Dietary β-Carotene Rescues Vitamin A Deficiency and Inhibits Atherogenesis in Apolipoprotein E-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Diet
2.3. Study Design
2.4. Lipid Analysis
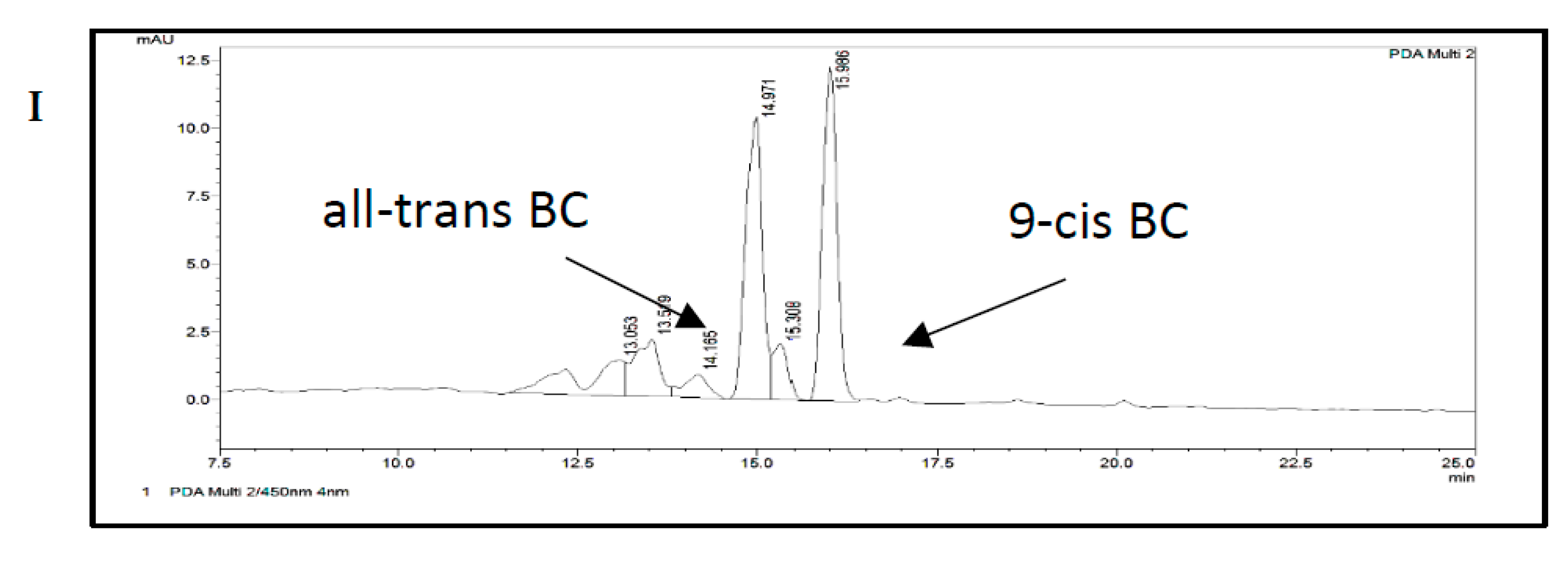
