An Evaluation of the Nutritional Value and Physical Properties of Blenderised Enteral Nutrition Formula: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Designs and Samples
2.2. Type of Population
2.3. Type of Intervention and Comparison
2.4. Types of Outcome
- Nutritional value of enteral nutrition formulas—Energy, Macronutrients (protein, carbohydrate, fat), Micronutrients (sodium, potassium, calcium, phosphorus, magnesium, zinc, iron, vitamin A, vitamin C)
- Physical properties—Viscosity, osmolality
- Any type of clinical outcomes
- Adverse events—diarrhoea, tube blockage
2.5. Inclusion and Exclusion Criteria
2.6. Search Strategy
2.7. Data Extraction
2.8. Statistical Analysis
2.9. Effect Size
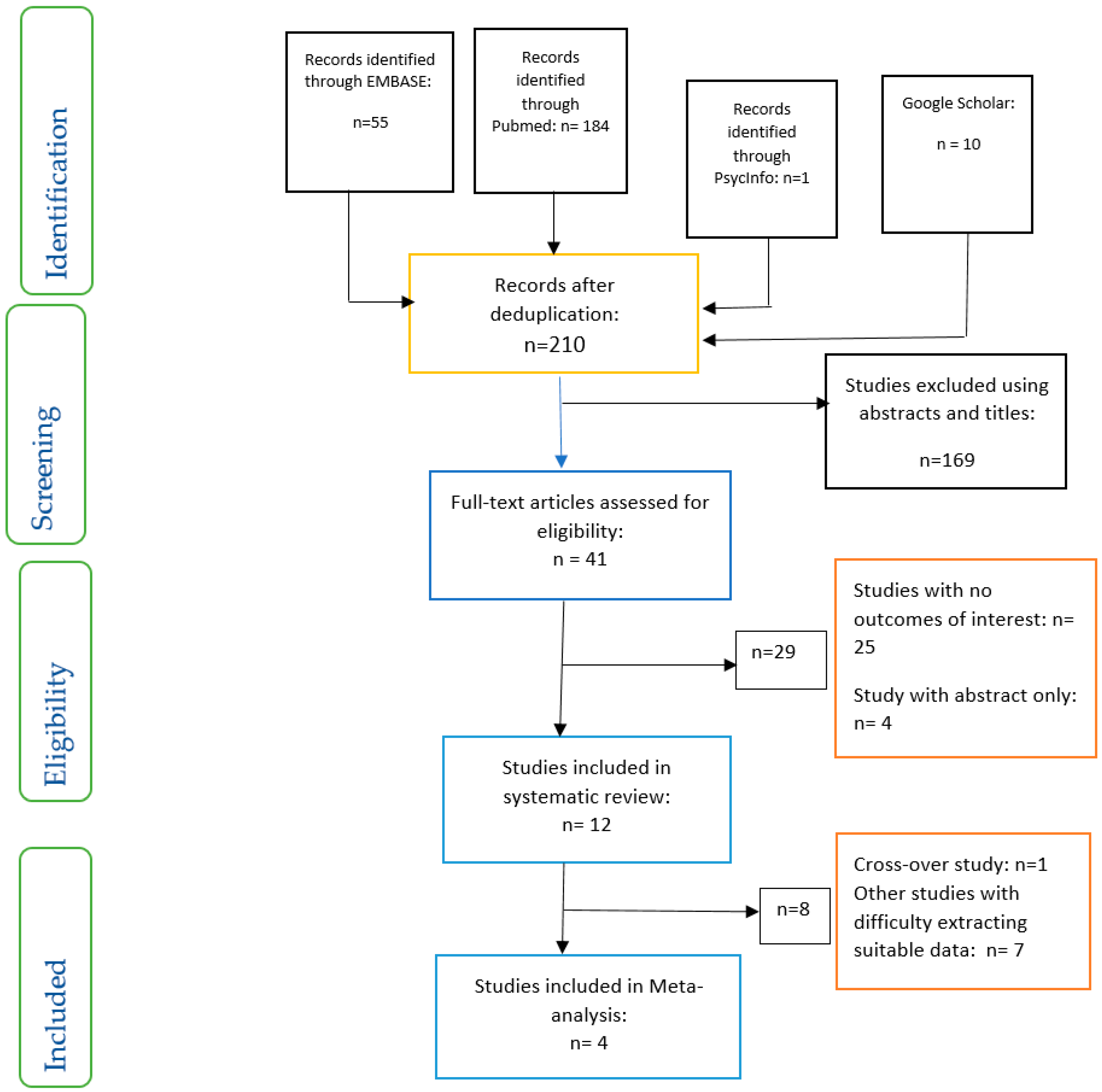
3. Results
3.1. Managing the Data for Meta-Analysis
3.2. Assessment of Risk of Bias and Evaluation of Quality
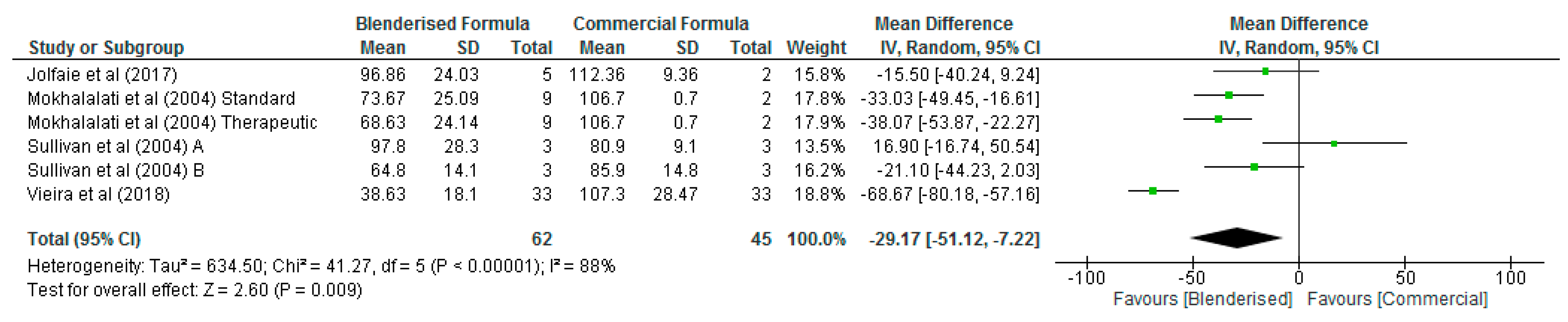
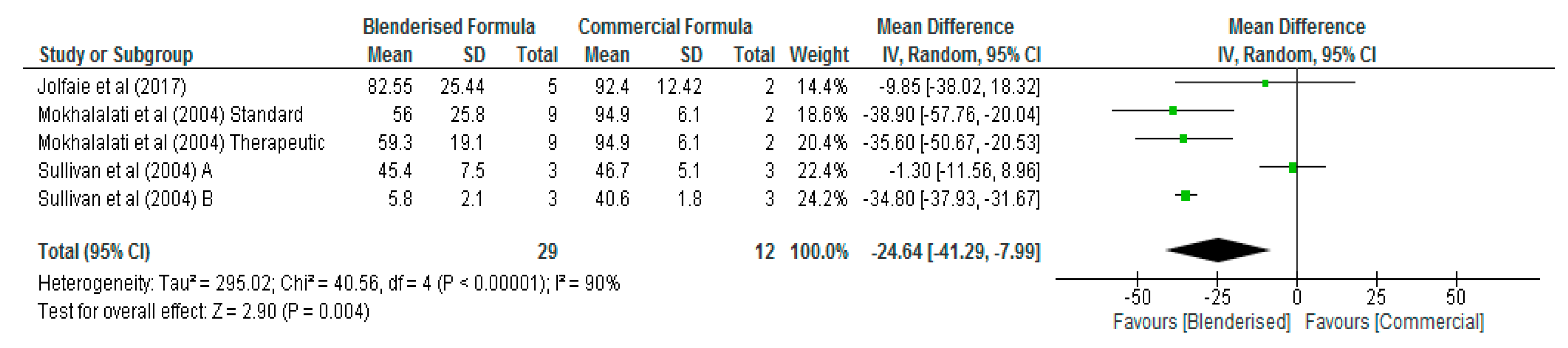
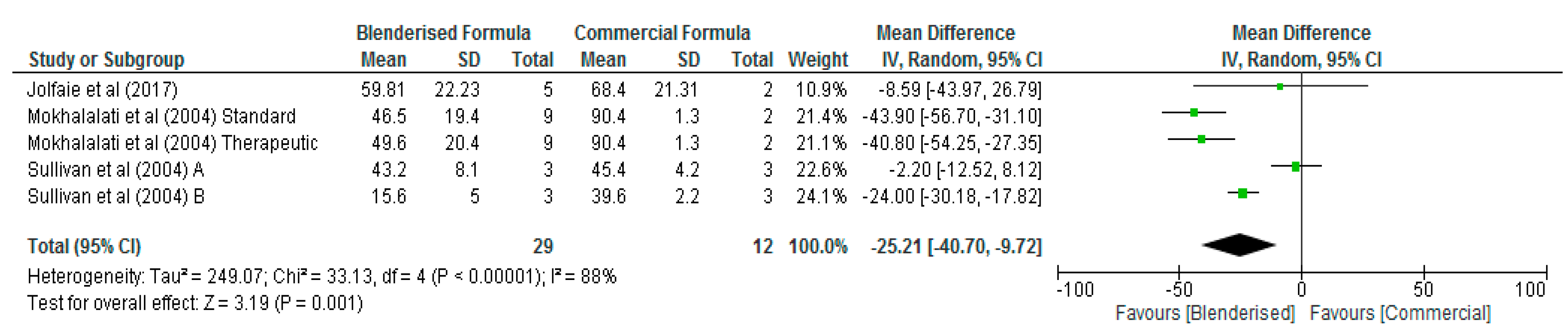
3.3. Nutritional Value of Blenderised Enteral Nutrtion Formulas
3.4. Physical Properties of the Blenderised Formulas
3.5. Clinical Outcomes
3.6. Adverse Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Klek et al. [29] | Jazayeri et al. [1] | Tiyapanjanit et al. [30] | Papakostas et al. [31] | |
|---|---|---|---|---|
| Section A | ||||
| 1. Did the study address a clearly focused issue? | YES | YES | YES | YES |
| 2. Was the cohort recruited in an acceptable way? | YES | YES | YES | YES |
| 3. Was the exposure accurately measured to minimise bias? | YES | YES | YES | YES |
| 4. Was the outcome accurately measured to minimise bias? | YES | YES | YES | YES |
| 5a. Have the authors identified all important confounding factors? | YES | YES | YES | YES |
