Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of LGG
2.2. Animal Care and LGG Administration
2.3. Leptin Resistance Test
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis of SOCS3 Expression
2.5. Histological Observation of Liver and Intestine
2.6. Metagenomic Analysis of Fecal Microbiome
2.7. Statistical Analysis
3. Results
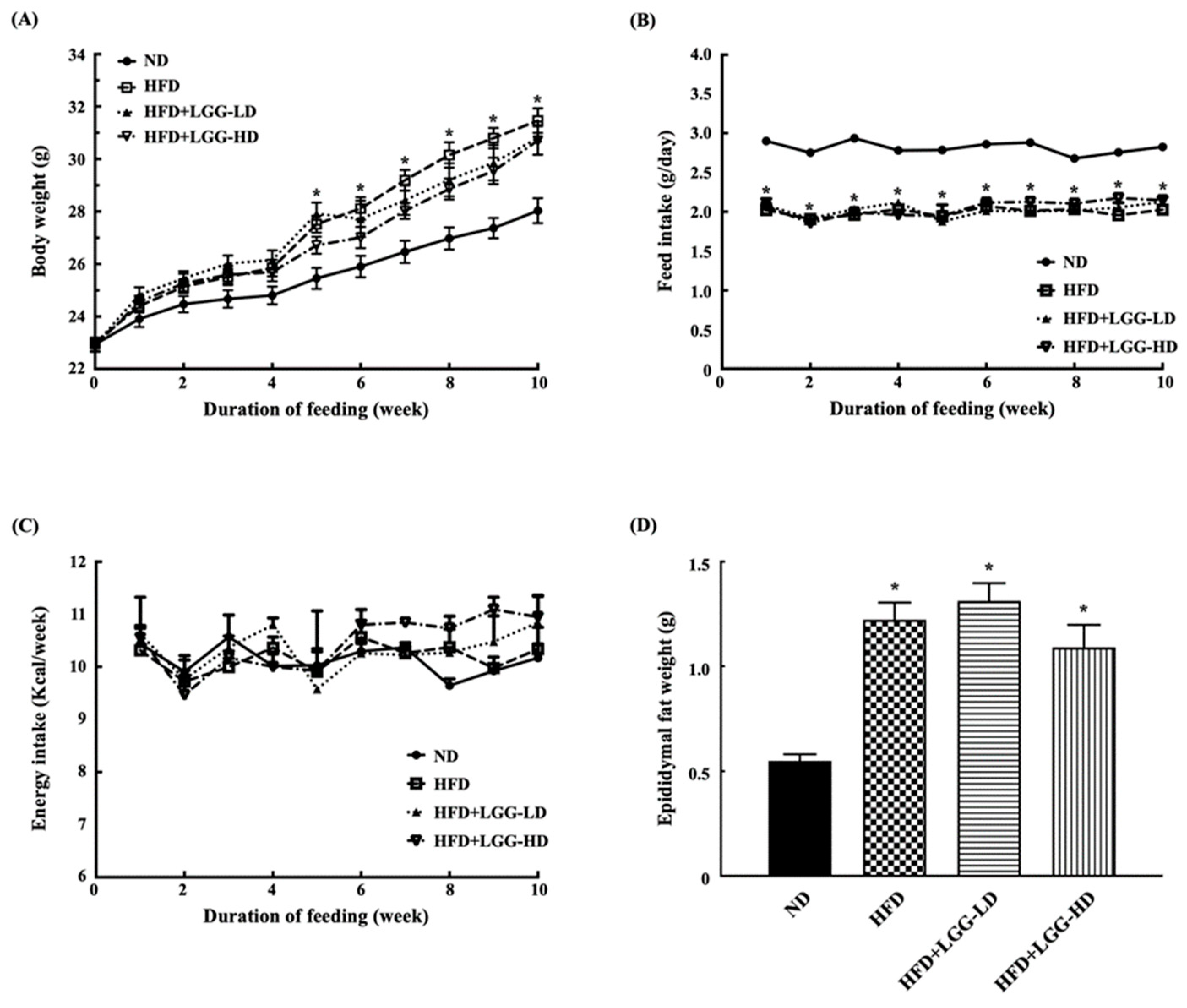
3.1. Effect of Administration of LGG on Body Weight and Feed Intake in Diet-Induced Obesity in Mice
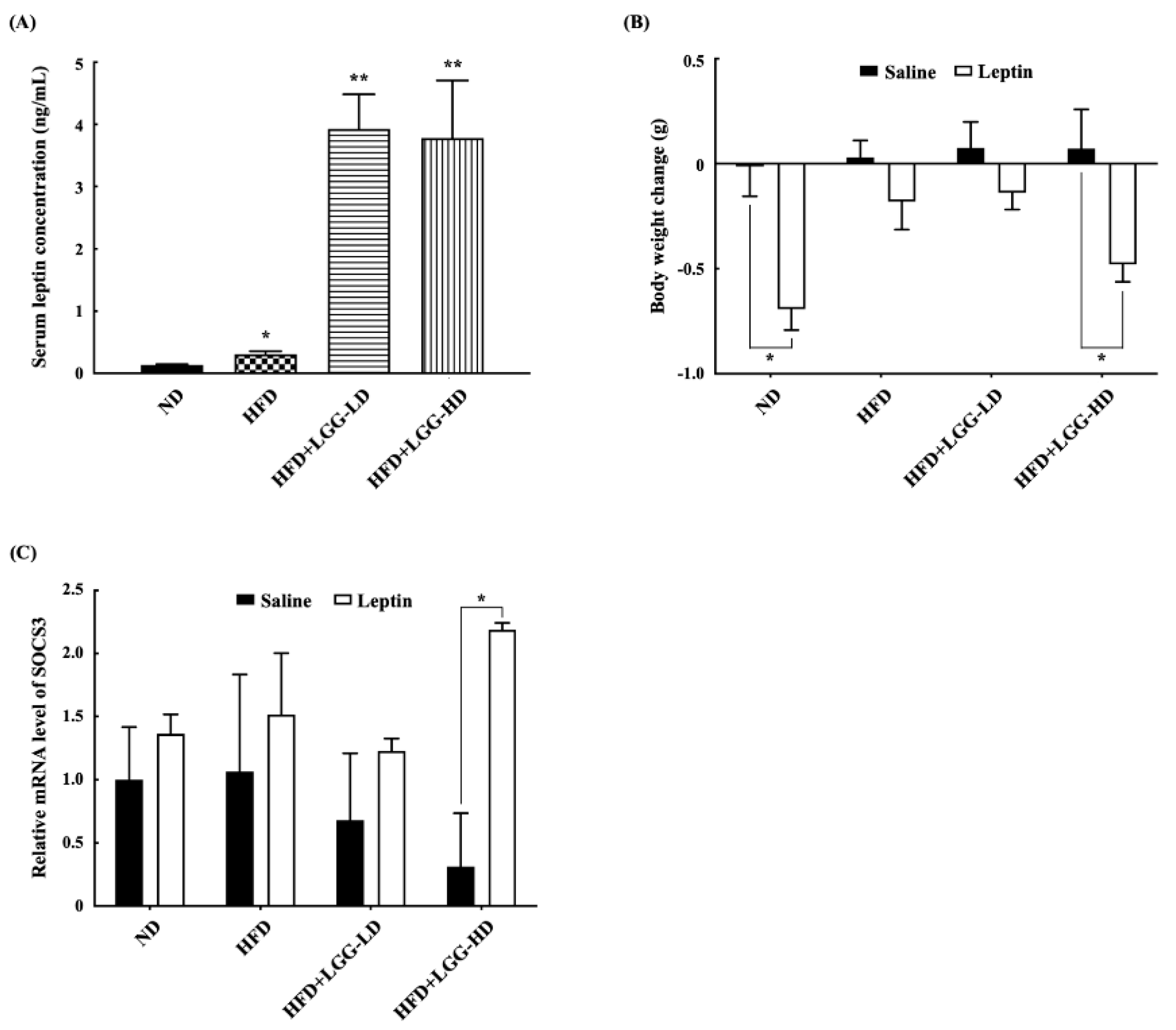
3.2. Effect of Administration of LGG on the Serum Leptin Concentrations, Leptin Resistance, and Hypothalamic SOCS3 Expression Levels in Mice
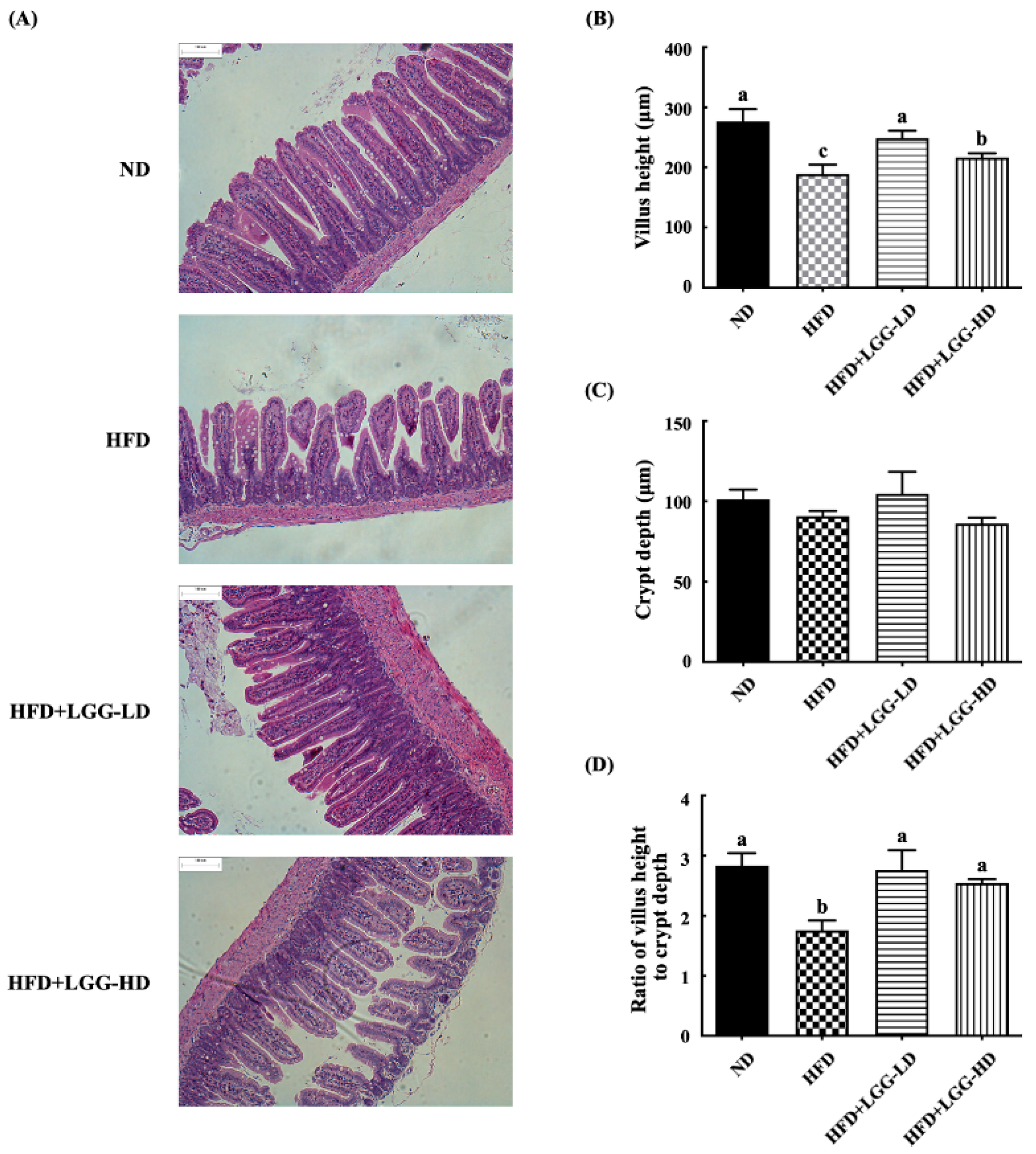
3.3. Effect of Administration of LGG on the Histological Morphology of the Ileum
3.4. Effect of Administration of LGG on Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 June 2019).
- Friedman, J.M. The function of leptin in nutrition; Weight, and physiology. Nutr. Rev. 2002, 60, S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Schéle, E.; Grahnemo, L.; Anesten, F.; Hallén, A.; Bäckhed, F.; Jansson, J.-O. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 2013, 154, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Mahony, R.; Ahmed, S.; Diskin, C.; Stevenson, N.J. SOCS3 revisited: A broad regulator of disease, now ready for therapeutic use? Cell. Mol. Life Sci. 2016, 73, 3323–3336. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Cani, P.D. Gutmicrobiota and obesity: Lessons from the microbiome. Brief. Funct. Genom. 2013, 12, 381–387. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Miele, L.; Giorgio, V.; Alberelli, M.A.; De Candia, E.; Gasbarrini, A.; Grieco, A. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr. Cardiol. Rep. 2015, 17, 120. [Google Scholar] [CrossRef]