2.5. Carotenoid and Retinol Analysis
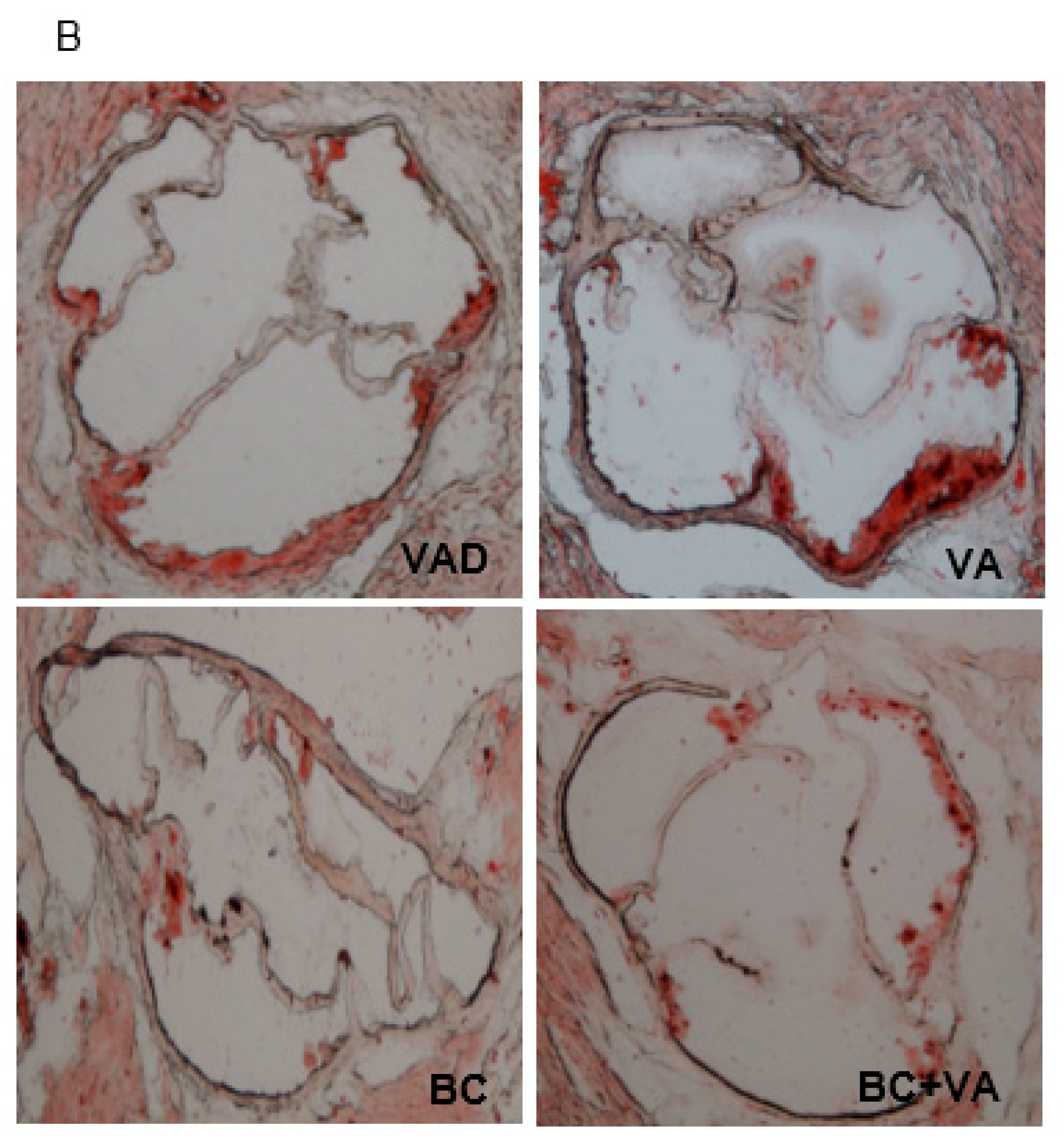
2.6. Assessment of Atherosclerosis in the Aortic Sinus
2.7. Analysis of Gene Expression by Real-Time PCR
2.8. Statistical Analyses
3. Results