| 5b. Have they taken account of the confounding factors in the design and analysis? | YES | YES | YES | YES |
| 6a. Was the follow up of subjects complete enough? | YES | YES | YES | YES |
| 6b. Was the follow up of subjects long enough? | YES | YES | Unclear why the first and third day of enteral feeding were monitored | YES |
| Section B | ||||
| 7. What are the results of the study? | SpecializedHETF care program reduces morbidity and costs related to long-term enteral feeding at home. | Albumin levels were significantly increased in the manufactured standard enteral formula compared to the hospital prepared blended formula. | Hospital blenderised formula resulted in significantly lower mean plasma glucose, cost, and number of capillary blood glucose tests, compared to commercial diabetic formula. | Commercially available, oncology specific enteral feeding formulas have significantly better nutritional profiles than the blenderised home-cooked diets. |
| 8. How precise are the results? | The results were significant and presented the CIs, demonstrating reasonable precision | Significant results were presented | Significant results were presented | Significant results were presented |
| 9. Do you believe the results? | YES | YES | YES | YES |
| Section C | ||||
| 10. Can the results be applied to the local population? | YES | YES | NO—sample of 10 participants | YES |
| 11. Do the results of this study fit with other available evidence? | YES | Unclear | NO | Unclear |
| 12. What are the implications of this study? | Patients receiving home enteral nutrition should be adequately supervised and provided with appropriate enteral diets to maximise the benefits of such a therapy. | Standard enteral formula has more benefits than hospital-prepared blended formulas for ICU