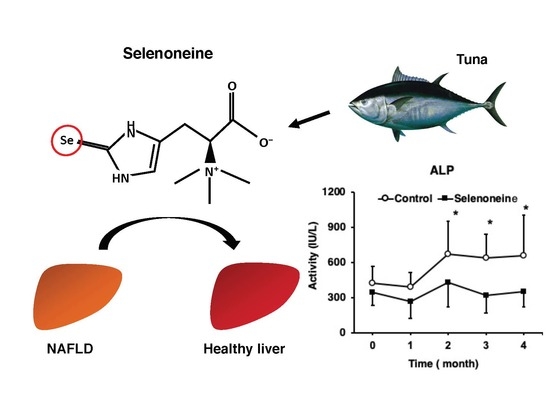
Selenoneine Ameliorates Hepatocellular Injury and Hepatic Steatosis in a Mouse Model of NAFLD
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Treatment, Sample Collection, and Histological Analysis
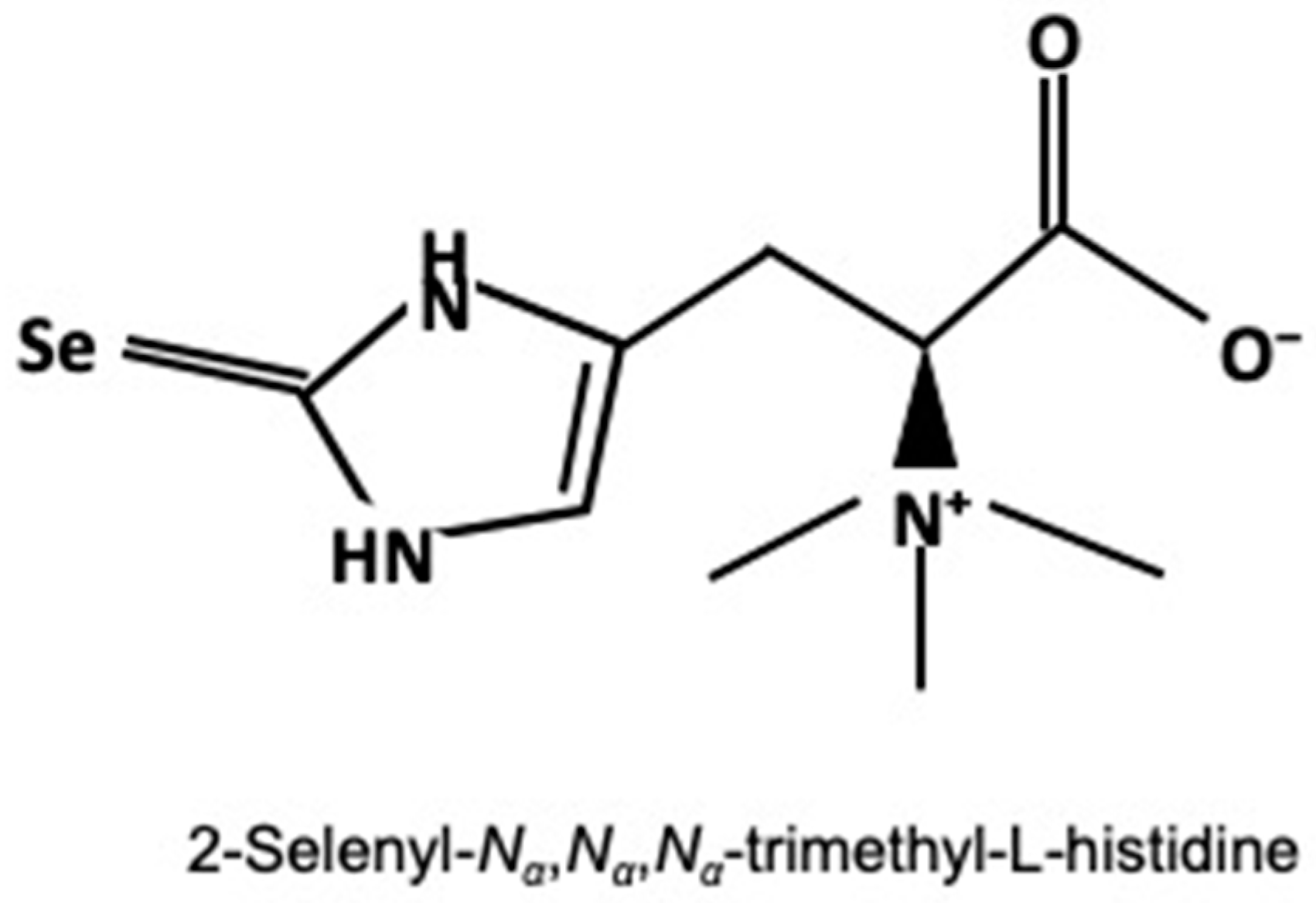
2.3. Determination of Selenoneine and Selenium Concentrations
2.4. Determination of Hepatic Damage-Associated Diagnostic Markers and Hepatic Lipid Levels
2.5. Determination of mRNA Levels
2.6. Statistical Analysis
3. Results
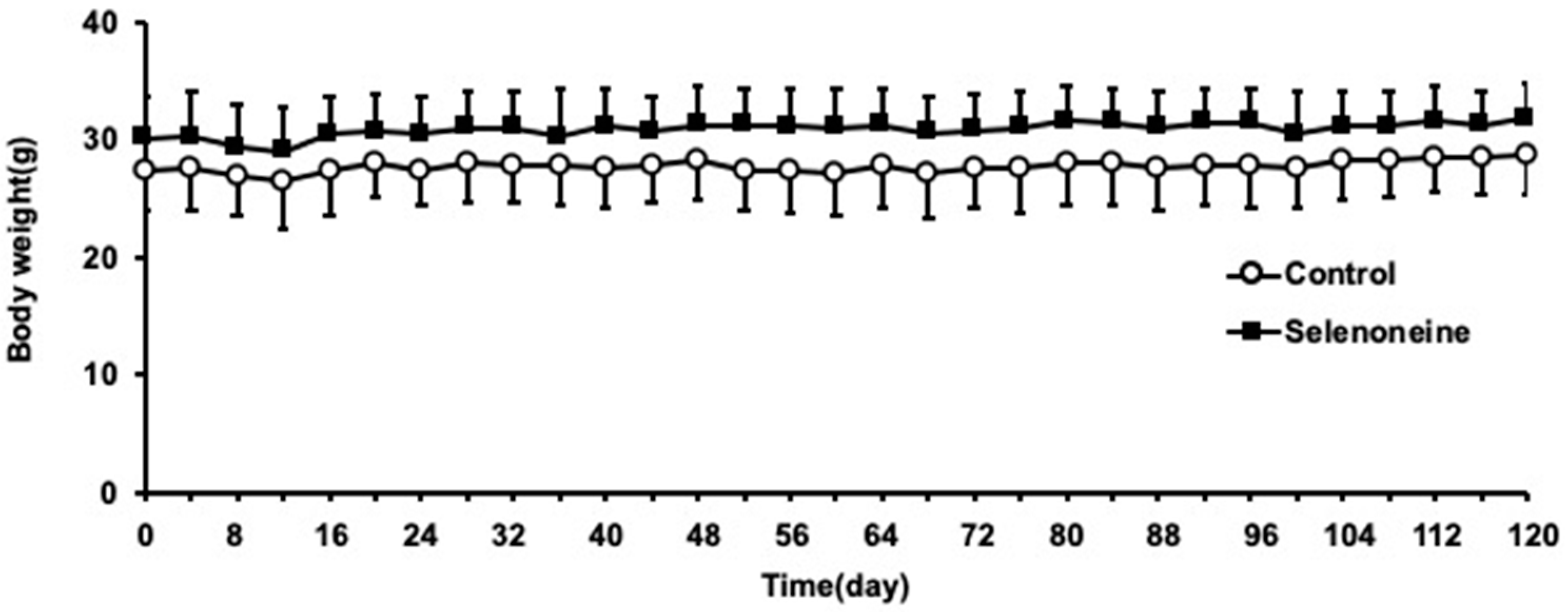
3.1. Body and Liver Weights
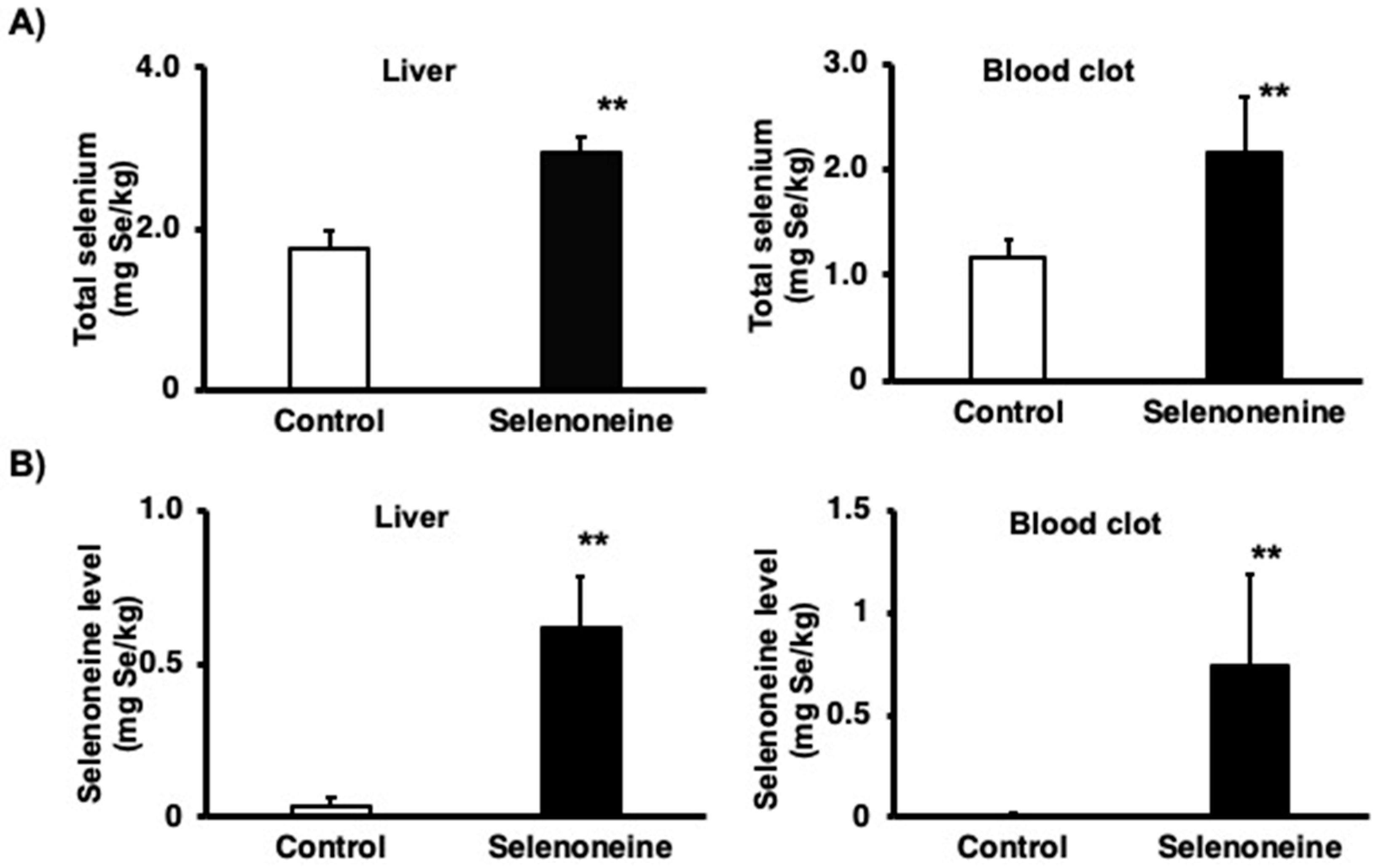
3.2. Total Selenium and Selenoneine Levels
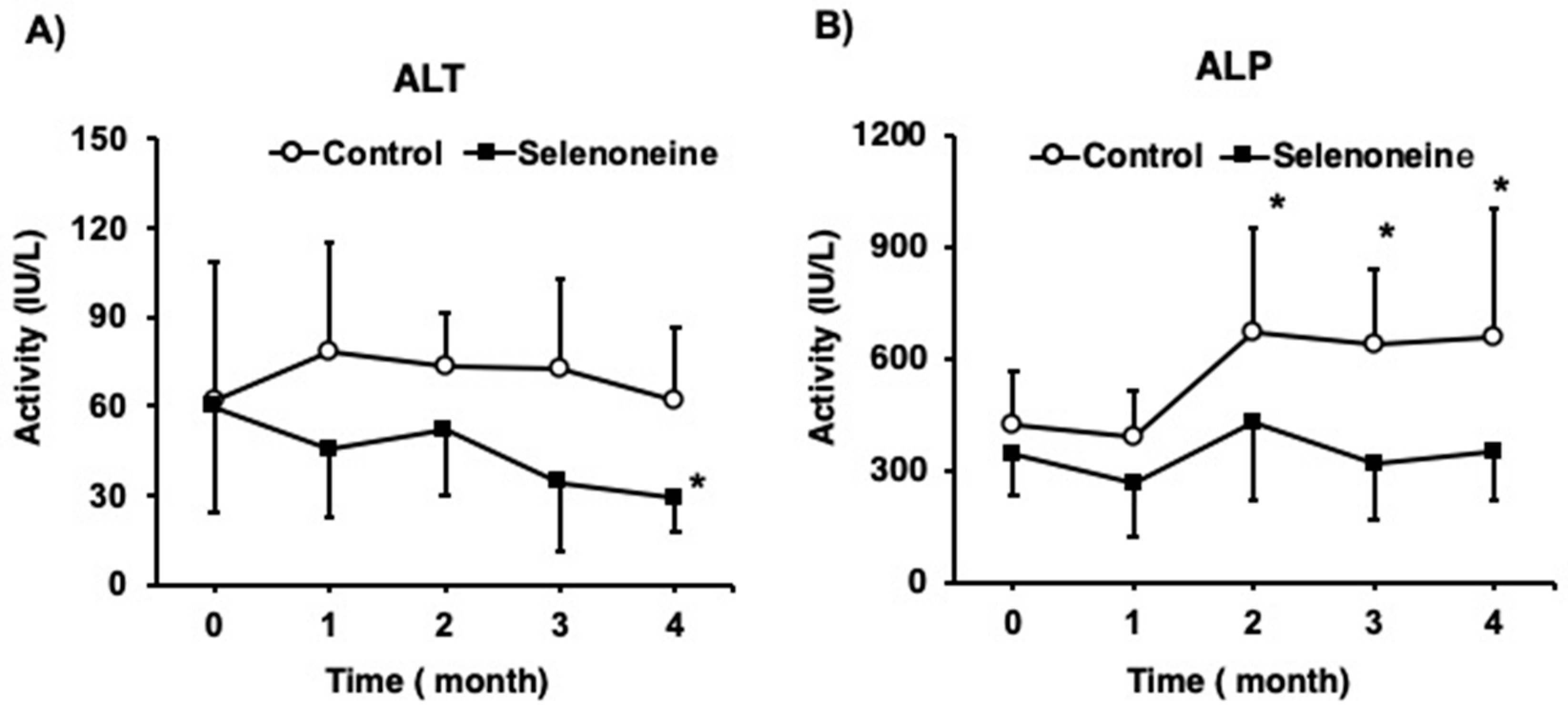
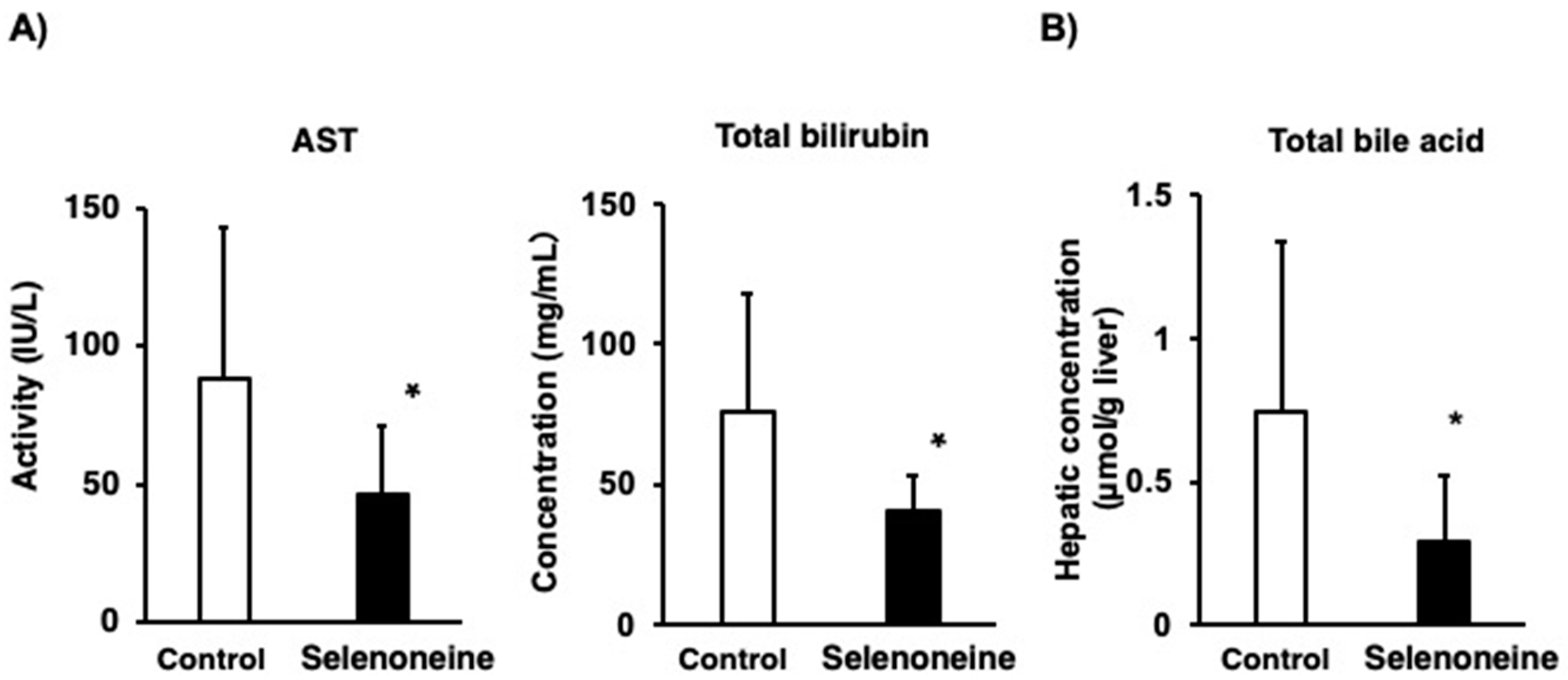
3.3. Hepatic Damage-Associated Diagnostic Marker
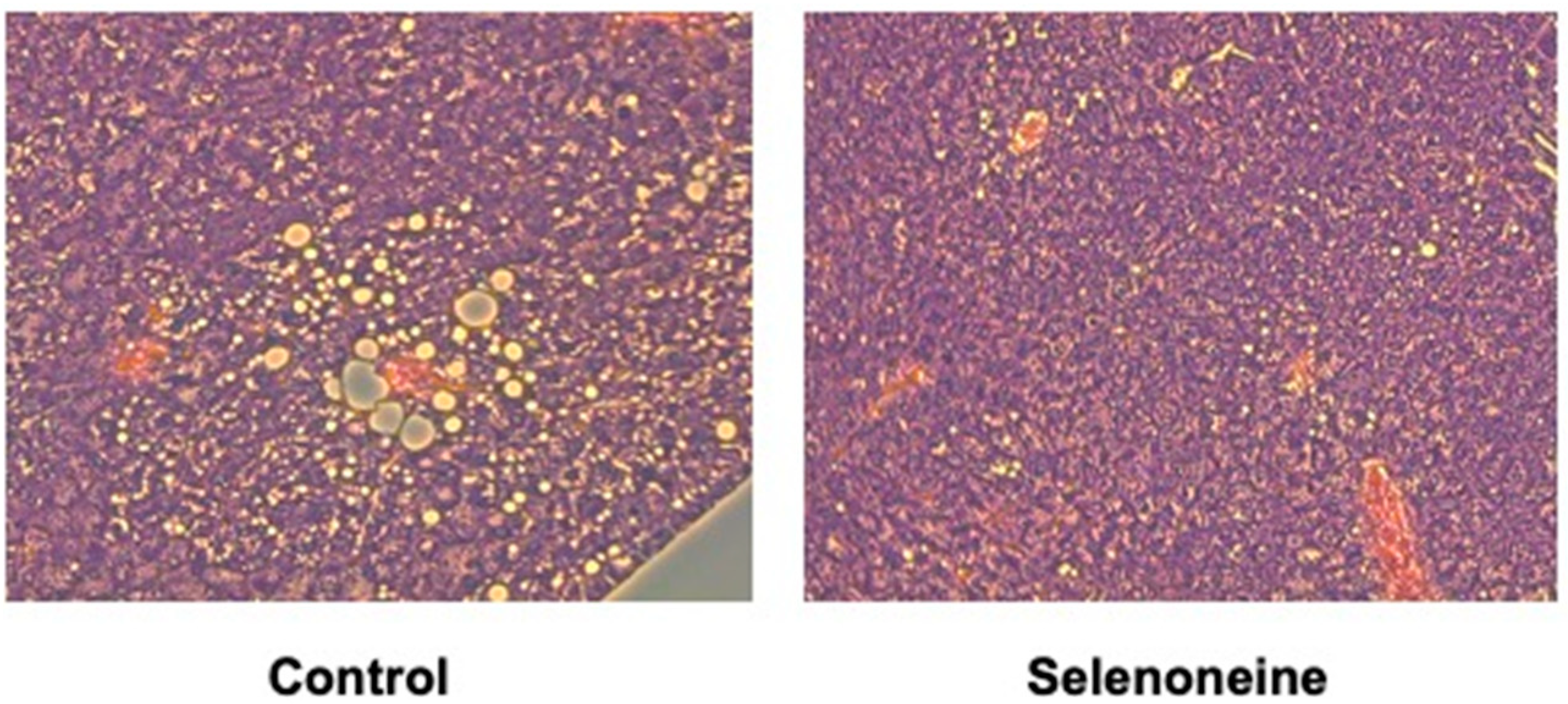
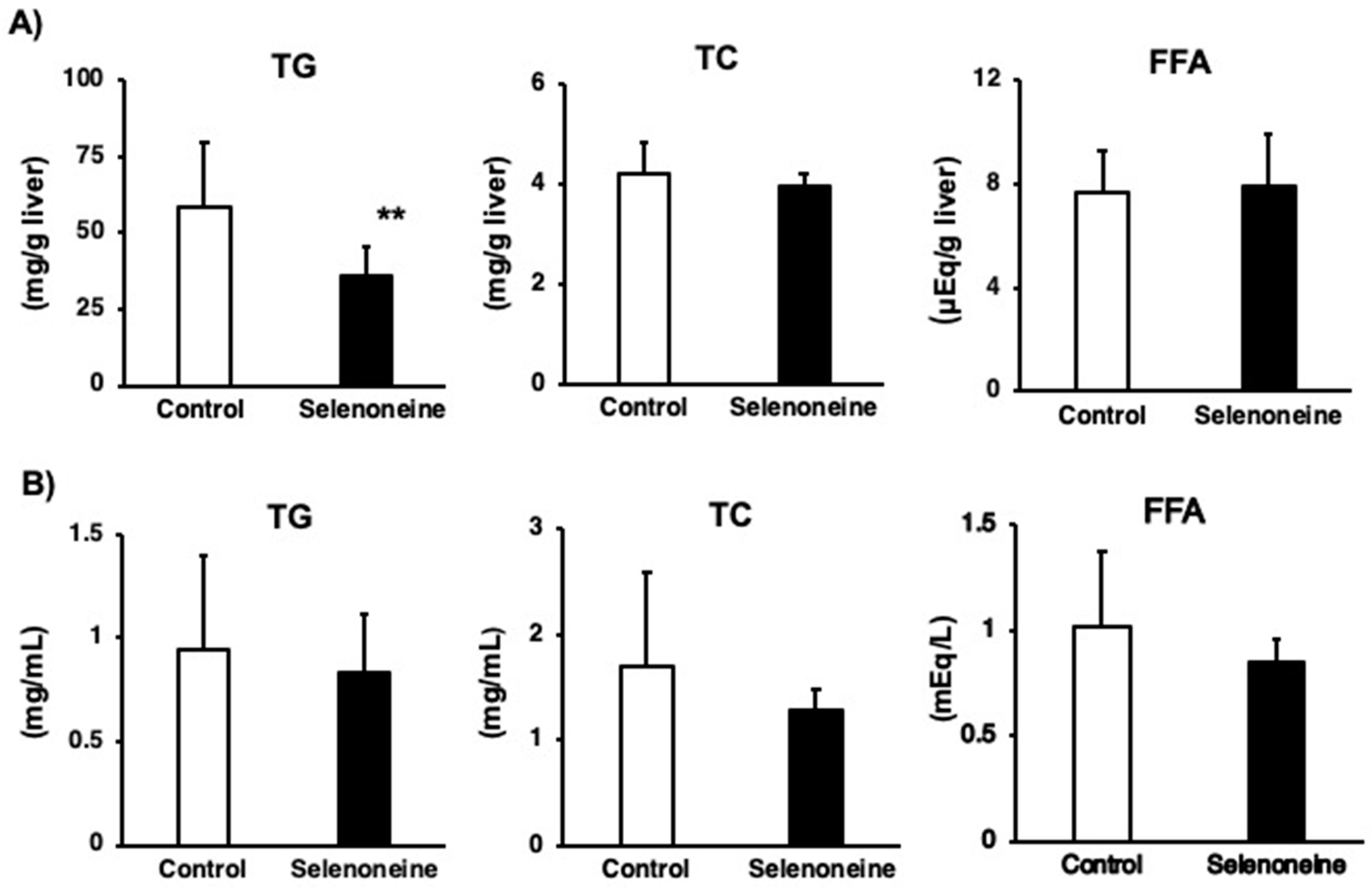
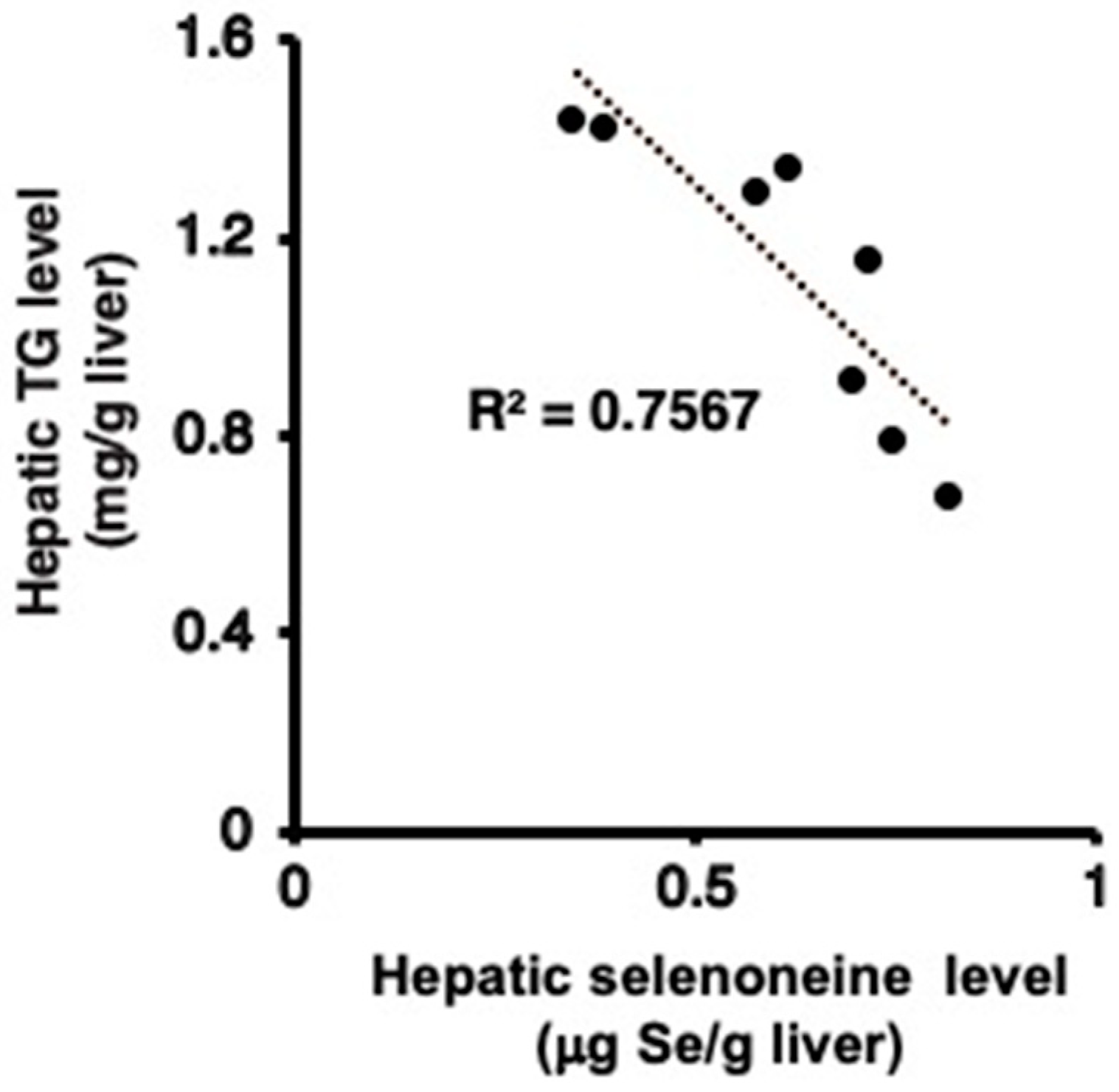
3.4. Hepatic and Serum Lipid Levels
3.5. Hepatic Gene Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Schomburg, L. Dietary Selenium and Human Health. Nutrients 2016, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.A. Selenium metabolism and bioavailability. Biol. Trace Elem. Res. 1996, 54, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 506. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.F.; Rea, H.M.; Friend, G.M.; Stewart, R.D.; Snow, P.C.; Thomson, C.D. On supplementing the selenium intake of New Zealanders. 2. Prolonged metabolic experiments with daily supplements of selenomethionine, selenite and fish. Br. J. Nutr. 1978, 39, 589–600. [Google Scholar] [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M. Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Cumming, B.M.; Chinta, K.C.; Reddy, V.P.; Steyn, A.J.C. Role of Ergothioneine in Microbial Physiology and Pathogenesis. Antioxid. Redox Signal. 2018, 28, 431–444. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yabu, T.; Yamashita, M. Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J. Biol. Chem. 2010, 1, 144–150. [Google Scholar] [CrossRef]
- Yamashita, Y.; Amlund, H.; Suzuki, T.; Hara, T.; Hossain, A.M.; Yabu, T.; Touhata, K.; Yamashita, M. Selenoneine, total selenium and total mercury content in the muscle of fishes. Fish. Sci. 2011, 77, 679–686. [Google Scholar] [CrossRef]
- Achouba, A.; Dumas, P.; Ouellet, N.; Little, M.; Lemire, M.; Ayotte, P. Selenoneine is a major selenium species in beluga skin and red blood cells of Inuit from Nunavik. Chemosphere 2019, 229, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Rohn, I.; Kroepfl, N.; Aschner, M.; Bornhorst, J.; Kuehnelt, D.; Schwerdtle, T. Selenoneine ameliorates peroxide-induced oxidative stress