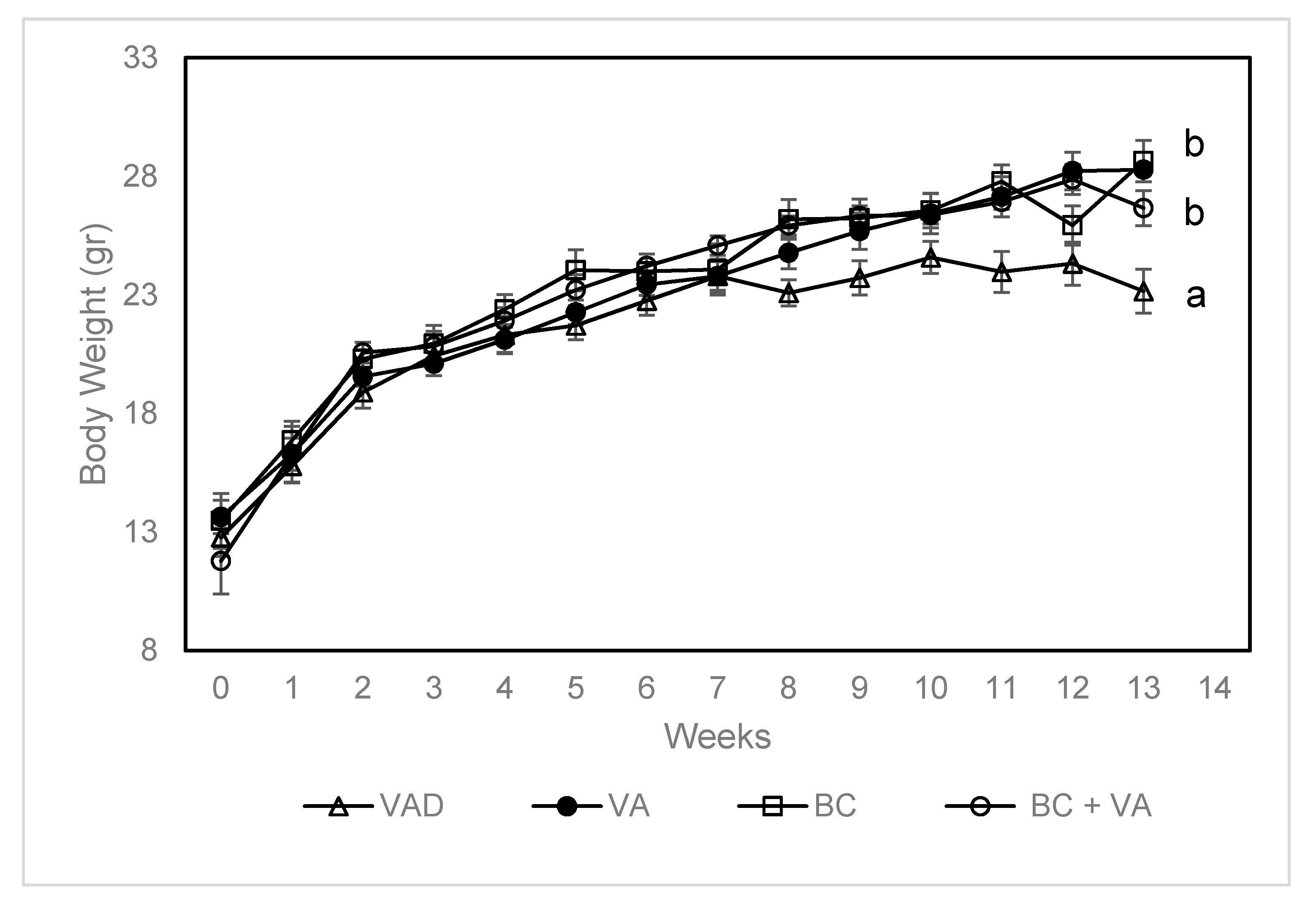
3.1. Body Weight
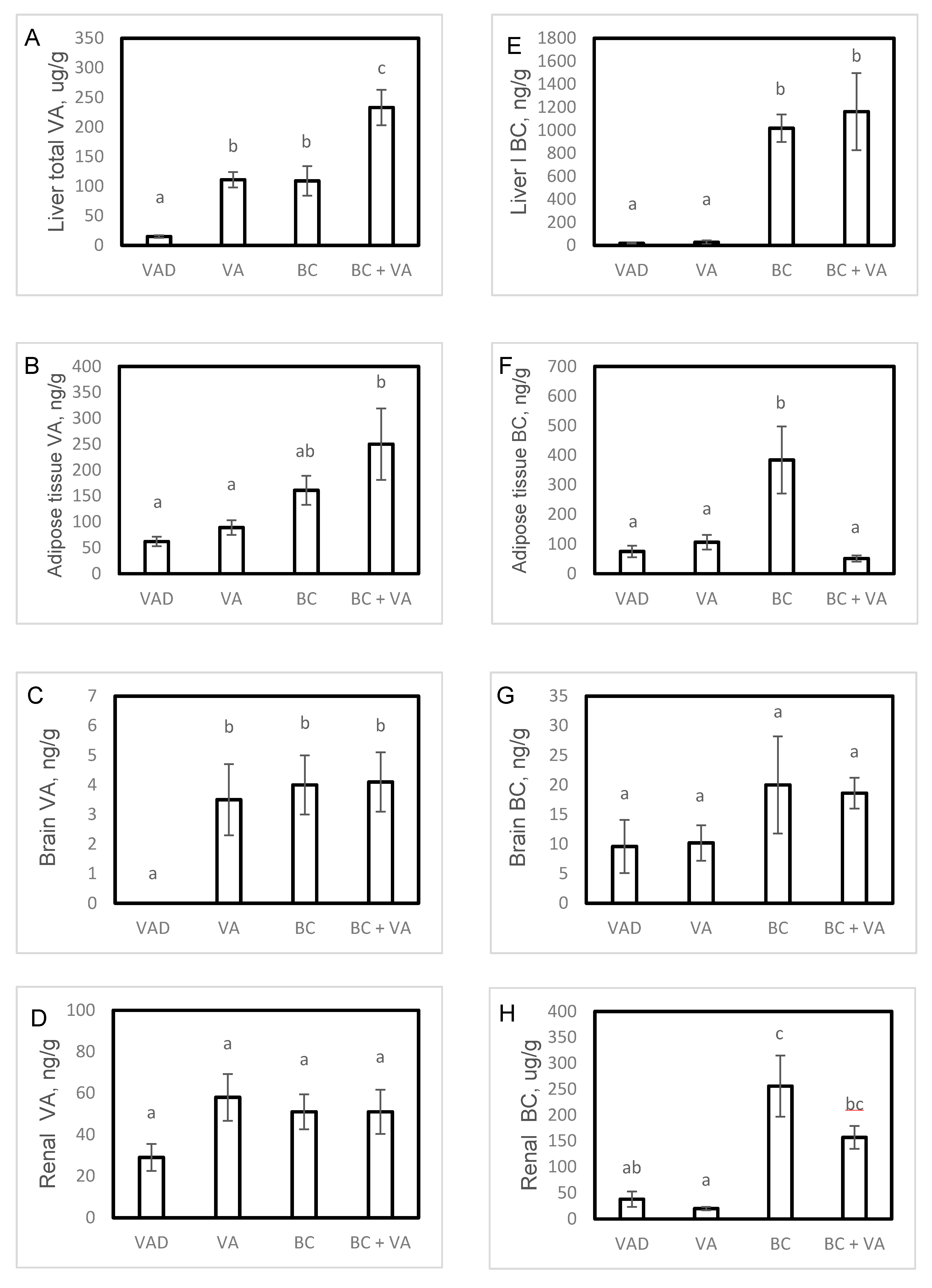
3.2. Tissue VA Levels
3.3. Tissue BC Levels
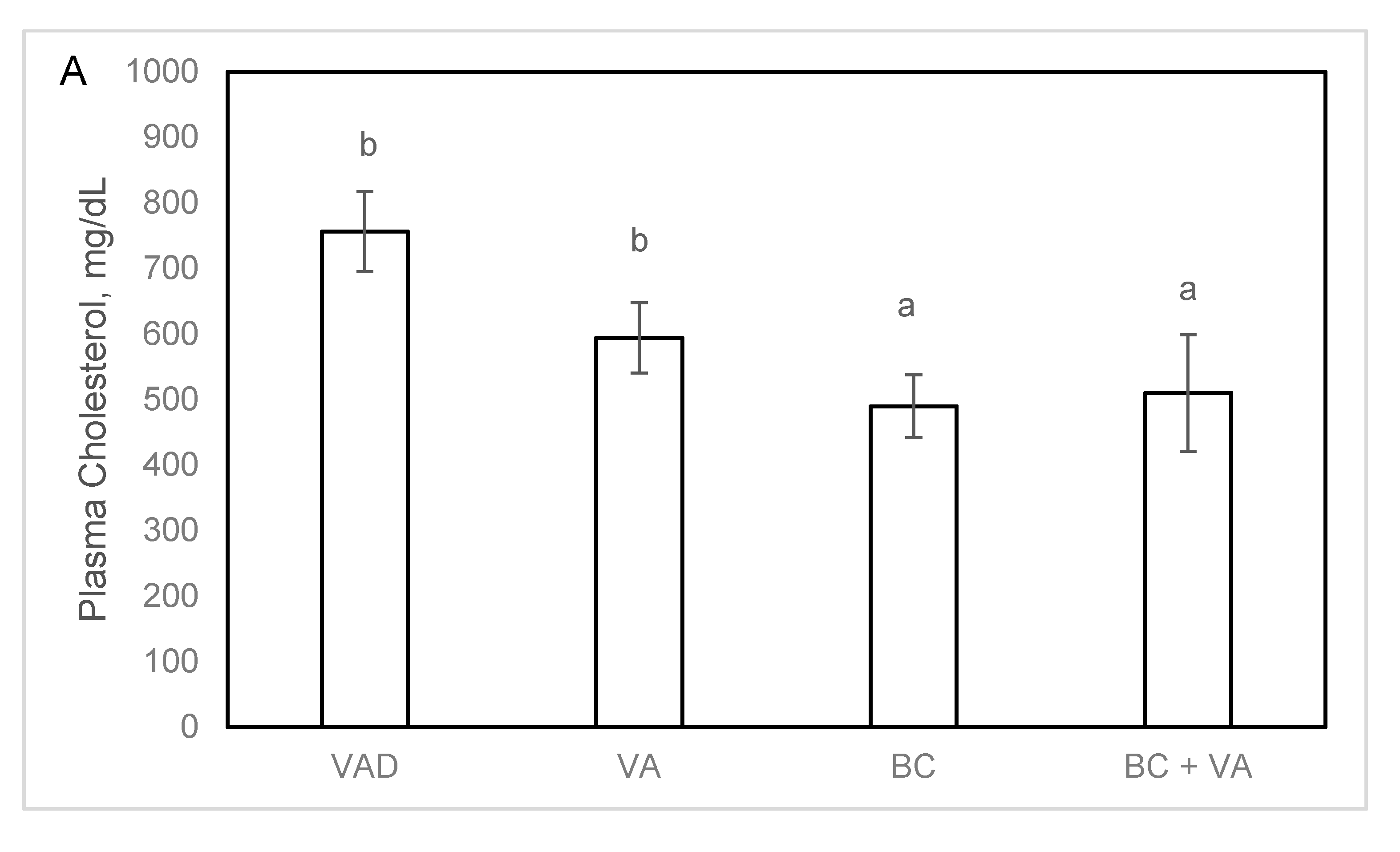
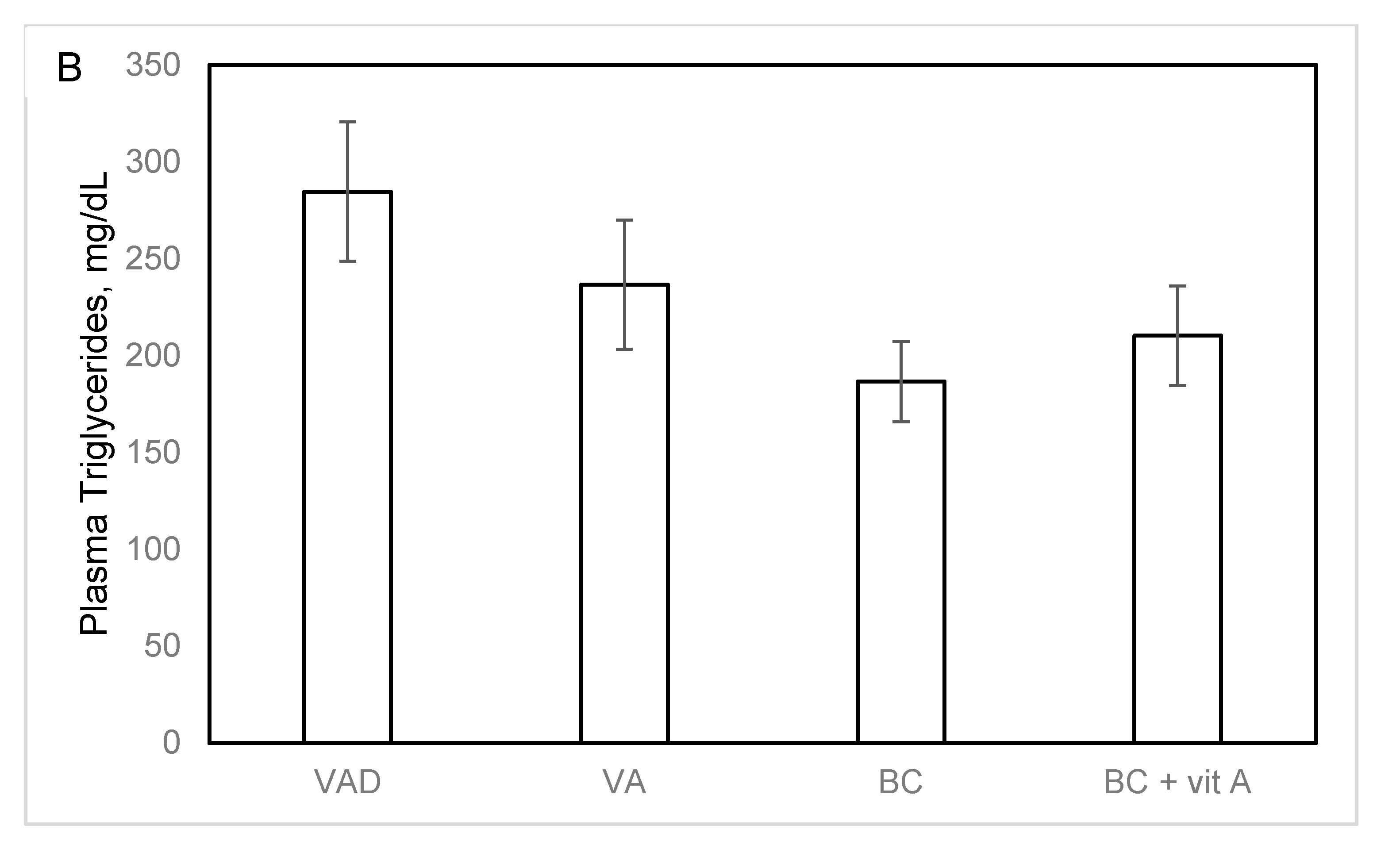