patients. | The hospital diabetic formula had significantly lower mean plasma glucose and was less expensive than the commercial diabetic formula. | Home-made and family-cooked food is inappropriate and may actually be harmful for malnourished HNC patients scheduled to receive concurrent treatment. |
| Hurt et al. [6] | Vieira et al. [25] | Jolfaie et al. [27] | |
|---|---|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | YES | YES | YES |
| 2. Were the study subjects and the setting described in detail? | YES | YES—commercial and non-commercial enteral feeds | YES—both participants and commercial and non-commercial formulas |
| 3. Was the exposure measured in a valid and reliable way? | YES | NOT APPLICALBE | YES |
| 4. Were objective, standard criteria used for measurement of the condition? | YES—received commercial enteral nutrition for 3 weeks | YES—for each commercial and non-commercial enteral feeds | YES—for commercial and non-commercial formulas |
| 5. Were confounding factors identified? | YES | NO | YES |
| 6. Were strategies to deal with confounding factors stated? | NOT APPLICABLE | NO | YES |
| 7. Were the outcomes measured in a valid and reliable way? | YES—developed and validated a survey, but analysis not provided | YES—the content of the feeds | YES—the content of the feeds |
| 8. Was appropriate statistical analysis used? | YES | YES | YES |
References
- Jazayeri, S.M.H.; Ostadrahimi, A.; Safaiyan, A.; Hashemzadeh, S.; Salehpour, F. Standard Enteral Feeding Improves Nutritional Status Compared with Hospital-Prepared Blended Formula Among Intensive Care Unit (ICU) Patients. Prog. Nutr. 2016, 18, 22–25. Available online: https://mattioli1885journals.com/index.php/progressinnutrition/article/view/4308 (accessed on 21 April 2020).
