Risk Factors for Muscle Loss in Hemodialysis Patients with High Comorbidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
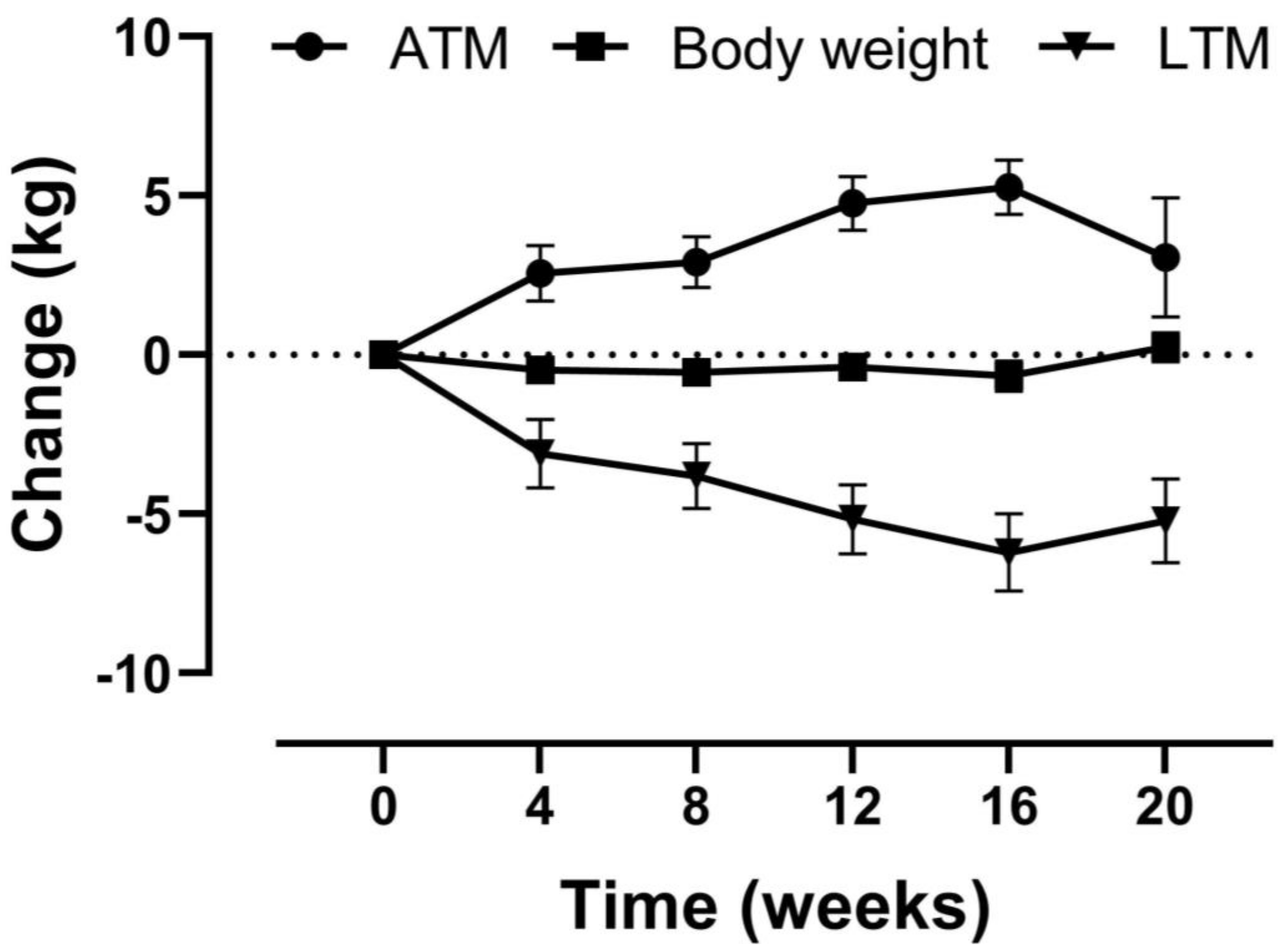
3.2. Lean Tissue Mass Is Replaced by Adipose Tissue Mass
3.3. Male Sex and Inflammation Accelerate Loss of Lean Tissue Mass
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinson, M.; Ikizler, T.A.; Morrell, G.; Wei, G.; Almeida, N.; Marcus, R.L.; Filipowicz, R.; Greene, T.H.; Beddhu, S. Associations of body size and body composition with functional ability and quality of life in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenvinkel, P.; Carrero, J.J.; Von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle wasting in end-stage renal disease promulgates premature death: Established, emerging and potential novel treatment strategies. Nephrol. Dial. Transplant. 2015, 31, 1070–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duenhas, M.R.; Draibe, S.A.; Avesani, C.M.; Sesso, R.; Cuppari, L. Influence of renal function on spontaneous dietary intake and on nutritional status of chronic renal insufficiency patients. Eur. J. Clin. Nutr. 2003, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossola, M.; Tazza, L.; Giungi, S.; Luciani, G. Anorexia in hemodialysis patients: An update. Kidney Int. 2006, 70, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Sherman, R.A.; Cody, R.P.; Rogers, M.E.; Solanchick, J.C. Interdialytic weight gain and nutritional parameters in chronic hemodialysis patients. Am. J. kidney Dis. 1995, 25, 579–583. [Google Scholar] [CrossRef]
- Ikizler, T.A. Effects of hemodialysis on protein metabolism. J. Ren. Nutr. 2005, 15, 39–43. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Pupim, L.B.; Brouillette, J.R.; Levenhagen, D.K.; Farmer, K.; Hakim, R.M.; Flakoll, P.J. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E107–E116. [Google Scholar] [CrossRef] [Green Version]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P. Inflammation in end-stage renal disease—A fire that burns within. In Cardiovascular Disorders in Hemodialysis; Karger Publishers: Basel, Switzerland, 2005; Volume 149, pp. 185–199. [Google Scholar]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Andrusev, A.M.; Aparicio-Madre, M.I.; Arribas Monzón, F.E.; Åsberg, A.; Barbullushi, M.; Beltrán, P.; et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: A summary. Clin. Kidney J. 2019, 12, 702–720. [Google Scholar] [CrossRef] [Green Version]
- Fouque, D. European Best Practice Guidelines (EBPG) Guideline on Nutrition. Nephrol. Dial. Transplant. 2007, 22, 2162–2166. [Google Scholar]
- Wabel, P.; Chamney, P.; Moissl, U.; Jirka, T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009, 27, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Mulasi, U.; Kuchnia, A.J.; Cole, A.J.; Earthman, C.P. Bioimpedance at the bedside: Current applications, limitations, and opportunities. Nutr. Clin. Pract. 2015, 30, 180–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moissl, U.; Wieskotten, S.; Chamney, P.; Wabel, P. Reference ranges for human body composition and fluid overload. J. Am. Soc. Nephrol. 2009, 20, 469A. [Google Scholar]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef] [Green Version]