in C. elegans. J. Trace Elem. Med. Biol. 2019, 55, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamashita, Y.; Suzuki, T.; Kani, Y.; Mizusawa, N.; Imamura, S.; Takemoto, K.; Hara, T.; Hossain, M.A.; Yabu, T.; et al. Selenoneine, a novel selenium-containing compound, mediates detoxification mechanisms against methylmercury accumulation and toxicity in zebrafish embryo. Mar. Biotechnol. (NY). 2013, 15, 559–570. [Google Scholar] [CrossRef]
- Masuda, J.; Umemura, C.; Yokozawa, M.; Yamauchi, K.; Seko, T.; Yamashita, M.; Yamashita, Y. Dietary Supplementation of Selenoneine-Containing Tuna Dark Muscle Extract Effectively Reduces Pathology of Experimental Colorectal Cancers in Mice. Nutrients 2018, 10, 1380. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Matsubara, T.; Li, F.; Gonzalez, F.J. FXR signaling in the enterohepatic system. Mol. Cell. Endocrinol. 2013, 368, 17–29. [Google Scholar] [CrossRef]
- Sinal, C.J.; Tohkin, M.; Miyata, M.; Ward, J.M.; Lambert, G.; Gonzalez, F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cellular 2000, 102, 731–744. [Google Scholar] [CrossRef]
- Ma, K.; Saha, P.K.; Chan, L.; Moore, D.D. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Investig. 2006, 116, 1102–1109. [Google Scholar] [CrossRef]