- Borghi, R.; Dutra Araujo, T.; Airoldi Vieira, R.I.; Theodoro de Souza, T.; Waitzberg, D.L. ILSI Task Force on enteral nutrition; estimated composition and costs of blenderized diets. Nutr. Hosp. 2013, 28, 2033–2038. [Google Scholar]
- Hassan-Ghomi, M.; Nikooyeh, B.; Motamed, S.; Neyestani, T.R. Efficacy of commercial formulas in comparison with home-made formulas for enteral feeding: A critical review. Med. J. Islamic Repub. Iran 2013, 31, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.; Keaveney, E.; Wang, X.-H.; Feng, P. The Effect of Enteral Tube Feeding on Patients’ Health-Related Quality of Life: A Systematic Review. Nutrients 2019, 11, 1046. [Google Scholar] [CrossRef] [Green Version]
- Jalali, M.; Sabzghabaee, A.M.; Badri, S.S.; Soltani, H.A.; Maracy, M.R. Bacterial contamination of hospital-prepared enteral tube feeding formulas in Isfahan, Iran. J. Res. Med. Sci. 2009, 14, 149–156. [Google Scholar]
- Hurt, R.T.; Edakkanambeth Varayil, J.; Epp, L.M.; Pattinson, A.K.; Lammert, L.M.; Lintz, J.E.; Mundi, M.S. Blenderized Tube Feeding Use in Adult Home Enteral Nutrition Patients: A Cross-Sectional Study. Nutr. Clin. Pract. 2015, 30, 824–829. [Google Scholar] [CrossRef]
- Ojo, O. The challenges of home enteral tube feeding: A global perspective. Nutrients 2015, 7, 2524–2538. [Google Scholar] [CrossRef] [Green Version]
- British Association for Parenteral and Enteral Nutrition (BAPEN, 2018). BANS Report 2018 Home Enteral Tube Feeding (HETF) in Adults (2010–2015). Available online: https://www.bapen.org.uk/pdfs/reports/bans/bans-report-2018.pdf (accessed on 21 April 2020).
- Zadák, Z.; Kent-Smith, L. Basics in clinical nutrition: Commercially prepared formulas. e-SPEN, Eur. e-J. Clin. Nutr. Metab. 2009, 4, e212–e215. [Google Scholar]
- Brown, B.; Roehl, K.; Betz, M. Enteral nutrition formula selection: Current evidence and implications for practice. Nutr. Clin. Pract. 2015, 30, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Epp, L.; Lammert, L.; Vallumsetla, N.; Hurt, R.T.; Mundi, M.S. Use of Blenderized Tube Feeding in Adult and Pediatric Home Enteral Nutrition Patients. Nutr. Clin. Pract. 2017, 32, 201–205. [Google Scholar] [CrossRef]
- Madden, A.M.; Baines, S.; Bothwell, S.; Chen, E.; Goh, S.; Jerome, L.; Sommariva-Nagle, C.; Szychta, M. A laboratory-based evaluation of tube blocking and microbial risks associated with one blended enteral feed recipe. J. Hum. Nutr. Diet. 2019, 32, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bobo, E. Reemergence of Blenderized Tube Feedings: Exploring the Evidence. Nutr Clin Pract. 2016, 31, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Milton, D.L.; Johnson, T.W.; Johnson, K.; Murphy, B.; Carter, H.; Hurt, R.T.; Mundi, M.S.; Epp, L.; Spurlock, A.Y.; Hussey, J. Accepted Safe Food-Handling Procedures Minimizes Microbial Contamination of Home-Prepared Blenderized Tube-Feeding. Nutr. Clin. Pract. 2020, 35, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Savino, P. Knowledge of Constituent Ingredients in Enteral Nutrition Formulas Can Make a Difference in Patient Response to Enteral Feeding. Nutr. Clin. Pract. 2018, 33, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Public Health England. Government Dietary Recommendations. 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/618167/government_dietary_recommendations.pdf (accessed on 6 April 2020).
