Eating Habits of Children Born after Maternal Bariatric Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. Meal Pattern
3.3. Food Intake Organized by Food Groups
3.3.1. Meat and Fish
3.3.2. Dairy Products
3.3.3. Vegetables and Fruits
3.3.4. Beverages
3.3.5. Snacks
4. Discussion
4.1. Meal Pattern
4.2. Food Intake
Author Contributions
Funding
Conflicts of Interest
References
- Campbell, K.; Crawford, D. Family food environments as determinants of preschool-aged children’s eating behaviours: Implications for obesity prevention policy. A review. Aust. J. Nutr. Diet. 2001, 58, 19–25. [Google Scholar]
- Osei-Assibey, G.; Dick, S.; Macdiarmid, J.; Semple, S.; Reilly, J.J.; Ellaway, A.; Cowie, H.; McNeill, G. The influence of the food environment on overweight and obesity in young children: A systematic review. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Min, J.; Khuri, J.; Li, M. A Systematic Examination of the Association between Parental and Child Obesity across Countries. Adv. Nutr. 2017, 8, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.R.; Reynolds, R.M. The risk of maternal obesity to the long-term health of the offspring. Clin. Endocrinol. 2013, 78, 9–16. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef]
- Daraki, V.; Roumeliotaki, T.; Koutra, K.; Georgiou, V.; Kampouri, M.; Kyriklaki, A.; Vafeiadi, M.; Papavasiliou, S.; Kogevinas, M.; Chatzi, L. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: The Rhea mother-child cohort, Crete, Greece. Eur. Child Adolesc. Psychiatry 2017, 26, 703–714. [Google Scholar] [CrossRef]
- Menting, M.D.; van de Beek, C.; de Rooij, S.R.; Painter, R.C.; Vrijkotte, T.G.M.; Roseboom, T.J. The association between pre-pregnancy overweight/obesity and offspring’s behavioral problems and executive functioning. Early Hum. Dev. 2018, 122, 32–41. [Google Scholar] [CrossRef]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Denison, F.C.; Räikkönen, K.; Norman, J.E.; Reynolds, R.M. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr. Res. 2017, 82, 47–54. [Google Scholar] [CrossRef]
- Mikkelsen, S.H.; Hohwü, L.; Olsen, J.; Bech, B.H.; Liew, Z.; Obel, C. Parental Body Mass Index and Behavioral Problems in Their Offspring: A Danish National Birth Cohort Study. Am. J. Epidemiol. 2017, 186, 593–602. [Google Scholar] [CrossRef]
- Flynn, A.C.; Dalrymple, K.; Barr, S.; Poston, L.; Goff, L.M.; Rogozińska, E.; van Poppel, M.N.; Rayanagoudar, G.; Yeo, S.; Barakat Carballo, R.; et al. Dietary interventions in overweight and obese pregnant women: A systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr. Rev. 2016, 74, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Stephansson, O.; Neovius, M. Outcomes of pregnancy after bariatric surgery. N. Engl. J. Med. 2015, 372, 2267. [Google Scholar] [CrossRef] [PubMed]
- Leahey, T.M.; Bond, D.S.; Raynor, H.; Roye, D.; Vithiananthan, S.; Ryder, B.A.; Sax, H.C.; Wing, R.R. Effects of bariatric surgery on food cravings: Do food cravings and the consumption of craved foods “normalize” after surgery? Surg. Obes. Relat. Dis. 2012, 8, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Guelinckx, I.; Devlieger, R.; Donceel, P.; Bel, S.; Pauwels, S.; Bogaerts, A.; Thijs, I.; Schurmans, K.; Deschilder, P.; Vansant, G. Lifestyle after bariatric surgery: A multicenter, prospective cohort study in pregnant women. Obes. Surg. 2012, 22, 1456–1464. [Google Scholar] [CrossRef]
- Walters-Bugbee, S.E.; McClure, K.S.; Kral, T.V.; Sarwer, D.B. Maternal child feeding practices and eating behaviors of women with extreme obesity and those who have undergone bariatric surgery. Surg. Obes. Relat. Dis. 2012, 8, 784–791. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheet on Obesity and Overweight. (Fact Sheet 311). 2016. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 14 August 2019).
- Ward, Z.J.; Long, M.W.; Resch, S.C.; Giles, C.M.; Cradock, A.L.; Gortmaker, S.L. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N. Engl. J. Med. 2017, 377, 2145–2153. [Google Scholar] [CrossRef]
- Van De Maele, K.; Gies, I.; Devlieger, R. Effect of bariatric surgery before pregnancy on the vascular function in the offspring: Protocol of a cross-sectional follow-up study. BMJ Paediatr. Open 2019, 3, e000405. [Google Scholar] [CrossRef]
- Flemish Growth Charts. Available online: https://www.vub.be/groeicurven/ (accessed on 24 August 2020).