- Al-muzafar, H.M.; Amin, K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef]
- Ji, Y.; Park, S.; Park, H.; Hwang, E.; Shin, H.; Pot, B.; Holzapfel, W.H. Modulation of active gut microbiota by Lactobacillus rhamnosus GG in a diet induced obesity murine model. Front. Microbiol. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Park, J.-H.; Seok, S.-H.; Baek, M.-W.; Kim, D.-J.; Lee, K.-E.; Paek, K.-S.; Lee, Y.; Park, J.-H. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. Acta 2006, 1761, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Halper, J.; Zhang, J.; Lewis, S.J.; Li, W.-I.O. Effect of Lactobacillus acidophilus supernatants on body weight and leptin expression in rats. BMC Complement. Altern. Med. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Park, K.-Y.; Kim, B.; Kim, E.; Hyun, C.-K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef]
- Darby, T.M.; Naudin, C.R.; Luo, L.; Jones, R.M. Lactobacillus rhamnosus GG–induced expression of leptin in the intestine orchestrates epithelial cell proliferation. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 627–639. [Google Scholar] [CrossRef]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Fernandez-Marcos, P.J.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef]
- Nossa, C.W.; Oberdorf, W.E.; Yang, L.; Aas, J.A.; Paster, B.J.; de Santis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135–4144. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Tan, C.K.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef]
- Zinocker, M.K.; Lindseth, I.A. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Reimer, R.A. Establishing the role of diet in the microbiota–disease axis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Obici, S.; Morgan, K.; Barzilai, N.; Feng, Z.H.; Rossetti, L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 2001, 50, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, C.; Fan, X.; Lu, Y.; Wang, Y.; Wang, R.; Tang, T.; Qi, K. Effects of gut microbiota on leptin expression and body weight are lessened by high-fat diet in mice. Br. J. Nutr. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.S.; Kim, H.N.; Park, H.J.; Lee, J.E.; Yeo, S.Y.; Yang, J.S.; Park, S.Y.; Yoon, H.S.; Cho, G.S.; Franz, C.M.A.P.; et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef. Microbes 2012, 3, 13–22. [Google Scholar] [CrossRef]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef]
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42. [Google Scholar] [CrossRef]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Lollmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid-content in mice—Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- Halaas, J.L.; Boozer, C.; BlairWest, J.; Fidahusein, N.; Denton, D.A.; Friedman, J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8878–8883. [Google Scholar] [CrossRef]
- Matarazzo, V.; Schaller, F.; Nédélec, E.; Benani, A.; Pénicaud, L.; Muscatelli, F.; Moyse, E.; Bauer, S. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J. Neurosci. 2012, 32, 17097–17107. [Google Scholar] [CrossRef]
- Lee, J.; Liu, J.; Feng, X.; Hernández, M.A.S.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef]
- Lee, J.S.; Paek, N.S.; Kwon, O.S.; Hahm, K.B. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: A novel mechanism. J. Gastroenterol. Hepatol. 2010, 25, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Latvala, S.; Miettinen, M.; Kekkonen, R.A.; Korpela, R.; Julkunen, I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin. Exp. Immunol. 2011, 165, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Machairas, N.; Pistiki, A.; Droggiti, D.-I.; Georgitsi, M.; Pelekanos, N.; Damoraki, G.; Kouraklis, G.; Giamarellos-Bourboulis, E.J. Pre-treatment with probiotics prolongs survival after experimental infection by multidrug-resistant Pseudomonas aeruginosa in rodents: An effect on sepsis-induced immunosuppression. Int. J. Antimicrob. Agents 2015, 45, 376–384. [Google Scholar] [CrossRef]