- Franca, S.C.; Paiva SAR de Borgato, M.H.; Fontes, C.M.B.; Simonetti, J.P.; Lima, S.A.M.; Papini, S.J. Homemade diet versusdiet industrialized for patients using alternative feeding tube at home—An integrative review. Nutr. Hosp. 2017, 34, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Carter, H.; Johnson, K.; Johnson, T.W.; Spurlock, A. Blended tube feeding prevalence, efficacy, and safety: What does the literature say? J. Am. Assoc. Nurse Pract. 2018, 30, 150–157. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzla_, J.; Altman, D.G.; Prisma, G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [Green Version]
- The Nordic Cochrane Centre, Review Manager (RevMan). Computer Program; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Mokhalalati, J.K.; Druyan, M.E.; Shott, S.B.; Comer, G.M. Microbial, nutritional and physical quality of commercial and hospital prepared tube feedings in Saudi Arabia. Saudi Med. J. 2004, 25, 331–341. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sullivan, M.M.; Sorreda-Esguerra, P.; Platon, M.B.; Castro, C.G.; Chou, N.R.; Shott, S.; Comer, G.M.; Alarcon, P. Nutritional analysis of blenderized enteral diets in the Philippines. Asia Pact. J. Clin. Nutr. 2004, 13, 385–391. [Google Scholar]
- Vieira, M.M.C.; Santos, V.F.N.; Bottoni, A.; Morais, T.B. Nutritional and microbiological quality of commercial and homemade blenderized whole food enteral diets for home-based enteral nutritional therapy in adults. Clin. Nutr. 2018, 37, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Jolfaie, N.R.; Rouhani, M.H.; Mirlohi, M.; Babashahi, M.; Abbasi, S.; Adibi, P.; Esmaillzadeh, A.; Azadbakht, L. Comparison of Energy and Nutrient Contents of Commercial and Noncommercial Enteral Nutrition Solutions. Adv. Biomed. Res. 2017, 6, 131. [Google Scholar] [CrossRef]
- Johnson, T.W.; Milton, D.L.; Johnson, K.; Carter, H.; Hurt, R.T.; Mundi, M.S.; Epp, L.; Spurlock, A.L. Comparison of Microbial Growth Between Commercial Formula and Blenderized Food for Tube Feeding. Nutr. Clin. Pract. 2019, 34, 257–263. [Google Scholar] [CrossRef]
- Klek, S.; Szybinski, P.; Sierzega, M.; Szczepanek, K.; Sumlet, M.; Kupiec, M.; Koczur-Szozda, E.; Steinhoff-Nowak, M.; Figula, K.; Kowalczyk, T.; et al. Commercial enteral formulas and nutrition support teams improve the outcome of home enteral tube feeding. Jpen. J. Parenter. Enter. Nutr. 2011, 35, 380–385. [Google Scholar] [CrossRef]
- Tiyapanjanit, T.; Boonyavarakul, A. Comparative study between the Phramongkutklao’s diabetic blenderized diets and commercial diabetic diets on glycemic variability in continuous tube fed patients with type 2 diabetes. J. Med. Assoc. Thail. 2014, 97, 1151–1156. [Google Scholar]
- Papakostas, P.; Tsaousi, G.; Stavrou, G.; Rachovitsas, D.; Tsiropoulos, G.; Rova, C.; Konstantinidis, I.; Michalopoulos, A.; Grosomanidis, V.; Kotzampassi, K. Percutaneous endoscopic gastrostomy feeding of locally advanced oro-pharygo-laryngeal cancer patients: Blenderized or commercial food? Oral. Oncol. 2017, 74, 135–141. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme CASP Cohort Study Checklist. 2018. Available online: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Cohort-Study-Checklist_2018.pdf (accessed on 2 May 2020).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk; Aromataris, E., Munn, Z., Eds.; Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute: Adelaide, Australia, 2017; Available online: https://reviewersmanual.joannabriggs.org/ (accessed on 2 May 2020).
- Martin, K.; Gardner, G. Home Enteral Nutrition: Updates, Trends, and Challenges. Nutr. Clin. Pract. 2017, 32, 712–721. [Google Scholar] [CrossRef]