- Norton, K.; Whittingham, N.; Carter, L.; Kerr, D.; Gore, C.; Marfell-Jones, M. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Underdale, Australia, 1996. [Google Scholar]
- Huybrechts, I.; De Bacquer, D.; Matthys, C.; De Backer, G.; De Henauw, S. Validity and reproducibility of a semi-quantitative food-frequency questionnaire for estimating calcium intake in Belgian preschool children. Br. J. Nutr. 2006, 95, 802–816. [Google Scholar] [CrossRef]
- Huybrechts, I.; De Backer, G.; De Bacquer, D.; Maes, L.; De Henauw, S. Relative validity and reproducibility of a food-frequency questionnaire for estimating food intakes among Flemish preschoolers. Int. J. Environ. Res. Public Health 2009, 6, 382–399. [Google Scholar] [CrossRef]
- Huybrechts, I.; Vereecken, C.; De Bacquer, D.; Vandevijvere, S.; Van Oyen, H.; Maes, L.; Vanhauwaert, E.; Temme, L.; De Backer, G.; De Henauw, S. Reproducibility and validity of a diet quality index for children assessed using a FFQ. Br. J. Nutr. 2010, 104, 135–144. [Google Scholar] [CrossRef]
- De Ridder, K.; Bel, S.; Brocatus, L.; Lebacq, T.; Ost, C.; Teppers, E. Food Consumption Survey 2014–2015: Summary of the Results; Tafforeau, J., Ed.; Scientific Institute of Public Health—WIV-ISP: Brussels, Belgium, 2016. [Google Scholar]
- Liu, X.; Chen, Q.; Tsai, H.J.; Wang, G.; Hong, X.; Zhou, Y.; Zhang, C.; Liu, C.; Liu, R.; Wang, H.; et al. Maternal preconception body mass index and offspring cord blood DNA methylation: Exploration of early life origins of disease. Environ. Mol. Mutagen. 2014, 55, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Cardenas, A.; Rifas-Shiman, S.L.; Pan, H.; Dreyfuss, J.M.; Oken, E.; Hivert, M.F.; James-Todd, T.; Patti, M.E.; Isganaitis, E. Association of Periconception Paternal Body Mass Index With Persistent Changes in DNA Methylation of Offspring in Childhood. JAMA Netw. Open 2019, 2, e1916777. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Toschke, A.M. Meal patterns and frequencies: Do they affect body weight in children and adolescents? Crit. Rev. Food Sci. Nutr. 2010, 50, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Ruszczynski, M. Systematic review demonstrating that breakfast consumption influences body weight outcomes in children and adolescents in Europe. Crit. Rev. Food Sci. Nutr. 2010, 50, 113–119. [Google Scholar] [CrossRef] [PubMed]
- De La Hunty, A.; Ashwell, M. Are people who regularly eat breakfast cereals slimmer than those who don’t? A systematic review of the evidence. Nutr. Bull. 2007, 32, 118–128. [Google Scholar] [CrossRef]
- de la Hunty, A.; Gibson, S.; Ashwell, M. Does regular breakfast cereal consumption help children and adolescents stay slimmer? A systematic review and meta-analysis. Obes. Facts 2013, 6, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Tabuchi, T.; Iso, H. Association between skipping breakfast in parents and children and childhood overweight/obesity among children: A nationwide 10.5-year prospective study in Japan. Int. J. Obes. 2018, 42, 1724–1732. [Google Scholar] [CrossRef]
- Blondin, S.A.; Anzman-Frasca, S.; Djang, H.C.; Economos, C.D. Breakfast consumption and adiposity among children and adolescents: An updated review of the literature. Pediatr. Obes. 2016, 11, 333–348. [Google Scholar] [CrossRef]
- Kaisari, P.; Yannakoulia, M.; Panagiotakos, D.B. Eating Frequency and Overweight and Obesity in Children and Adolescents: A Meta-analysis. Pediatrics 2013, 131, 958. [Google Scholar] [CrossRef]
- Daniels, S.R.; Hassink, S.G. The Role of the Pediatrician in Primary Prevention of Obesity. Pediatrics 2015, 136, e275. [Google Scholar] [CrossRef]