- Liu, N.; Meng, Z.; Lou, G.; Zhou, W.; Wang, X.; Zhang, Y.; Zhang, L.; Liu, X.; Yen, Y.; Lai, L.; et al. Hepatocarcinogenesis in FXR-/- mice mimics human HCC progression that operates through HNF1alpha regulation of FXR expression. Mol. Endocrinol. 2012, 26, 775–785. [Google Scholar] [CrossRef]
- Bjursell, M.; Wedin, M.; Admyre, T.; Hermansson, M.; Bottcher, G.; Goransson, M.; Linden, D.; Bamberg, K.; Oscarsson, J.; Bohlooly, Y.M. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS ONE 2013, 8, e64721. [Google Scholar] [CrossRef] [PubMed]
- Kitada, H.; Miyata, M.; Nakamura, T.; Tozawa, A.; Honma, W.; Shimada, M.; Nagata, K.; Sinal, C.J.; Guo, G.L.; Gonzalez, F.J.; et al. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J. Biol. Chem. 2003, 278, 17838–17844. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, M.; Miyata, M.; Yin, S.; Kurata, Y.; Shimada, M.; Yoshinari, K.; Gonzalez, F.J.; Suzuki, K.; Shibasaki, S.; Kurosawa, T.; et al. Bile acid-induced elevated oxidative stress in the absence of farnesoid X receptor. Biol. Pharm. Bull. 2009, 32, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef]
- Miyata, M.; Kinoshita, Y.; Shinno, K.; Sugiura, Y.; Harada, K. Hepatic n-3/n-6 polyunsaturated fatty acid shift improves hepatic steatosis in farnesoid X receptor-null mice. Fish. Sci. 2016, 82, 529–536. [Google Scholar] [CrossRef]