- Urea kinetic modelling in chronic hemodialysis: Benefits, problems, and practical solutions. In Seminars in Dialysis; Jindal, K.K.; Goldstein, M.B. (Eds.) Wiley Online Library; Blackwell Publishing Ltd.: Oxford, UK, 1988. [Google Scholar]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Singer, J.D.; Willett, J.B.; Willett, J.B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Verbeke, G. Linear mixed models for longitudinal data. In Linear Mixed Models in Practice; Springer: Berlin/Heidelberg, Germany, 1997; pp. 63–153. [Google Scholar]
- Leal, V.O.; Mafra, D.; Fouque, D.; Anjos, L.A. Use of handgrip strength in the assessment of the muscle function of chronic kidney disease patients on dialysis: A systematic review. Nephrol. Dial. Transplant. 2010, 26, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Vogt, B.P.; Borges, M.C.C.; de Goés, C.R.; Caramori, J.C.T. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin. Nutr. 2016, 35, 1429–1433. [Google Scholar] [CrossRef] [Green Version]
- Molina, P.; Vizcaíno, B.; Molina, M.D.; Beltrán, S.; González-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Ávila, A.I.; Górriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol. Dial. Transplant. 2018, 33, 1223–1235. [Google Scholar] [CrossRef]
- Marcelli, D.; Brand, K.; Ponce, P.; Milkowski, A.; Marelli, C.; Ok, E.; Godino, J.I.; Gurevich, K.; Jirka, T.; Rosenberger, J.; et al. Longitudinal Changes in Body Composition in Patients After Initiation of Hemodialysis Therapy: Results From an International Cohort. J. Ren. Nutr. 2016, 26, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Keane, D.; Gardiner, C.; Lindley, E.; Lines, S.; Woodrow, G.; Wright, M. Changes in body composition in the two years after initiation of haemodialysis: A retrospective cohort study. Nutrients 2016, 8, 702. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Abraham, G.; Vijayan, M.; Thandavan, T.; Mathew, M.; Veerappan, I.; Revathy, L.; Alex, M.E. Body composition monitoring and nutrition in maintenance hemodialysis and CAPD patients—A multicenter longitudinal study. Ren. Fail. 2015, 37, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.C.; Di-Gioia, M.; Gallar, P.; Rodriguez, I.; Rodríguez, I.; Laso, N.; Callejas, R.; Ortega, O.; Herrero, J.C.; Herrero, J.C.; et al. Changes in body composition parameters in patients on haemodialysis and peritoneal dialysis. Nefrología 2012, 32, 108–113. [Google Scholar] [PubMed]
- Pupim, L.B.; Heimburger, O.; Qureshi, A.R.; Ikizler, T.; Stenvinkel, P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005, 68, 2368–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, K.L.; Kaysen, G.A.; Young, B.S.; Hung, A.M.; da Silva, M.; Chertow, G.M. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am. J. Clin. Nutr. 2003, 77, 842–846. [Google Scholar] [CrossRef]