- Gaal, S.; Kerr, M.A.; Ward, M.; McNulty, H.; Livingstone, M.B.E. Breakfast Consumption in the UK: Patterns, Nutrient Intake and Diet Quality. A Study from the International Breakfast Research Initiative Group. Nutrients 2018, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, M.; Gustafsson, P.E.; Wennberg, P.; Hammarström, A. Poor breakfast habits in adolescence predict the metabolic syndrome in adulthood. Public Health Nutr. 2015, 18, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Siega-Riz, A.M.; Popkin, B.M.; Carson, T. Trends in breakfast consumption for children in the United States from 1965–1991. Am. J. Clin. Nutr. 1998, 67, 748S–756S. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Breslin, M.C.; McNaughton, S.A.; Gall, S.L.; Blizzard, L.; Venn, A.J. Skipping breakfast among Australian children and adolescents; findings from the 2011–2012 National Nutrition and Physical Activity Survey. Aust. N. Z. J. Public Health 2017, 41, 572–578. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Figueiredo, R.A.; Viljakainen, J.; Viljakainen, H.; Roos, E.; Rounge, T.B.; Weiderpass, E. Identifying eating habits in Finnish children: A cross-sectional study. BMC Public Health 2019, 19, 312. [Google Scholar] [CrossRef]
- Ali, R.A.; Abdel Razeq, N.M.; Alnuaimi, K.M.; Alzoubi, F.A. Maternal Sociodemographic Characteristics and Behaviors as Correlates of Preadolescent’s Breakfast Habits. J. Pediatr. Nurs. 2018, 39, 61–67. [Google Scholar] [CrossRef]
- Sjöberg, A.; Hallberg, L.; Höglund, D.; Hulthén, L. Meal pattern, food choice, nutrient intake and lifestyle factors in The Göteborg Adolescence Study. Eur. J. Clin. Nutr. 2003, 57, 1569–1578. [Google Scholar] [CrossRef]
- Mota, J.; Fidalgo, F.; Silva, R.; Ribeiro, J.C.; Santos, R.; Carvalho, J.; Santos, M.P. Relationships between physical activity, obesity and meal frequency in adolescents. Ann. Hum. Biol. 2008, 35, 1–10. [Google Scholar] [CrossRef]
- Vaezghasemi, M.; Lindkvist, M.; Ivarsson, A.; Eurenius, E. Overweight and lifestyle among 13–15 year olds: A cross-sectional study in northern Sweden. Scand. J. Public Health 2012, 40, 221–228. [Google Scholar] [CrossRef]
- Medin, A.C.; Myhre, J.B.; Diep, L.M.; Andersen, L.F. Diet quality on days without breakfast or lunch—Identifying targets to improve adolescents’ diet. Appetite 2019, 135, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Azemati, B.; Heshmat, R.; Qorbani, M.; Ahadi, Z.; Azemati, A.; Shafiee, G.; Ziaodini, H.; Motlagh, M.E.; Kelishadi, R. Association of meal skipping with subjective health complaints in children and adolescents: The CASPIAN-V study. Eat. Weight Disord. 2020, 25, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Pourrostami, K.; Heshmat, R.; Hemati, Z.; Heidari-Beni, M.; Qorbani, M.; Motlagh, M.E.; Raeisi, A.; Shafiee, G.; Ziaodini, H.; Beshtar, S.; et al. Association of fruit and vegetable intake with meal skipping in children and adolescents: The CASPIAN-V study. Eat. Weight Disord. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mathias, K.C.; Jacquier, E.; Eldridge, A.L. Missing Lunch is Associated with Lower Intakes of Micronutrients from Foods and Beverages among Children and Adolescents in the United States. J. Acad. Nutr. Diet. 2016, 116, 667–676.e6. [Google Scholar] [CrossRef]
- Wijtzes, A.I.; Jansen, W.; Bouthoorn, S.H.; van Lenthe, F.J.; Franco, O.H.; Hofman, A.; Jaddoe, V.W.; Raat, H. Meal-Skipping Behaviors and Body Fat in 6-Year-Old Children. J. Pediatr. 2016, 168, 118–125.e2. [Google Scholar] [CrossRef]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef]
- Mehranfar, S.; Jalilpiran, Y.; Surkan, P.J.; Azadbakht, L. Association between protein-rich dietary patterns and anthropometric measurements among children aged 6 years. Nutr. Diet. 2020, 77, 359–367. [Google Scholar] [CrossRef]
- Dougkas, A.; Barr, S.; Reddy, S.; Summerbell, C.D. A critical review of the role of milk and other dairy products in the development of obesity in children and adolescents. Nutr. Res. Rev. 2019, 32, 106–127. [Google Scholar] [CrossRef]
- Spence, L.A.; Cifelli, C.J.; Miller, G.D. The Role of Dairy Products in Healthy Weight and Body Composition in Children and Adolescents. Curr. Nutr. Food Sci. 2011, 7, 40–49. [Google Scholar] [CrossRef]