- Miyata, M.; Funaki, A.; Fukuhara, C.; Sumiya, Y.; Sugiura, Y. Taurine attenuates hepatic steatosis in a genetic model of fatty liver disease. J. Toxicol. Sci. 2020, 45, 87–94. [Google Scholar] [CrossRef]
- Miyata, M.; Shinno, K.; Kinoshita, T.; Kinoshita, Y.; Sugiura, Y. Fish oil feeding reverses hepatomegaly and disrupted hepatic function due to the lack of FXR signaling. J. Toxicol. Sci. 2017, 42, 671–681. [Google Scholar] [CrossRef]
- Miyata, M.; Nomoto, M.; Sotodate, F.; Mizuki, T.; Hori, W.; Nagayasu, M.; Yokokawa, S.; Ninomiya, S.; Yamazoe, Y. Possible protective role of pregnenolone-16 alpha-carbonitrile in lithocholic acid-induced hepatotoxicity through enhanced hepatic lipogenesis. Eur. J. Pharmacol. 2010, 636, 145–154. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, K.; Lei, X.G. Selenium and diabetes—Evidence from animal studies. Free Radic Biol. Med. 2013, 65, 1548–1556. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Liu, G.; Niu, Y.; Zhang, W.; Lu, S.; Li, X.; Zhang, H.; Ning, G.; Fan, J.; et al. Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: A cross-sectional analysis. Sci. Rep. 2016, 6, 37288. [Google Scholar] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Little, M.; Achouba, A.; Dumas, P.; Ouellet, N.; Ayotte, P.; Lemire, M. Determinants of selenoneine concentration in red blood cells of Inuit from Nunavik (Northern Quebec, Canada). Environ. Int. 