- Vendrely, B.; Chauveau, P.; Barthe, N.; El Haggan, W.; Castaing, F.; De Précigout, V.; Combe, C.; Aparicio, M. Nutrition in hemodialysis patients previously on a supplemented very low protein diet. Kidney Int. 2003, 63, 1491–1498. [Google Scholar] [CrossRef] [Green Version]
- Ishimura, E.; Okuno, S.; Kim, M.; Yamamoto, T.; Izumotani, T.; Otoshi, T.; Shoji, T.; Inaba, M.; Nishizawa, Y. Increasing body fat mass in the first year of hemodialysis. J. Am. Soc. Nephrol. 2001, 12, 1921–1926. [Google Scholar]
- Carrero, J.J.; Chmielewski, M.; Axelsson, J.; Snaedal, S.; Heimburger, O.; Barany, P.; Suliman, M.E.; Lindholm, B.; Stenvinkel, P.; Qureshi, A.R. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin. Nutr. 2008, 27, 557–564. [Google Scholar] [CrossRef]
- Avesani, C.M.; Carrero, J.J.; Axelsson, J.; Qureshi, A.R.; Lindholm, B.; Stenvinkel, P. Inflammation and wasting in chronic kidney disease: Partners in crime. Kidney Int. 2006, 70, S8–S13. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P.; Gillespie, I.A.; Tunks, J.; Addison, J.; Kronenberg, F.; Drueke, T.B.; Marcelli, D.; Schernthaner, G.; Eckardt, K.U.; Floege, J.; et al. Inflammation Modifies the Paradoxical Association between Body Mass Index and Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2016, 27, 1479–1486. [Google Scholar] [CrossRef]
- Wang, Y.W.; Lin, T.Y.; Peng, C.H.; Huang, J.L.; Hung, S.C. Factors Associated with Decreased Lean Tissue Index in Patients with Chronic Kidney Disease. Nutrients 2017, 9, 434. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Greene, T.; Daugirdas, J.T.; Kimmel, P.L.; Schulman, G.W.; Toto, R.D.; Levin, N.W.; Yan, G.; HEMO Study Group. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am. J. Kidney Dis. 2003, 42, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- van de Logt, A.E.; Rijpma, S.R.; Vink, C.H.; Prudon-Rosmulder, E.; Wetzels, J.F.; van Berkel, M. Albumin assays and clinical decision-making in nephrotic syndrome patients reply. Kidney Int. 2019, 96, 249. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Hirayama, S.; Ito, M.; Nishioka, E.; Fukushima, Y.; Satoh, T.; Idei, M.; Horiuchi, Y.; Shoji, H.; Ohmura, H.; et al. Albumin concentration determined by the modified bromocresol purple method is superior to that by the bromocresol green method for assessing nutritional status in malnourished patients with inflammation. Ann. Clin. Biochem. 2013, 50, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrero, J.J. Gender differences in chronic kidney disease: Underpinnings and therapeutic implications. Kidney Blood Press. Res. 2010, 33, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Barany, P.; Chung, S.H.; Lindholm, B.; Heimburger, O. A comparative analysis of nutritional parameters as predictors of outcome in male and female ESRD patients. Nephrol. Dial. Transplant. 2002, 17, 1266–1274. [Google Scholar] [CrossRef]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019, 10, 43. [Google Scholar] [CrossRef]