- Grenov, B.; Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F. Role of Milk and Dairy Products in Growth of the Child. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 77–90. [Google Scholar]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Totzauer, M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin. Perinatol. 2019, 43, 151153. [Google Scholar] [CrossRef] [PubMed]
- Do, B.; Yang, C.H.; Lopez, N.V.; Mason, T.B.; Margolin, G.; Dunton, G.F. Investigating the momentary association between maternal support and children’s fruit and vegetable consumption using ecological momentary assessment. Appetite 2020, 150, 104667. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Bilger, M.; van Dam, R.M.; Finkelstein, E.A. Consumption of Specific Foods and Beverages and Excess Weight Gain Among Children and Adolescents. Health Aff. 2015, 34, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Faith, M.S.; Dennison, B.A.; Edmunds, L.S.; Stratton, H.H. Fruit juice intake predicts increased adiposity gain in children from low-income families: Weight status-by-environment interaction. Pediatrics 2006, 118, 2066–2075. [Google Scholar] [CrossRef]
- Shefferly, A.; Scharf, R.J.; DeBoer, M.D. Longitudinal evaluation of 100% fruit juice consumption on BMI status in 2-5-year-old children. Pediatr. Obes. 2016, 11, 221–227. [Google Scholar] [CrossRef]
- Sonneville, K.R.; Long, M.W.; Rifas-Shiman, S.L.; Kleinman, K.; Gillman, M.W.; Taveras, E.M. Juice and water intake in infancy and later beverage intake and adiposity: Could juice be a gateway drink? Obesity 2015, 23, 170–176. [Google Scholar] [CrossRef]
- Lee, A.K.; Chowdhury, R.; Welsh, J.A. Sugars and adiposity: The long-term effects of consuming added and naturally occurring sugars in foods and in beverages. Obes. Sci. Pract. 2015, 1, 41–49. [Google Scholar] [CrossRef]
- Newby, P.K.; Peterson, K.E.; Berkey, C.S.; Leppert, J.; Willett, W.C.; Colditz, G.A. Beverage consumption is not associated with changes in weight and body mass index among low-income preschool children in North Dakota. J. Am. Diet. Assoc. 2004, 104, 1086–1094. [Google Scholar] [CrossRef]
- Skinner, J.D.; Carruth, B.R. A longitudinal study of children’s juice intake and growth: The juice controversy revisited. J. Am. Diet. Assoc. 2001, 101, 432–437. [Google Scholar] [CrossRef]
- Zheng, M.; Allman-Farinelli, M.; Heitmann, B.L.; Toelle, B.; Marks, G.; Cowell, C.; Rangan, A. Liquid versus solid energy intake in relation to body composition among Australian children. J. Hum. Nutr. Diet. 2015, 28 (Suppl. 2), 70–79. [Google Scholar] [CrossRef]
- Zheng, M.; Rangan, A.; Olsen, N.J.; Andersen, L.B.; Wedderkopp, N.; Kristensen, P.; Grøntved, A.; Ried-Larsen, M.; Lempert, S.M.; Allman-Farinelli, M.; et al. Substituting sugar-sweetened beverages with water or milk is inversely associated with body fatness development from childhood to adolescence. Nutrition 2015, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- Millar, L.; Rowland, B.; Nichols, M.; Swinburn, B.; Bennett, C.; Skouteris, H.; Allender, S. Relationship between raised BMI and sugar sweetened beverage and high fat food consumption among children. Obesity 2014, 22, E96–E103. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Neate, D. Sugar intake, soft drink consumption and body weight among British children: Further analysis of National Diet and Nutrition Survey data with adjustment for under-reporting and physical activity. Int. J. Food Sci. Nutr. 2007, 58, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Bucher Della Torre, S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child. Obes. 2015, 11, 338–346. [Google Scholar] [CrossRef]
- Reedy, J.; Krebs-Smith, S.M. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J. Am. Diet. Assoc. 2010, 110, 1477–1484. [Google Scholar] [CrossRef]
- Frantsve-Hawley, J.; Bader, J.D.; Welsh, J.A.; Wright, J.T. A systematic review of the association between consumption of sugar-containing beverages and excess weight gain among children under age 12. J. Public Health Dent. 2017, 77 (Suppl. 1), S43–S66. [Google Scholar] [CrossRef]