2019, 127, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamashita, Y.; Ando, T.; Wakamiya, J.; Akiba, S. Identification and determination of selenoneine, 2-selenyl-N alpha, N alpha, N alpha -trimethyl-l-histidine, as the major organic selenium in blood cells in a fish-eating population on remote Japanese Islands. Biol. Trace Elem. Res. 2013, 156, 36–44. [Google Scholar] [CrossRef]
- Gonzalez-Reimers, E.; Monedero-Prieto, M.J.; Gonzalez-Perez, J.M.; Duran-Castellon, M.C.; Galindo-Martin, L.; Abreu-Gonzalez, P.; Sanchez-Perez, M.J.; Santolaria-Fernandez, F. Relative and combined effects of selenium, protein deficiency and ethanol on hepatocyte ballooning and liver steatosis. Biol. Trace Elem. Res. 2013, 154, 281–287. [Google Scholar]
- Zhang, Q.; Qian, Z.Y.; Zhou, P.H.; Zhou, X.L.; Zhang, D.L.; He, N.; Zhang, J.; Liu, Y.H.; Gu, Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef]
- Hu, X.F.; Sharin, T.; Chan, H.M. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J. Trace Elem. Med. Biol. 2017, 44, 322–330. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Yang, J.; Fernandez-Galilea, M.; Martinez-Fernandez, L.; Gonzalez-Muniesa, P.; Perez-Chavez, A.; Martinez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Pinto, A.; Sies, H. High selenium intake and increased diabetes risk: Experimental evidence for