- Lochs, H.; Allison, S.P.; Meier, R.; Pirlich, M.; Kondrup, J.; Schneider, S.; van den Berghe, G.; Pichard, C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin Nutr. 2006, 25, 180–186. [Google Scholar] [CrossRef] [Green Version]
- National Collaborating Centre for Acute Care. Nutrition Support in Adults Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. 2006. Available online: https://www.nice.org.uk/guidance/cg32/evidence/full-guideline-194889853 (accessed on 13 March 2020).
- Iacone, R.; Scanzano, C.; Santarpia, L.; D’Isanto, A.; Contaldo, F.; Pasanisi, F. Micronutrient content in enteral nutrition formulas: Comparison with the dietary reference values for healthy populations. Nutr. J. 2016, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Stroud, M.; Duncan, H.; Nightingale, J. Guidelines for enteral feeding in adult hospital patients. Gut 2003, 52 (Suppl. 70), vii1–vii12. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R.; Cifelli, A.M.; Kostas, G.; Kim, I.-Y. Optimizing Protein Intake in Adults: Interpretation and Application of the Recommended Dietary Allowance Compared with the Acceptable Macronutrient Distribution Range. Adv. Nutr. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Gramlich, L.; Hurt, R.T.; Jin, J.; Mundi, M.S. Home Enteral Nutrition: Towards a Standard of Care. Nutrients 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef] [Green Version]

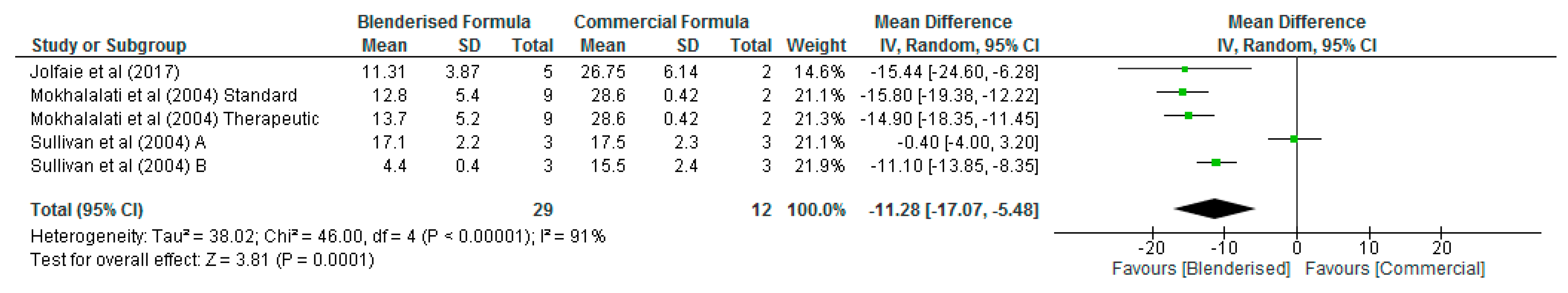
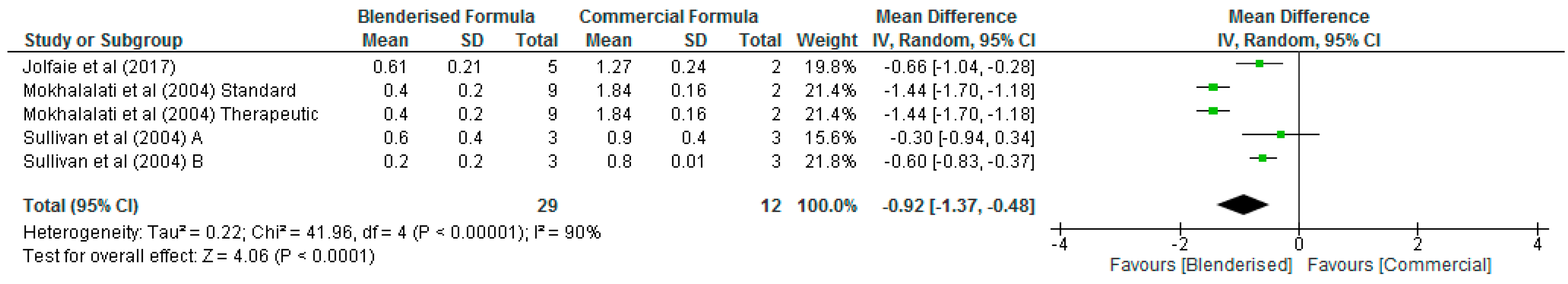
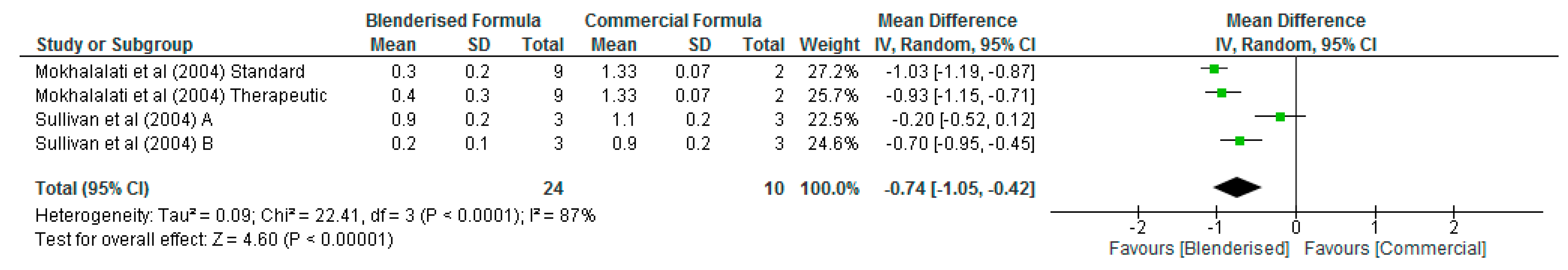
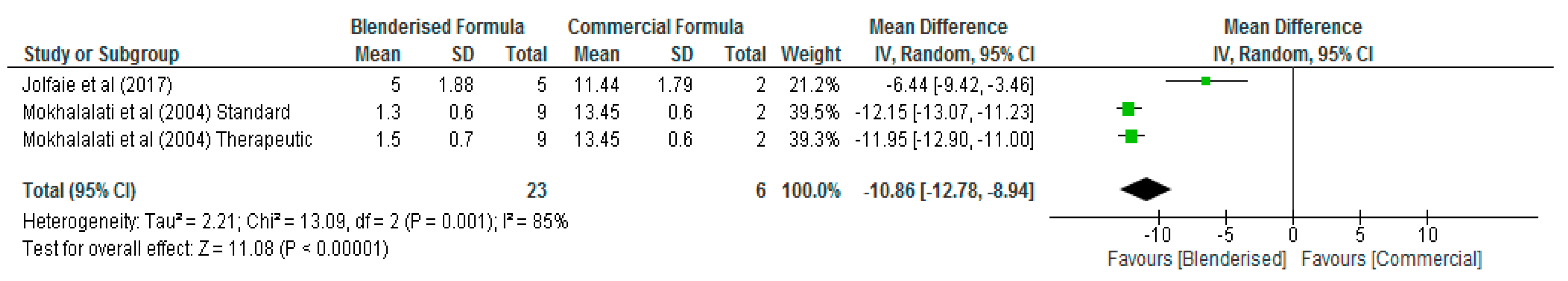

| Citation | Country | Aim/Objective of Study | Study Design | Study Method/Sample Size/Description | Age (Years) | STUDY Results/Conclusion |
|---|---|---|---|---|---|---|
| Jazayeri et al. [1] | Iran | To evaluate the effects of standard enteral feeding compared with hospital-prepared blended formula among Intensive Care Unit (ICU) patients. | Case-control study | A total of 80 patients were involved in the study. These included 40 patients in a standard enteral feeding group and another 40 patients in the hospital-prepared blended formula group. | Hospital-prepared blended formulas: 50.1 ± 18.7 Years Standard enteral feeding: 49.9 ± 18.1 Years | There was increased macronutrient intake in the standard ENF group and this helped in patients’ recovery. The standard enteral ENF has more benefits than hospital-prepared blended ENF for ICU patients. |
| Borghi et al. [2] | Brazil | To evaluate the nutritional quality and cost of blenderised tube diets (BTD). | In-vitro experimental study | Only five BTD out of 14 collected BDT recipes were analysed for their nutritional properties while the commercial foods were based on portion size and manufacturer’s information | Not Applicable | Blenderised tube feeding diets were highly variable and with inconsistent nutritional value. |
| Hurt et al. [6] | USA | To determine the prevalence and use of BTF and frequency of use in adults receiving HEN. | Cross-sectional study | All patients who had follow-up appointments at the HEN clinic were approached during their appointment to participate in completing the survey electronically or fill in a paper questionnaire. The survey consisted of 15 questions. | 60.5 Years | Most of the adult HEN patients use BTF as part of their nutrition regimen during tube feeding. Patients did not report any significant concerns with BTF. |