- Wang, H.; Jeong, H.; Kim, N.H.; Kang, Y.; Hwang, K.; Lee, H.; Hong, J.H.; Oh, K.S. Association between beverage intake and obesity in children: The Korea National Health and Nutrition Examination Survey (KNHANES) 2013–2015. Nutr. Res. Pract. 2018, 12, 307–314. [Google Scholar] [CrossRef]
- Cantoral, A.; Téllez-Rojo, M.M.; Ettinger, A.S.; Hu, H.; Hernández-Ávila, M.; Peterson, K. Early introduction and cumulative consumption of sugar-sweetened beverages during the pre-school period and risk of obesity at 8–14 years of age. Pediatr. Obes. 2016, 11, 68–74. [Google Scholar] [CrossRef]
- Bigornia, S.J.; LaValley, M.P.; Noel, S.E.; Moore, L.L.; Ness, A.R.; Newby, P.K. Sugar-sweetened beverage consumption and central and total adiposity in older children: A prospective study accounting for dietary reporting errors. Public Health Nutr. 2015, 18, 1155–1163. [Google Scholar] [CrossRef]
- De Ruyter, J.C.; Olthof, M.R.; Kuijper, L.D.; Katan, M.B. Effect of sugar-sweetened beverages on body weight in children: Design and baseline characteristics of the Double-blind, Randomized INtervention study in Kids. Contemp. Clin. Trials 2012, 33, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, L.M.; Marini, M.; Francis, L.A.; Smiciklas-Wright, H.; Birch, L.L. Beverage intake of girls at age 5 y predicts adiposity and weight status in childhood and adolescence. Am. J. Clin. Nutr. 2009, 90, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Rangan, A.; Olsen, N.J.; Bo Andersen, L.; Wedderkopp, N.; Kristensen, P.; Grøntved, A.; Ried-Larsen, M.; Lempert, S.M.; Allman-Farinelli, M.; et al. Sugar-sweetened beverages consumption in relation to changes in body fatness over 6 and 12 years among 9-year-old children: The European Youth Heart Study. Eur. J. Clin. Nutr. 2014, 68, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101–108. [Google Scholar] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. Artificially sweetened beverages: Cause for concern. JAMA 2009, 302, 2477–2478. [Google Scholar] [CrossRef]
- Azad, M.B.; Archibald, A.; Tomczyk, M.M.; Head, A.; Cheung, K.G.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Nonnutritive sweetener consumption during pregnancy, adiposity, and adipocyte differentiation in offspring: Evidence from humans, mice, and cells. Int. J. Obes. 2020. [Google Scholar] [CrossRef]
- Reid, A.E.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Abou-Setta, A.M.; Fiander, M.; MacKay, D.S.; McGavock, J.; et al. Early Exposure to Nonnutritive Sweeteners and Long-term Metabolic Health: A Systematic Review. Pediatrics 2016, 137, e20153603. [Google Scholar] [CrossRef]
- Archibald, A.J.; Dolinsky, V.W.; Azad, M.B. Early-Life Exposure to Non-Nutritive Sweeteners and the Developmental Origins of Childhood Obesity: Global Evidence from Human and Rodent Studies. Nutrients 2018, 10, 194. [Google Scholar] [CrossRef]
- Larson, N.; Story, M. A review of snacking patterns among children and adolescents: What are the implications of snacking for weight status? Child. Obes. 2013, 9, 104–115. [Google Scholar] [CrossRef]
- Miller, R.; Benelam, B.; Stanner, S.A.; Buttriss, J.L. Is snacking good or bad for health: An overview. Nutr. Bull. 2013, 38, 302–322. [Google Scholar] [CrossRef]
- Keast, D.R.; Nicklas, T.A.; O’Neil, C.E. Snacking is associated with reduced risk of overweight and reduced abdominal obesity in adolescents: National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am. J. Clin. Nutr. 2010, 92, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Toschke, A.M.; Thorsteinsdottir, K.H.; von Kries, R.; GME Study Group. Meal frequency, breakfast consumption and childhood obesity. Int. J. Pediatr. Obes. 2009, 4, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar] [CrossRef]
- Ocké, M.C.; Larrañaga, N.; Grioni, S.; van den Berg, S.W.; Ferrari, P.; Salvini, S.; Benetou, V.; Linseisen, J.; Wirfält, E.; Rinaldi, S.; et al. Energy intake and sources of energy intake in the European Prospective Investigation into Cancer and Nutrition. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S3–S15. [Google Scholar] [CrossRef]