interplay between selenium and carbohydrate metabolism. J. Clin. Biochem. Nutr. 2011, 48, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Mita, Y.; Nakayama, K.; Inari, S.; Nishito, Y.; Yoshioka, Y.; Sakai, N.; Sotani, K.; Nagamura, T.; Kuzuhara, Y.; Inagaki, K.; et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat. Commun. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Matsushita, Y.; Poudel-Tandukar, K.; Kato, M.; Oba, S.; Inoue, M.; Tsugane, S.; et al. Fish intake and type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2011, 94, 884–891. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control | Selenoneine |
|---|---|---|
| Body weight (g) | 28.7 ± 3.2 | 31.8 ± 3.0 |
| Liver weight (g) | 2.15 ± 0.51 | 1.98 ± 0.29 |
| Liver/body weight ratio (%) | 7.49 ± 1.98 | 5.19 ± 0.59 * |
| Gene Symbol | Control | Selenoneine |
|---|---|---|
| Tnf α | 1.00 ± 0.41 | 0.74 ± 0.66 |
| IL-1 β | 1.00 ± 0.65 | 0.61 ± 0.50 |
| IL-6 | 1.00 ± 1.08 | 0.62 ± 0.55 |
| Hmox1 | 1.00 ± 1.04 | 0.19 ± 0.23 * |
| Gsta1 | 1.00 ± 1.06 | 0.02 ± 0.03 * |
| Gsta2 | 1.00 ± 0.87 | 0.08 ± 0.08 * |
| Gene Symbol | Control | Selenoneine |
|---|---|---|
| Acc1 | 1.00 ± 0.60 | 0.53 ± 0.42 * |
| Scd1 | 1.00 ± 0.55 | 0.24 ± 0.22 ** |
| Fasn | 1.00 ± 0.86 | 0.34 ± 0.26 * |
| Ppar α | 1.00 ± 0.77 | 0.79 ± 0.54 |
| Srebp1c | 1.00 ± 0.67 | 0.47 ± 0.54 |