- Stavrou, G.; Giamarellos-Bourboulis, E.J.; Kotzampassi, K. The role of probiotics in the prevention of severe infections following abdominal surgery. Int. J. Antimicrob. Agents 2015, 46, S2–S4. [Google Scholar] [CrossRef]
- Adelman, D.C.; Murray, J.; Wu, T.-T.; Mäki, M.; Green, P.H.; Kelly, C.P. Measuring change in small intestinal histology in patients with celiac disease. Am. J. Gastroenterol. 2018, 113, 339–347. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Senthong, V.; Wang, Z.; Li, X.S.; Fan, Y.; Wu, Y.; Tang, W.H.W.; Hazen, S.L. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: The contributory role of intestinal microbiota in a COURAGE-like patient cohort. J. Am. Heart Assoc. 2016, 5, e002816. [Google Scholar] [CrossRef]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Raber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.; OKeefe, J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019, 6, e000890. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-C.; Liu, J.-R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. https://doi.org/10.3390/nu12092557
Cheng Y-C, Liu J-R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients. 2020; 12(9):2557. https://doi.org/10.3390/nu12092557
Chicago/Turabian StyleCheng, Yu-Chieh, and Je-Ruei Liu. 2020. "Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity" Nutrients 12, no. 9: 2557. https://doi.org/10.3390/nu12092557
APA StyleCheng, Y.-C., & Liu, J.-R. (2020). Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients, 12(9), 2557. https://doi.org/10.3390/nu12092557