- Ovaskainen, M.L.; Reinivuo, H.; Tapanainen, H.; Hannila, M.L.; Korhonen, T.; Pakkala, H. Snacks as an element of energy intake and food consumption. Eur. J. Clin. Nutr. 2006, 60, 494–501. [Google Scholar] [CrossRef]
- Yanetz, R.; Kipnis, V.; Carroll, R.J.; Dodd, K.W.; Subar, A.F.; Schatzkin, A.; Freedman, L.S. Using biomarker data to adjust estimates of the distribution of usual intakes for misreporting: Application to energy intake in the US population. J. Am. Diet. Assoc. 2008, 108, 455–464. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Graubard, B.I.; Kahle, L.L.; Subar, A.F.; Cleveland, L.E.; Ballard-Barbash, R. Low energy reporters vs others: A comparison of reported food intakes. Eur. J. Clin. Nutr. 2000, 54, 281–287. [Google Scholar] [CrossRef]
- Bjorklund, G.; Semenova, Y.; Pivina, L.; Costea, D.O. Follow-up after bariatric surgery: A review. Nutrition 2020, 78, 110831. [Google Scholar] [CrossRef]
| NW (n = 35) | OW/OB (n = 71) | BS (n = 36) | p-Value | Post-Hoc | |
|---|---|---|---|---|---|
| Age (years) | 10.6 (0.2) | 10.2 (1.9) | 6.5 (1.3) | <0.001 | BS < NW and OW/OB |
| Sex (female) | 18 (51.4%) | 37 (52.1%) | 17 (47.2%) | 0.89 | - |
| Height (cm) | 146.4 (5.4) | 143.4 (13.0) | 123.2 (9.6) | <0.001 | BS < NW and OW/OB |
| Height (SD score) | 0.42 (0.81) | 0.36 (0.91) | 0.64 (0.92) | 0.31 | - |
| Weight (kg) | 35.6 (6.2) | 37.6 (11.6) | 26.3 (7.5) | <0.001 | BS < NW and OW/OB |
| Weight (SD score) | −0.086 (0.84) | 0.24 (0.95) | 0.70 (1.27) | 0.005 | NW < BS |
| BMI (kg/m²) | 16.5 (2.3) | 17.9 (3.6) | 17.1 (2.9) | 0.09 | - |
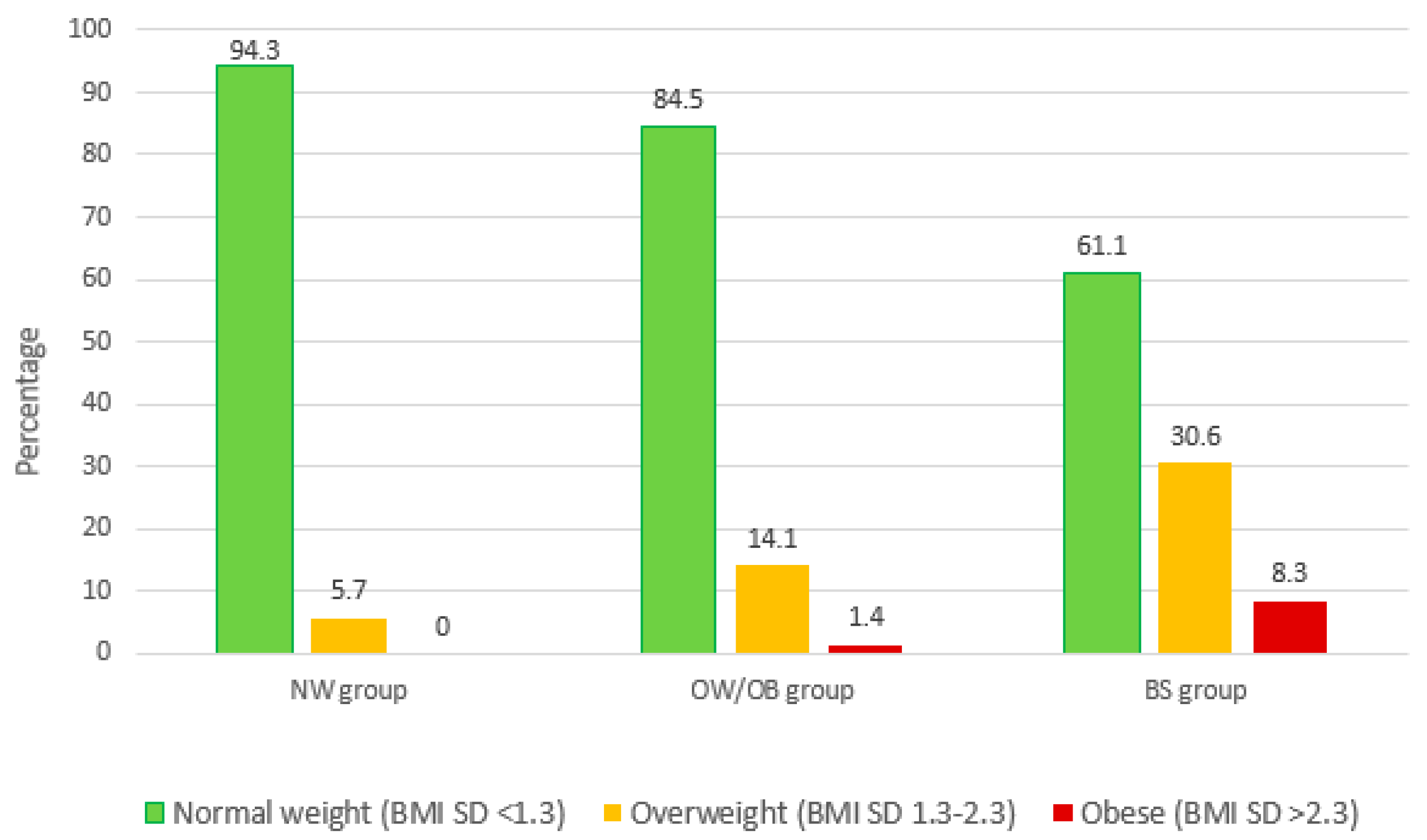
| BMI (SD score) | −0.42 (1.06) | 0.68 (1.03) | 0.47 (1.50) | 0.007 | NW < BS |
| Total body fat (%) (BIA) | 20.2 (4.4) | 22.5 (6.5) | 23.4 (5.2) | 0.06 | - |
| Neck circumference (cm) | 28.3 (1.8) | 28.9 (2.7) | 26.9 (1.8) | <0.001 | BS < NW and OW/OB |
| Waist (cm) | 60.7 (7.2) | 62.8 (9.5) | 57.8 (8.2) | 0.02 | BS < OW/OB |
| Hip (cm) | 73.3 (5.2) | 76.1 (10.5) | 67.5 (8.8) | <0.001 | BS < NW and OW/OB |
| Skinfold biceps (mm) | 9.7 (4.7) | 11.1 (5.7) | 11.5 (5.1) | 0.32 | - |
| Skinfold triceps (mm) | 11.9 (4.2) | 13.4 (5.8) | 12.4 (4.2) | 0.32 | - |
| Skinfold Subscapularis (mm) | 7.5 (4.0) | 9.0 (6.8) | 8.6 (4.1) | 0.47 | - |
| Skinfold Supraspinalis (mm) | 8.7 (5.3) | 10.5 (7.6) | 9.9 (5.8) | 0.44 | - |
| Sum of skinfolds (SSF, mm) | 37.9 (17.2) | 44.0 (24.7) | 42.5 (17.6) | 0.39 | - |
| Hours of night sleep | 9.8 (0.8) | 9.6 (1.1) | 10.3 (1.3) | 0.009 | OW/OB < BS |
| Screen time/day (h) | 2.2 (1.2) | 2.7 (1.1) | 2.4 (1.1) | 0.10 | - |
| Physical activity/week (h) | 3.5 (2.1) | 3.5 (2.7) | 2.6 (1.8) | 0.18 | - |
| Pre-pregnancy BMI | 21.8 (1.9) | 32.4 (4.1) | 29.5 (5.0) | <0.001 | NW < BS < OW/OB |
| Actual maternal BMI | 23.1 (3.1) | 31.4 (5.5) | 30.4 (6.3) | <0.001 | NW < BS and OW/OB |
| Mother education level: college or university | 30 (85.7%) | 54 (77.1%) | 16 (44.4%) | <0.001 | BS < NW and OW/OB |
| Actual paternal BMI | 24.7 (3.2) | 26.6 (4.5) | 28.5 (4.8) | 0.003 | NW < BS |
| Father education level: college or university | 24 (70.6%) | 28 (41.8%) | 7 (20.6%) | <0.001 | BS and OW/OB < NW |
| National Population | NW (n = 35) | OW/OB (n = 71) | BS (n = 36) | |
|---|---|---|---|---|
| Water (/day) | 589 mL | 54.3% > 600 mL | 47.9% > 600 mL | 31.4% >6 00 mL |
| Other sugar-free beverages (/day) | 24 mL | 28 mL (±73 mL) | 62 mL (±123 mL) | 110 mL (±185 mL) |
| Fruit juice (/day) | 90 mL | 135 mL (±109 mL) | 113 mL (±102 mL) | 62 mL (±57 mL) |
| Sugar-Sweetened Beverages | 145 mL 52% min 2x/week o/w 20% daily | 112 mL (±111 mL) 29% min 2x/week o/w 12% daily | 147 mL (±141 mL) 35% min 2x/week o/w 14% daily | 200 mL (±198 mL) 28% min 2x/week o/w 19% daily |
| Vegetables (/day) | 96 g | 114 g (±57 g) | 115 g (±63 g) | 119 g (±50 g) |
| Fruit (/day) Daily | 120 g 62% | 133 g (±64 g) 71% | 137 g (±52 g) 72% | 121 g (±55 g) 50% |
| Meat (/day) | 112 g | NA | NA | NA |