| Bsep | 1.00 ± 0.71 | 0.47 ± 0.34 |
| Cyp7a1 | 1.00 ± 0.60 | 1.77 ± 1.47 |
| Gpx1 | 1.00 ± 0.86 | 0.34 ± 0.35 * |
| Gpx2 | 1.00 ± 1.97 | 1.01 ± 1.65 |
| Gpx4 | 1.00 ± 0.66 | 0.46 ± 0.50 |
| Txnrd1 | 1.00 ± 0.78 | 0.49 ± 0.49 |
| Selenop | 1.00 ± 0.86 | 0.38 ± 0.34 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyata, M.; Matsushita, K.; Shindo, R.; Shimokawa, Y.; Sugiura, Y.; Yamashita, M. Selenoneine Ameliorates Hepatocellular Injury and Hepatic Steatosis in a Mouse Model of NAFLD. Nutrients 2020, 12, 1898. https://doi.org/10.3390/nu12061898
Miyata M, Matsushita K, Shindo R, Shimokawa Y, Sugiura Y, Yamashita M. Selenoneine Ameliorates Hepatocellular Injury and Hepatic Steatosis in a Mouse Model of NAFLD. Nutrients. 2020; 12(6):1898. https://doi.org/10.3390/nu12061898
Chicago/Turabian StyleMiyata, Masaaki, Koki Matsushita, Ryunosuke Shindo, Yutaro Shimokawa, Yoshimasa Sugiura, and Michiaki Yamashita. 2020. "Selenoneine Ameliorates Hepatocellular Injury and Hepatic Steatosis in a Mouse Model of NAFLD" Nutrients 12, no. 6: 1898. https://doi.org/10.3390/nu12061898
APA StyleMiyata, M., Matsushita, K., Shindo, R., Shimokawa, Y., Sugiura, Y., & Yamashita, M. (2020). Selenoneine Ameliorates Hepatocellular Injury and Hepatic Steatosis in a Mouse Model of NAFLD. Nutrients, 12(6), 1898. https://doi.org/10.3390/nu12061898