- Rymarz, A.; Matyjek, A.; Gomolka, M.; Niemczyk, S. Lean Tissue Index and Body Cell Mass Can Be Predictors of Low Free Testosterone Levels in Men on Hemodialysis. J. Ren. Nutr. 2019, 29, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Chertow, G.M.; Johansen, K.L.; Lew, N.; Lazarus, J.M.; Lowrie, E.G. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000, 57, 1176–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beddhu, S.; Bruns, F.J.; Saul, M.; Seddon, P.; Zeidel, M.L. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am. J. Med. 2000, 108, 609–613. [Google Scholar] [CrossRef]
- Chae, J.W.; Song, C.S.; Kim, H.; Lee, K.B.; Seo, B.S.; Kim, D.I. Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson Comorbidity Index using ICD-10 database. Nephron Clin. Pract. 2011, 117, c379–c384. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.T.; Kiberd, B.A.; Royston, J.P.; Alfaadhel, T.; Soroka, S.D.; Hemmelgarn, B.R.; Tennankore, K.K. Comorbidity burden at dialysis initiation and mortality: A cohort study. Can. J. Kidney Health Dis. 2015, 2, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrero, J.J.; Johansen, K.L.; Lindholm, B.; Stenvinkel, P.; Cuppari, L.; Avesani, C.M. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016, 90, 53–66. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Ammann, T.; Lai, S.; Grosso, A.; Lionetto, L.; Spagnoli, A.; Simmaco, M.; Monti, M.; Laviano, A.; et al. Longitudinal physical activity change during hemodialysis and its association with body composition and plasma BAIBA levels. Front. Physiol. 2019, 10, 805. [Google Scholar] [CrossRef] [Green Version]
- Pupim, L.B.; Flakoll, P.J.; Brouillette, J.R.; Levenhagen, D.K.; Hakim, R.M.; Ikizler, T.A. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J. Clin. Investig. 2002, 110, 483–492. [Google Scholar] [CrossRef]
| General Characteristics | Age (Years) | 66 (52, 74) |
| Males, n (%) | 38 (71) | |
| Comorbidities | Charlson Comorbidity Index | 9.0 ± 3.4 |
| Diabetes, n (%) | 20 (37) | |
| Cardiovascular disease, n (%) | 38 (71) | |
| Dialysis characteristics | Dialysis vintage (months) | 19 (7, 40) |
| spKt/V | 1.49 ± 0.24 | |
| Laboratory values | Albumin, g/L | 38 (36.6, 39.2) |
| C-reactive protein, mg/L | 11 (7.6, 14.3) | |
| Body composition | Body weight, kg | 76.7 (71.9, 81.6) |
| Body mass index for dry weight, kg/m2 | 25.6 (22.3, 28.5) | |
| Predialysis overhydration (mL) | 1278 (835, 1712) | |
| Total body water (L) | 34.5 (29.1, 40.5) | |
| Extracellular water | 16.6 (14.5, 19.3) | |
| Intracellular water | 17.5 (14.1, 21.3) | |
| Body cell mass (kg) | 18.3 (12.6, 23.7) | |
| Lean tissue mass, kg | 37.7 (34.7, 40.6) | |
| Lean tissue index < P10, n (%) | 26 (48) | |
| Adipose tissue mass, kg | 37.6 (32.6, 42.5) | |
| Fat tissue index > P90, n (%) | 14 (26) | |
| Phase angle,° | 4.35 ± 1.1 | |
| Muscle strength | Handgrip strength, kg | 23 (19.8, 25.2) |