| Fish (/week) | 44% <1x | 46% <1x | 67% <1x | 67% <1x |
| Rest group (per day) | 356 g (632 kcal) | NA | NA | NA |
| Biscuits and cake (/day) | 58 g 86% >2x/week o/w 45% daily | 48 g (±21 g) 87% >2x/week o/w 37% daily | 49 g (±23 g) 86% >2x/week o/w 48% daily | 42 g (±23 g) 86% >2x/week o/w 28% daily |
| Sweets and chocolate (/day) | 40 g | NA | NA | NA |
| NW (n = 35) | OW/OB (n = 71) | BS (n = 36) | p-Value | |
|---|---|---|---|---|
| Breakfast | 0.83 | |||
| Daily | 31/35 (88.6%) | 59/70 (84.3%) | 29/36 (80.6%) | |
| 5–6 times/week | 2/35 (5.7%) | 5/70 (7.1%) | 2/36 (5.5%) | |
| ≤4 times/week | 2/35 (5.7%) | 6/70 (8.6%) | 5/36 (13.9%) | |
| Lunch | 0.72 | |||
| Daily | 34/35 (97.1%) | 67/70 (95.7%) | 33/36 (91.7%) | |
| 5–6 times/week | 1/35 (2.9%) | 3/70 (4.3%) | 3/36 (8.3%) | |
| Dinner | 0.05 | |||
| Daily | 35/35 (100%) | 70/70 (100%) | 33/36 (91.7%) | |
| 5–6 times/week | 1/36 (2.8%) | |||
| ≤4 times/week | 2/36 (5.5%) |
| NW (n = 35) | OW/OB (n = 71) | BS (n = 36) | p-Value | |
|---|---|---|---|---|
| Meat | ||||
| Frequency per week | 0.53 | |||
| ≥5 times | 5/35 (14.3%) | 13/70 (18.6%) | 9/36 (25 %) | |
| ≤4 times | 30/35 (85.7%) | 57/70 (81.4%) | 27/36 (75%) | |
| Quantity per average consumption day | 0.36 | |||
| >75 g | 18/32 (56.3%) | 43/69 (62.3%) | 16/34 (47.1%) | |
| ≤75 g | 14/32 (43.8%) | 26/69 (37.7%) | 18/34 (52.9%) | |
| Poultry | ||||
| Frequency per week | 0.11 | |||
| <2 times | 17/35 (48.6%) | 21/71 (29.6%) | 16/36 (44.4%) | |
| ≥2 times | 18/35 (51.4%) | 50 /71 (70.4%) | 20/36 (55.6%) | |
| Quantity per average consumption day | 0.008 Post-hoc: BS < OW/OB | |||
| >75 g | 16/34 (47.1%) | 38/67 (56.7%) | 9/36 (25%) | |
| ≤75 g | 18/34 (52.9%) | 29/67 (43.3%) | 25/36 (75%) | |
| Fish | ||||
| Frequency per week | 0.10 | |||
| <1 time | 16/35 (45.7%) | 46/69 (66.7%) | 24/36 (66.7%) | |
| ≥1 time | 19/35 (54.3%) | 23/69 (33.3%) | 12/36 (33.3%) | |
| Quantity per average consumption day | 0.21 | |||
| >75 g | 13/29 (44.8%) | 24/41 (58.5%) | 10/27 (37%) | |
| ≤75 g | 16/29 (55.2%) | 17/41 (41.5%) | 17/27 (63%) | |
| Milk | ||||
| Frequency per week | 0.81 | |||
| <1 time | 13/35 (37.1%) | 28/70 (40.0%) | 16/36 (44.4%) | |
| ≥2 times | 22/35 (62.9%) | 42/70 (60.0%) | 20/36 (55.6%) | |
| Quantity per average consumption day | 0.38 | |||
| >200 mL | 15/24 (62.5%) | 23/45 (51.1%) | 10/24 (41.7%) | |
| ≤200 mL | 9/24 (37.5%) | 22/45 (48.9%) | 14/24 (58.3%) | |
| Sugared yoghurt | ||||
| Frequency per week | 0.91 | |||
| <1 time | 29/35 (82.9%) | 61/71 (85.9%) | 31/36 (86.1%) | |
| ≥2 times | 6/35 (17.1%) | 10/71 (14.1%) | 5/36 (13.9%) | |
| Quantity per average consumption day | 0.01 Post-hoc: BS < NW | |||
| >65 g | 19/20 (95.0%) | 27/33 (81.8%) | 8/15 (53.3%) | |
| ≤65 g | 1/20 (5.0%) | 6/33 (18.2%) | 7/15 (46.7%) | |
| Fruit | ||||
| Frequency per week | 0.97 | |||
| ≥5 times | 27/35 (77.1%) | 54/70 (77.1%) | 27/36 (75%) | |
| ≤4 times | 8/35 (22.9%) | 16/70 (22.9%) | 9/36 (25%) | |
| Quantity per average consumption day | 0.55 | |||
| >75 g | 31/35 (88.6%) | 65/69 (94.2%) | 33/36 (91.7%) | |
| ≤75 g | 4/35 (11.4%) | 4/69 (5.8%) | 3/36 (8.3%) | |
| Vegetables | ||||
| Frequency per week | 0.38 | |||
| ≥5 times | 19/35 (54.3%) | 36/71 (50.7%) | 14/36 (38.9%) | |
| ≤4 times | 16/35 (45.7%) | 35/71 (49.3%) | 22/36 (61.1%) | |
| Quantity per average consumption day | 0.32 | |||
| >180 g | 5/35 (14.3%) | 4/68 (5.9%) | 2/33 (6.1%) | |
| ≤180 g | 30/35 (85.7%) | 64/68 (94.1%) | 31/33 (93.9%) | |
| NW (n = 35) | OW/OB (n = 71) | BS (n = 36) | p-Value | |
|---|---|---|---|---|
| Fruit juice | ||||
| Frequency per week | 0.01 Post-hoc: BS < OW/OB and NW | |||
| ≥2 days | 13/35 (37.1%) | 21/70 (30.0%) | 3/36 (8.3%) | |
| ≤1 day | 22/35 (62.9%) | 49/70 (70.0%) | 33/36 (91.7%) | |
| Quantity per average consumption day | 0.53 | |||
| >200 mL | 8/28 (28.6%) | 19/49 (38.8%) | 5/19 (26.3%) | |
| ≤200 mL | 20/28 (71.4%) | 30/49 (61.2%) | 14/19 (38.9%) | |
| Low-calorie sweetened beverages | ||||