| Madden et al. [12] | UK | To examine the risks of blended formula providing nutritionally adequate intake. | In-vitro experimental study | A blended formula was made using three different methods (professional, jug, and stick blenders) and three storage procedures. The feed samples were delivered through 10-, 12-, and 14-French (Fr) enteral feeding tubes and both blockages and the time taken were recorded. | Not Applicable | There was no risk of tube blockages when one blended ENF recipe made using three methods was delivered via a 14-Fr tube. After removing the waste (residues remaining on utensils and unsieved fraction), the remaining feed provided less than 95% of the estimated requirements for energy, fibre, iron, zinc, selenium, and vitamins A, D, E, and B6 |
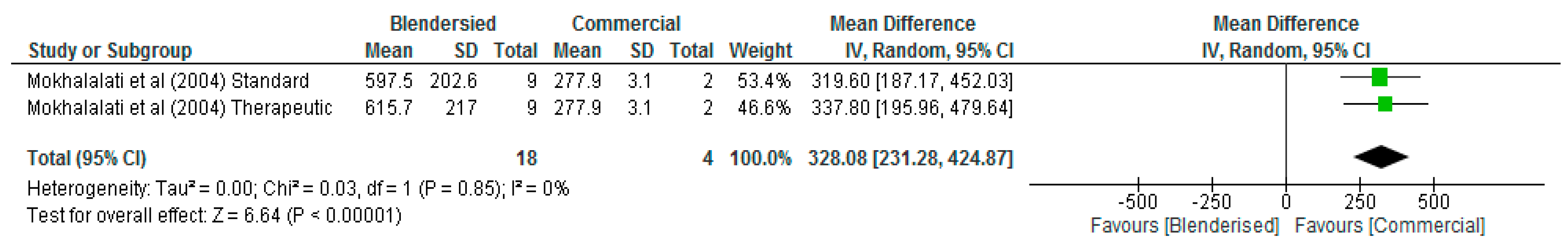
| Mokhalalati et al. [22] | Saudi Arabia | To compare the microbial safety, nutritional content, and physical properties of BTF and commercially prepared formulas (CPF). | In-vitro experimental study | 18 samples of BTF were collected from 3 hospitals. Samples of a CPF were also collected for comparison. | There is a high degree of variability in nutrient content and physical properties of BTF. Cholesterol, sodium, vitamin A, and vitamin B6 levels for all BTF were higher than the commercial ENF. However, the values for unsaturated fat, nonferrous extract (NFE), calories, calcium, phosphorus, magnesium, zinc, iron, copper, and vitamins D, E, B3, and C were lower for all BTF compared with commercial ENF. | |
| Sullivan et al. [24] | Philippines | To evaluate the nutritional content and viscosity of hospital-prepared BTF. | In-vitro experimental study | Two different BTFs (one standard and one modified) were collected from each hospital on three separate occasions and analysed for macronutrients, micronutrients, and viscosity. | Not Applicable | Hospital prepared BTF showed unpredictable levels of micronutrients and macronutrients and may provide less than the required amounts of nutrients. In addition, the viscosity of these formulas may not be suitable for infusion through feeding tubes. |
| Vieira et al. [25] | Brazil | To evaluate the nutritional and microbiological quality of commercial enteral and homemade blenderised whole foods. | Cross-sectional study | 66 samples of commercial (n = 33) and noncommercial (n = 33) enteral diets were collected at the homes of patients on HEN | 73 Years (20–100 Years) | The homemade blenderised ENF demonstrated low values of energy and macronutrients and provided less than 50% of the recommended values. |
| Jolfaie et al. [27] | Iran | To compare the nutritional quality of commercial enteral nutrition and blenderised enteral formula | Cross-sectional study | 150 patients were fed blended formula and 120 patients were fed commercial ENF | Blenderised formula: 55.46 ± 20.19 Years Commercial Formula: 53.13 ± 20.35 Years | Commercial ENF contained more energy and nutrients compared with blended ENF and they are more effective in meeting the nutritional requirements of patients who are fed enterally. |
| Johnson et al. [28] | USA | To compare microbial loads of a standard polymeric commercial formula (CF), a BTF made using baby food (BTF-BF), and a BTF prepared from blending whole food (BTF-WF). | In-vitro experimental study | Three tube-feeding formulas (CF, BTF-BF, BTF-WF) were compared. | Not Applicable | The results show that BTF recipe selection and adherence to safe food handling provide a safe feeding that is comparable to CF in the hospital setting. |