| Handgrip strength < P10, n (%) | 33 (61) | |
| Protein intake | Normalized protein catabolic rate, g/kg | 0.9 ± 0.2 |
| Subjective Global Assessment | Severely malnourished (1–2), n (%) | 1 (2) |
| Moderately malnourished (3–5), n (%) | 40 (74) | |
| Well nourished (6–7), n (%) | 13 (24) |
| Baseline | 20 Weeks | Difference in Time | |||
|---|---|---|---|---|---|
| % | p−Value | ||||
| Lean tissue mass, kg | 37.7 (34.7, 40.6) | 31.3 (28.2, 34.3) | −6.4 (−8.1, −4.8) | −17.1 | <0.001 |
| Body weight, kg | 76.7 (71.9, 81.6) | 76.2 (71.4, 81.1) | −0.5 (−1.0, 0.1) | −0.6 | 0.09 |
| Adipose tissue mass, kg | 37.6 (32.6, 42.5) | 42.1 (37.1, 47.07) | 4.5 (2.7, 6.2) | 11.9 | <0.001 |
| Handgrip strength, kg | 22.5 (19.8, 25.2) | 20.6 (17.8, 23.3) | −1.9 (−3.1, −0.7) | −8.6 | 0.002 |
| Pre−dialysis OH, mL | 1278 (835, 1712) | 1509 (1031, 1973) | 236 (−205, 678) | 18.5 | 0.292 |
| Total body water, L | 34.5 (29.1, 40.5) | 31.7 (28.9, 33.9) | −2.79 (−3.5, −2.0) | −8.1 | <0.001 |
| Extracellular water, L | 16.6 (14.5, 19.3) | 15.9 (14.7, 17.0) | −0.69 (−1.4, −0.5) | −4.3 | <0.001 |
| Intracellular water, L | 17.5 (14.1, 21.3) | 15.4 (13.8, 16.8) | −2.11 (−2.9, −1.4) | −12 | <0.001 |
| Body cell mass, kg | 18.3 (12.6, 23.7) | 14.0 (12.4, 17.4) | −4.30 (−5.9, −2.9) | −23.5 | <0.001 |
| Phase angle, ° | 4.35 (4.04, 4.66) | 4.21 (3.89, 4.53) | −0.14 (−0.28, −0.01) | −3.2 | 0.07 |
| Serum albumin, g/L | 38.0 (36.6, 39.2) | 37.2 (35.7, 38.4) | −0.8 (−2.0, 0.3) | −2.2 | 0.1 |
| Serum CRP, mg/L | 11 (7.6, 14.3) | 9.7 (6.0, 13.21) | −1.3 (−5.5, 2.8) | −12.3 | 0.5 |
| nPCR, g/kg | 0.9 (0.77, 1.03) | 1.0 (0.82, 1.09) | +0.1 (−0.05, 0.16) | 11.1 | 0.4 |
| Baseline | 20 Weeks | Difference | |||||
|---|---|---|---|---|---|---|---|
| kg | p-Value | kg | kg | % | p-Value | ||
| Age | <65 years | 41.9 | 36.1 | −5.8 | −13.7 | <0.001 | |
| ≥65 years | 33.7 | 26.8 | −6.9 | −20.5 | <0.001 | ||
| difference | 8.2 | 0.004 | 9.3 | 1.1 | 6.8 | 0.5 | |
| Sex | male | 42.3 | 34.9 | −7.4 | −17.5 | <0.001 | |
| female | 26.8 | 22.6 | −4.2 | −15.7 | <0.001 | ||
| difference | 15.5 | <0.001 | 12.3 | 3.2 | −1.8 | 0.07 | |
| LTI (percentile) | <P10 | 37.0 | 31.2 | −5.8 | −15.6 | <0.001 | |
| ≥P10 | 39.3 | 32.1 | −7.2 | −18.4 | <0.001 | ||
| difference | −2.3 | <0.001 | −0.9 | −1.4 | −2.8 | <0.001 | |
| C-reactive protein | <median | 37.1 | 31.6 | −5.5 | −14.7 | <0.001 | |
| ≥median | 40.0 | 29.7 | −10.3 | −25.6 | <0.001 | ||
| difference | 2.9 | 0.03 | −1.9 | −4.8 | −10.9 | 0.005 | |
| Serum albumin | <median | 38.9 | 31.5 | −7.4 | −18.9 | <0.001 | |
| ≥median | 37.1 | 30.9 | −6.2 | −16.8 | < 0.001 | ||
| difference | 1.8 | 0.1 | 0.6 | −1.2 | −2.1 | 0.5 | |
| Dialysis vintage | <12 months | 41.1 | 32.8 | −8.3 | −20.2 | <0.001 | |
| ≥12 months | 35.6 | 30.0 | −5.6 | −15.8 | <0.001 | ||
| difference | 5.5 | 0.07 | 2.8 | −2.7 | −4.4 | 0.1 | |
| Diabetes mellitus | no diabetes | 39.7 | 34.1 | −5.6 | −14.1 | <0.001 | |
| diabetes | 34.2 | 26.3 | −7.9 | −23.3 | <0.001 | ||