| Frequency per week | 0.01 Post-hoc: BS > NW | |||
| ≥2 days | 3/35 (8.6%) | 19/71 (26.8%) o/w 9/71 (13%) daily | 14/36 (38.9%) o/w 7/36 (19%) daily | |
| ≤1 day | 32/35 (91.4%) | 52/71 (73.2%) | 22/36 (61.1%) | |
| Quantity per average consumption day | 0.94 | |||
| >200 mL | 7/13 (53.8%) | 19/34 (55.9%) | 8/17 (47.1%) o/w 3/17 (18%) > 600 mL | |
| ≤200 mL | 6/13 (46.2%) | 15/34 (44.1%) | 9/17 (52.9%) | |
| Sugar-sweetened beverages | ||||
| Frequency per week | 0.73 | |||
| ≥2 days | 10/34 (29.4%) o/w 4/34 (12%) daily | 25/71 (35.2%) o/w 10/71 (14%) daily | 10/36 (27.8%) o/w 7/36 (19%) daily | |
| ≤1 day | 24/34 (70.6%) | 46/71 (64.8%) | 26/36 (72.2%) | |
| Quantity per average consumption day | 0.35 | |||
| >200 mL | 13/26 (50%) | 23/50 (46%) o/w 4/50 (8%) > 400 | 6/20 (30.0%) o/w 2/20 (10%) > 400 | |
| ≤200 mL | 13/26 (50%) | 27/50 (54%) | 14/20 (70%) | |
| Water | ||||
| Frequency per week | 0.43 | |||
| ≤6 days | 2/35 (5.7%) | 3/71 (4.2%) | 4/36 (11.1%) | |
| Daily | 33/35 (94.3%) | 68/71 (95.8%) | 32/36 (88.9%) | |
| Quantity per average consumption day | 0.14 | |||
| <600 mL/day | 16/35 (45.7%) | 37/71 (52.1%) | 24/35 (68.6%) | |
| >600 mL/day | 19/35 (54.3%) | 34/71 (47.9%) | 11/35 (31.4%) | |
| NW (n = 35) | OW/OB (n = 71) | BS (n = 36) | p-Value | |
|---|---|---|---|---|
| Chocolate | ||||
| Frequency per week | 0.86 | |||
| ≥2 times | 9/35 (25.7%) | 22/70 (31.4%) | 10/36 (27.8%) | |
| ≤1 time | 26/35 (74.3%) | 48/70 (68.6%) | 26/36 (72.2%) | |
| Quantity per average consumption day | 0.80 | |||
| >25 g | 10/31 (32.3%) | 21/55 (38.2%) | 10/31 (32.3%) | |
| ≤25 g | 21/31 (67.7%) | 34/55 (61.8%) | 21/31 (67.7%) | |
| Sweet snacks | ||||
| Frequency per week | 1.0 | |||
| ≥2 times | 31/35 (88.6%) | 61/71 (85.9%) | 31/36 (86.1%) | |
| ≤1 time | 4/35 (11.4%) | 10/71 (14.1%) | 5/36 (13.9%) | |
| Quantity per average consumption day | 0.19 | |||
| >25 g | 26/35 (74.3%) | 45/66 (68.2%) | 19/35 (54.3%) | |
| ≤25 g | 9/35 (25.7%) | 21/66 (31.8%) | 16/35 (45.7%) | |
| Salty snacks | ||||
| Frequency per week | 0.84 | |||
| ≥2 times | 7/35 (20%) | 18/71 (25.4%) | 9/36 (25%) | |
| ≤1 time | 28/35 (80%) | 53/71 (74.6%) | 27/36 (75%) | |
| Quantity per average consumption day | 0.01 Post-hoc: BS < OW/OB | |||
| >25 g | 28/34 (82.4%) | 52/63 (82.5%) | 19/34 (55.9%) | |
| ≤25 g | 6/34 (17.6%) | 11/63 (17.5%) | 16/34 (44.1%) | |
| Chocolate mousse/ice cream | ||||
| Frequency per week | 0.01 Post-hoc: NW > OW/OB | |||
| ≥2 times | 10/35 (28.6%) | 5/69 (7.2%) | 5/36 (13.9%) | |
| ≤1 time | 25/35 (71.4%) | 64/69 (92.8%) | 31/36 (86.1%) | |
| Quantity per average consumption day | 0.34 | |||
| >65 g | 19/29 (65.5%) | 29/49 (59.2%) | 14/30 (46.7%) | |
| ≤65 g | 10/29 (34.5%) | 20/49 (40.8) | 16/30 (53.3%) | |
| Milk desserts | ||||
| Frequency per week | 0.01 Post-hoc: BS > OW/OB | |||
| ≥2 times | 6/34 (17.6%) | 4/69 (5.8%) | 9/36 (25%) | |
| ≤1 time | 28/34 (82.4%) | 65/69 (94.2%) | 27/36 (75%) | |
| Quantity per average consumption day | 0.59 | |||
| >65 g | 13/16 (81.2%) | 34/41 (82.9%) | 19/26 (73.1%) | |
| ≤65 g | 3/16 (18.8%) | 7/41 (17.1%) | 7/26 (26.9%) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van De Maele, K.; De Geyter, C.; Vandenplas, Y.; Gies, I.; Devlieger, R. Eating Habits of Children Born after Maternal Bariatric Surgery. Nutrients 2020, 12, 2577. https://doi.org/10.3390/nu12092577
Van De Maele K, De Geyter C, Vandenplas Y, Gies I, Devlieger R. Eating Habits of Children Born after Maternal Bariatric Surgery. Nutrients. 2020; 12(9):2577. https://doi.org/10.3390/nu12092577
Chicago/Turabian StyleVan De Maele, Karolien, Charlotte De Geyter, Yvan Vandenplas, Inge Gies, and Roland Devlieger. 2020. "Eating Habits of Children Born after Maternal Bariatric Surgery" Nutrients 12, no. 9: 2577. https://doi.org/10.3390/nu12092577
APA StyleVan De Maele, K., De Geyter, C., Vandenplas, Y., Gies, I., & Devlieger, R. (2020). Eating Habits of Children Born after Maternal Bariatric Surgery. Nutrients, 12(9), 2577. https://doi.org/10.3390/nu12092577