| Klek et al. [29] | Poland | To examine the effect of commercial enteral nutrition (specialised home enteral nutrition) programme on clinical outcomes. | Cohort | All patients who had received home enteral tube feeding (HETF) with homemade blenderised diets for 12 months before starting a specialized nutrition programme for another 12 months consisting of the provision of commercial enteral nutrition formulas and guidance on nutrition support. | 52.5 Years | It was demonstrated that the specialized HETF care programme consisting of commercial ENF and nutrition support team reduces morbidity and costs related to long-term enteral feeding at home. |
| Tiyapanjanit & Boonyavarakul [30] | Thailand | To compare blood glucose parameters and cost between the Phramongkutklao’s diabetic formula and commercial diabetic formula in patients with type 2 diabetes. | Cross-over study | Participants were fed using 24 h continuous feeding for three days. The Phramongkutklao’s diabetic formula was followed by commercial diabetic formula continuously for 36 h each. | 79.80 ± 11.03 Years | The Phramongkutklao’s diabetic formula had significantly lower mean plasma glucose and was less expensive than the commercial diabetic formula. |
| Papakostas et al. [31] | Greece | To evaluate the body composition characteristics and nutritional status in HNC patients who are receiving either the prescribed commercial enteral nutrition formula or decided on home-made BTF | Quasi-experimental design | All patients were prescribed to receive, on an out-patient basis, a commercially available enteral nutrition formula. Patients with low income and no public health insurance were recommended to have equivalent home-made enteral formula. Both groups were also advised to consume, yogurt with honey, ice-cream, and fruit and vegetables. 212 patients including 112 who received the commercial formula, 69 who switched to BTF, and 31 that were prescribed to receive a home-made formula of standard ingredients were involved in the study. | Commercial: 56.4 ± 3.6 Years Home-made: 55.9 ± 3.5 Years Blenderised Family Food: 56.2 ± 3.8 Years | The results show that home-made and blenderised foods do not adequately support the nutritional requirements of patients with HNC. |
| Outcomes | Number of Studies/Experiments | Number of Samples | Mean Difference (95% CI) | p-Value | I2 % |
|---|---|---|---|---|---|
| Fat (g/100 mL) | 6 | 107 | −0.63 [−1.41, 0.14] | 0.11 | 74 |
| Protein (g/100 mL) | 6 | 107 | −0.76 [−1.64, 0.12] | 0.09 | 76 |
| Sodium (mg/100 mL) | 5 | 41 | −29.22 [−65.90, 7.46] | 0.12 | 81 |
| Potassium (mg/100 mL) | 5 | 41 | −27.68 [−74.88, 19.53] | 0.25 | 88 |
| Vitamin A (mcg/100 mL) | 4 | 34 | −2.03 [−37.73, 33.68] | 0.91 | 82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojo, O.; Adegboye, A.R.A.; Ojo, O.O.; Wang, X.; Brooke, J. An Evaluation of the Nutritional Value and Physical Properties of Blenderised Enteral Nutrition Formula: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1840. https://doi.org/10.3390/nu12061840
Ojo O, Adegboye ARA, Ojo OO, Wang X, Brooke J. An Evaluation of the Nutritional Value and Physical Properties of Blenderised Enteral Nutrition Formula: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(6):1840. https://doi.org/10.3390/nu12061840
Chicago/Turabian StyleOjo, Omorogieva, Amanda Rodrigues Amorim Adegboye, Osarhumwese Osaretin Ojo, Xiaohua Wang, and Joanne Brooke. 2020. "An Evaluation of the Nutritional Value and Physical Properties of Blenderised Enteral Nutrition Formula: A Systematic Review and Meta-Analysis" Nutrients 12, no. 6: 1840. https://doi.org/10.3390/nu12061840