| difference | 5.5 | 0.07 | 7.8 | −2.3 | −9.2 | 0.2 | |
| Charlson Comorbidity Index | <mean | 42.6 | 36.2 | −6.4 | −15.1 | <0.001 | |
| ≥mean | 32.8 | 26.3 | −6.5 | −19.6 | <0.001 | ||
| difference | 9.8 | <0.001 | 9.9 | 0.1 | 4.5 | 0.9 | |
| Effect 1 | Time × Effect 2 | |||
|---|---|---|---|---|
| Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value | |
| Intercept | 46.7 (43.4, 49.9) | <0.001 | −5.2 (−9.1, −1.4) | 0.007 |
| Age ≥ 65 years | −8.4 (−11.8, −4.9) | <0.001 | 0.595 (−3.2, 4.3) | 0.8 |
| Male gender | 12.4 (11.6, 13.3) | <0.001 | −5.2 (−6.6, −3.8) | 0.02 |
| LTI ≥ P10 | 2.6 (2.1, 3.1) | <0.001 | −1.6 (−2.1. −1.0) | <0.001 |
| Serum CRP ≥ median | 0.08 (0.004, 0.1) | 0.04 | −0.1 (−0.2, 0.001) | 0.03 |
| Serum albumin < median | −0.08 (−2.3, 2.1) | 0.9 | 0.1 (−3.3, 3.6) | 0.9 |
| Dialysis vintage ≥ 12 M | −2.6 (−5.8, 0.07) | 0.1 | 1.5 (−2.3, 5.2) | 0.5 |
| Diabetes mellitus | −1.9 (−5.1, 1.3) | 0.3 | −1.5 (−4.9, 1.9) | 0.4 |
| CCI ≥ mean | −0.141 (−3.7, 3.4) | 0.9 | −3.5 (−7.6, 0.6) | 0.09 |
| 1st Auth. | Visser | Molina | Marcelli | Keane | Mathew | Di Goia | Pupim | Johansen | Vendrely | Ishimura |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2020 | 2018 | 2016 | 2016 | 2014 | 2012 | 2005 | 2003 | 2003 | 2001 |
| Ref. | - | [23] | [24] | [25] | [26] | [27] | [28] | [29] | [30] | [31] |
| Number | 54 | 32 | 8227 | 299 | 99 | 84 | 142 | 54 | 30 | 72 |
| Design | P | P | R | R | P | P | P | P | P | R |
| Mod. | HD | HD | HD | HD | HD + PD | HD + PD | HD | HD | HD | HD |
| Vintage | Any | Any | Start | Start | Start | Any | Start | Any | Start | Start |
| Age | 66 | 60 | 61 | 63 | 55 | 57 | 53 | 51 | 58 | 62 |
| %Male | 71 | 58 | 64 | 62 | 78 | 34 | 63 | 67 | 66 | 59 |
| %DM | 37 | 18 | 32 | 42 | 39 | - | 31 | - | 10 | 50 |
| %CVD | 70 | 21 | 52 | - | - | - | 33 | - | - | - |
| F-U | 20 w | 1 y | 2 y | 2 y | 2 y | 6 m | 1 y | 1 y | 1 y | 1 y |
| Method | BIS | BIS | BIS | BIS | BIS | BIS | DEXA | DEXA | DEXA | DEXA |
| LTM ** | −6.4 | −0.5 vs. −6.8 | −1.2 | −0.9 | −1.8 | −0.2 | −3.4 vs. −1.1 | +1.1 | 0 | −0.7 |
| ATM | +4.5 | −0.1 + 9.8 | +2.6 | +0.7 | +0.6 | +0.3 | +1.6 | −0.4 | +2.4 | +1.3 |
| CRP | 11 | 3.3 | 6 | - | - | 5.2 | <10 | 20 | - | - |
| Albumin | 38 | 39 | 38 | - | 36 | 37.6 | 32.5 | 39 | 38.5 | 39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visser, W.J.; de Mik-van Egmond, A.M.E.; Timman, R.; Severs, D.; Hoorn, E.J. Risk Factors for Muscle Loss in Hemodialysis Patients with High Comorbidity. Nutrients 2020, 12, 2494. https://doi.org/10.3390/nu12092494
Visser WJ, de Mik-van Egmond AME, Timman R, Severs D, Hoorn EJ. Risk Factors for Muscle Loss in Hemodialysis Patients with High Comorbidity. Nutrients. 2020; 12(9):2494. https://doi.org/10.3390/nu12092494
Chicago/Turabian StyleVisser, Wesley J., Anneke M.E. de Mik-van Egmond, Reinier Timman, David Severs, and Ewout J. Hoorn. 2020. "Risk Factors for Muscle Loss in Hemodialysis Patients with High Comorbidity" Nutrients 12, no. 9: 2494. https://doi.org/10.3390/nu12092494





