hyperparathyroidim (%) | 26.2 | 29.3 | 31.5 | <0.001 |
| 25-OH-VitD (ng/mL) | 26.8 (17–35.7) | 27.3 (19–36) | 24.8 (18.3–32.9) | 0.91 |
| Calcitriol (pg/mL) | 34 (22.1–43.9) | 33 (22.6–44.4) | 33.2 (24–43.6) | 0.98 |
| Urine | ||||
| Proteinuria (mg/mmol creatinine) | 19.1 (3–88.6) | 22 (6.8–77.8) | 19.2 (3–88.6) | 0.84 |
| ACR (mg/mmol creatinine) | 3.4 (1.3–27.4) | 4.6 (1.4–28.2) | 5.1 (1.7–38.4) | 0.005 |
| Creatinine (mmol/kg/day) | 0.17 (0.13–0.23) | 0.15 (0.12–0.18) | 0.14 (0.12–0.17) | <0.0001 |
| Dietary Data | Visit 1 | Visit 2 | Visit 3 | p-Value |
|---|---|---|---|---|
| Urine volume (mL/day) | 2175 (1716–2700) | 2203 (1639–2743) | 2159 (1778–2794) | 0.80 |
| Estimated sodium chloride intake (g/day) | 8.3 (6–10.8) | 7.8 (5.4–10.7) | 7.7 (5.8–9.6) | 0.25 |
| Estimated protein intake (g/kg/day) | 1.29 (1.05–1.47) | 1.23 (0.96–1.42) | 1.18 (0.95–1.41) | 0.02 |
| Estimated Osmole intake (osm/day) | 779.9 (655–935) | 745.2 (592–889) | 740.4 (616–857) | 0.006 |
| Phosphate excretion (mmol/day) | 20.1 (14.9–27.8) | 20.5 (13.3–30.4) | 20.1 (14.4–27.6) | 0.18 |
| Calcium excretion (mmol/day) | 1.47 (0.81–2.78) | 1.52 (0.66–2.80) | 1.33 (0.61–2.53) | 0.03 |
| Ammonium excretion (mmol/day) | 20.2 (13.1–31.7) | 18.3 (11.1–27.3) | 17.6 (11.3–26.5) | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Nabey, M.; Saint-Jacques, C.; Boffa, J.-J.; Frochot, V.; Livrozet, M.; Daudon, M.; Flamant, M.; Letavernier, E.; Haymann, J.-P., on behalf of the NephroTest Study Group. 24-h Urine Collection: A Relevant Tool in CKD Nutrition Evaluation. Nutrients 2020, 12, 2615. https://doi.org/10.3390/nu12092615
Abdel-Nabey M, Saint-Jacques C, Boffa J-J, Frochot V, Livrozet M, Daudon M, Flamant M, Letavernier E, Haymann J-P on behalf of the NephroTest Study Group. 24-h Urine Collection: A Relevant Tool in CKD Nutrition Evaluation. Nutrients. 2020; 12(9):2615. https://doi.org/10.3390/nu12092615
Chicago/Turabian StyleAbdel-Nabey, Moustafa, Camille Saint-Jacques, Jean-Jacques Boffa, Vincent Frochot, Marine Livrozet, Michel Daudon, Martin Flamant, Emmanuel Letavernier, and Jean-Philippe Haymann on behalf of the NephroTest Study Group. 2020. "24-h Urine Collection: A Relevant Tool in CKD Nutrition Evaluation" Nutrients 12, no. 9: 2615. https://doi.org/10.3390/nu12092615
APA StyleAbdel-Nabey, M., Saint-Jacques, C., Boffa, J.-J., Frochot, V., Livrozet, M., Daudon, M., Flamant, M., Letavernier, E., & Haymann, J.-P., on behalf of the NephroTest Study Group. (2020). 24-h Urine Collection: A Relevant Tool in CKD Nutrition Evaluation. Nutrients, 12(9), 2615. https://doi.org/10.3390/nu12092615