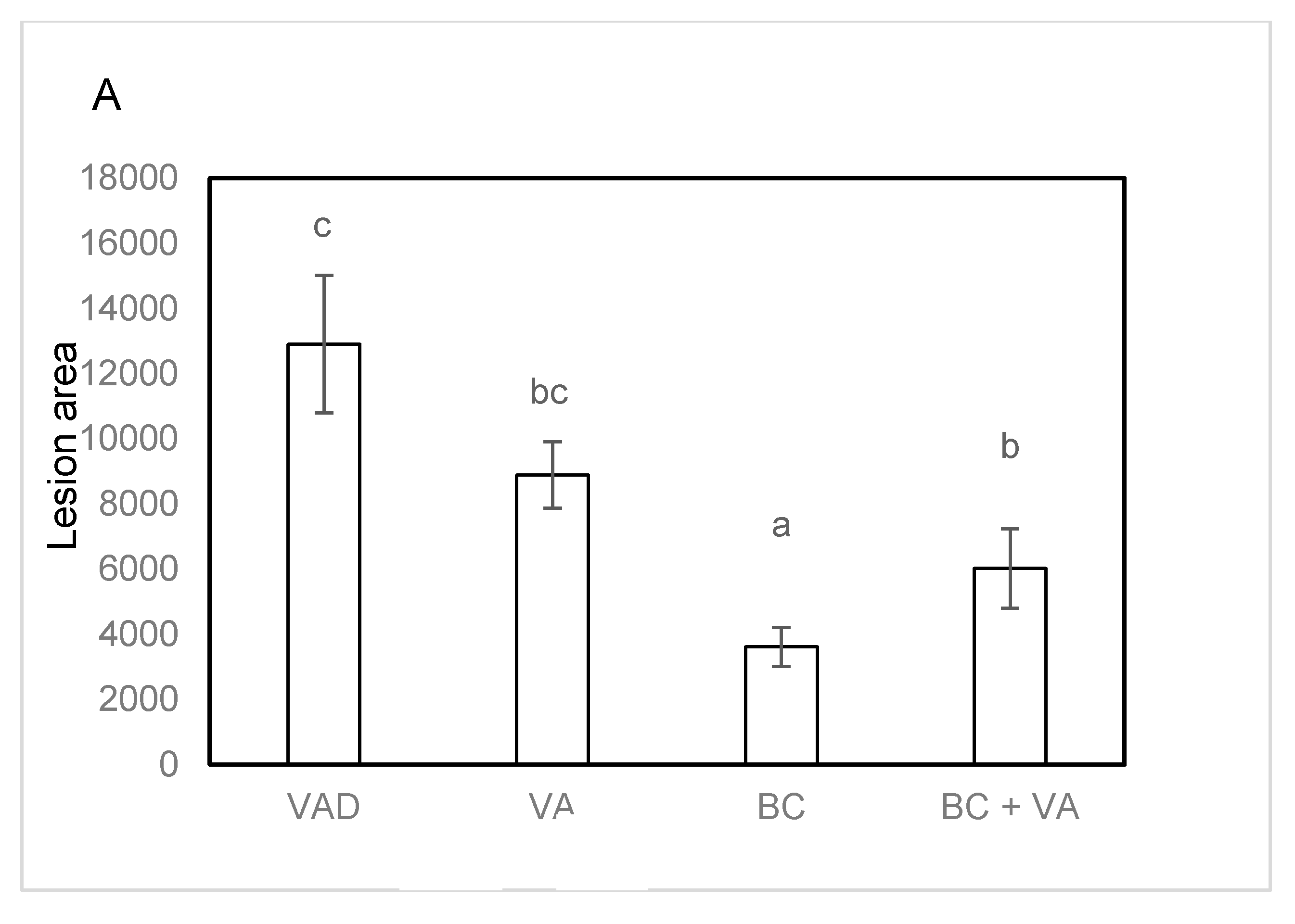
3.4. Plasma Cholesterol and Atherogenesis
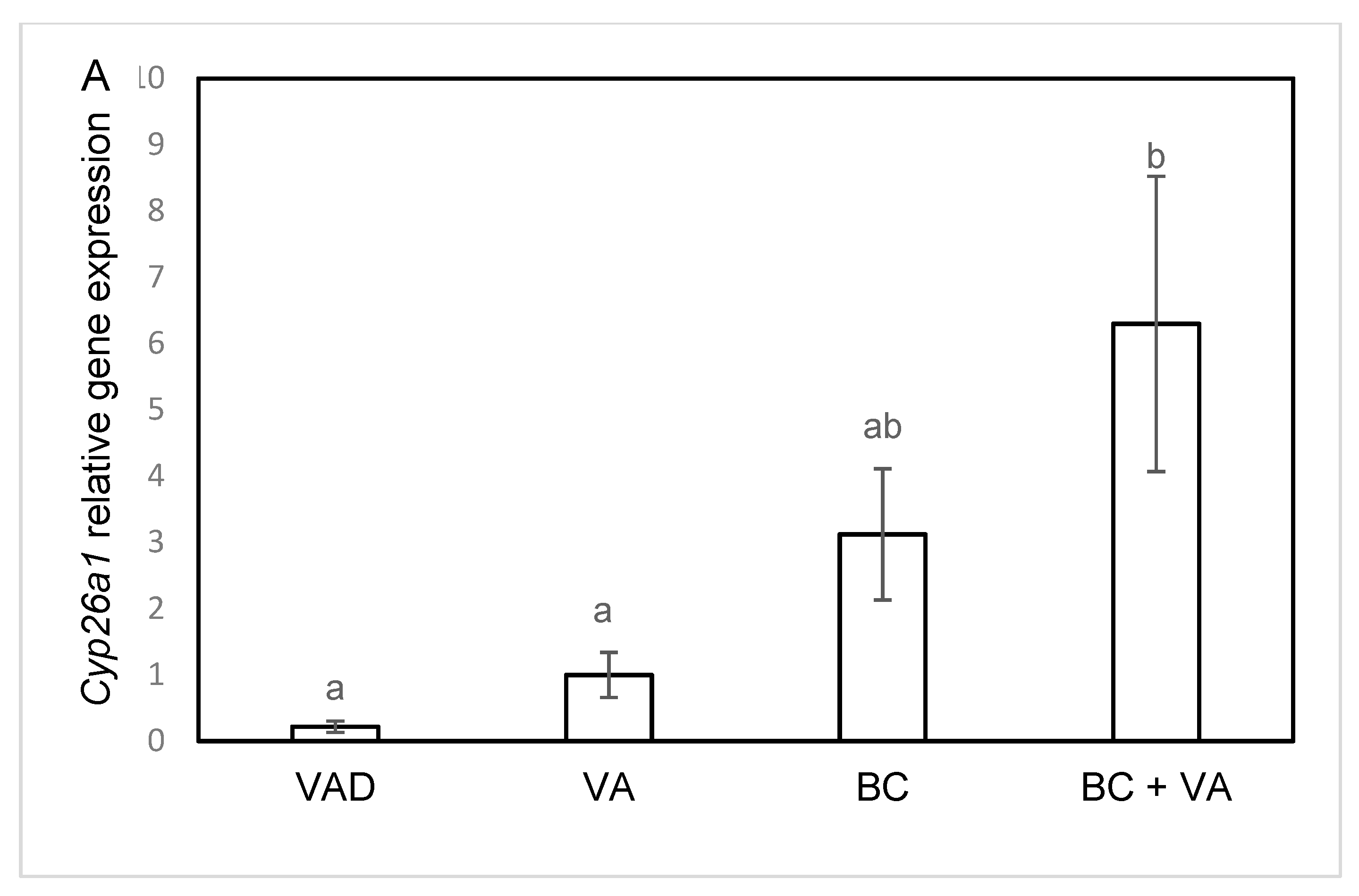
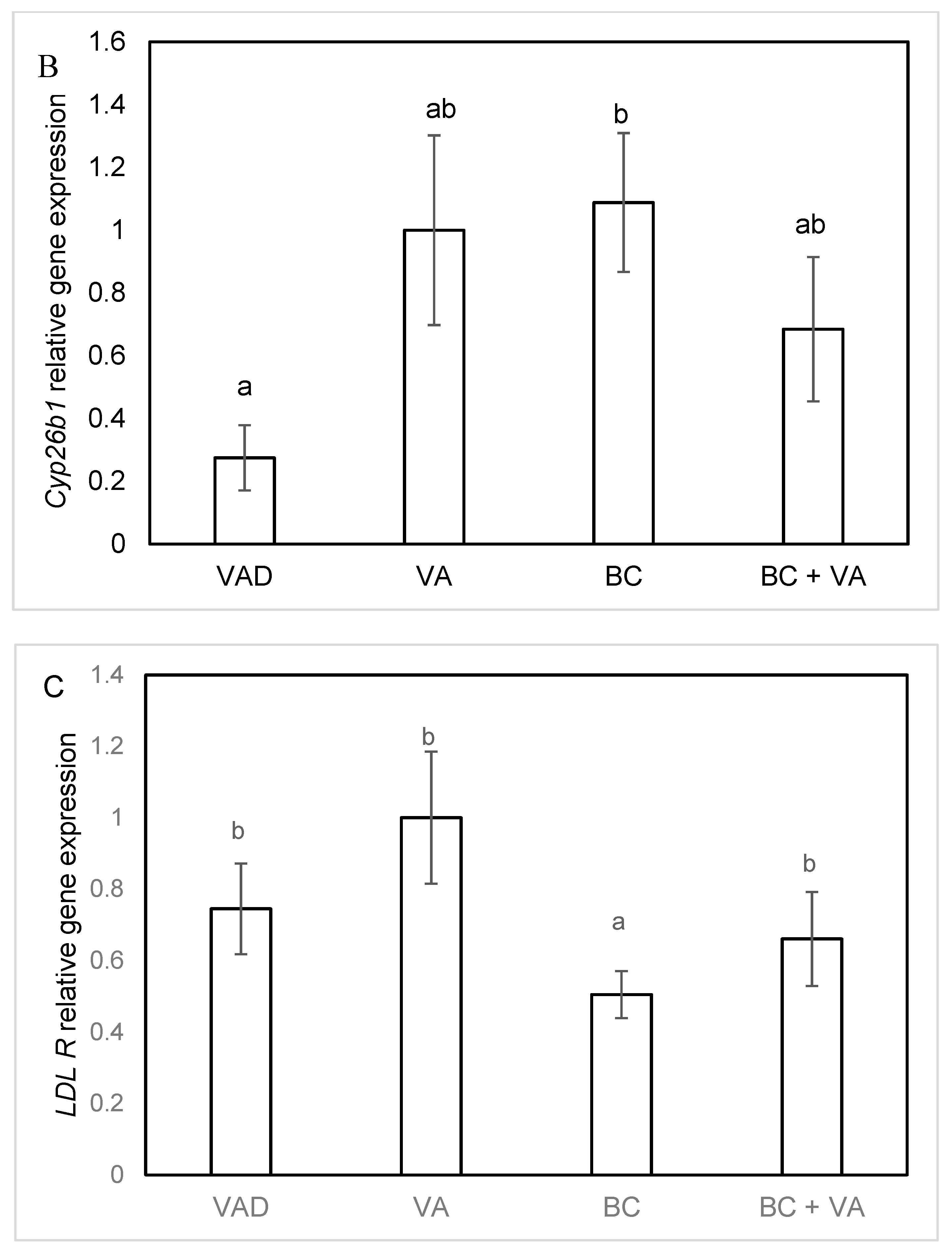
3.5. The mRNA Levels of RA Catalyzing Enzymes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ste vens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Wiseman, E.M.; Bar-El Dadon, S.; Reifen, R. The vicious cycle of vitamin a deficiency: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Cabezuelo, M.T.; Zaragoza, R.; Barber, T.; Vina, J.R. Role of Vitamin A in Mammary Gland Development and Lactation. Nutrients 2019, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Tanumihardjo, S.A. Vitamin A: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 658S–665S. [Google Scholar] [CrossRef]
- Barker, D.J.; Fall, C.H. Fetal and infant origins of cardiovascular disease. Arch. Dis. Child. 1993, 68, 797–799. [Google Scholar] [CrossRef]
- Wei, X.; Peng, R.; Cao, J.; Kang, Y.; Qu, P.; Liu, Y.; Xiao, X.; Li, T. Serum vitamin A status is associated with obesity and the metabolic syndrome among school-age children in Chongqing, China. Asia Pac. J. Clin. Nutr. 2016, 25, 563–570. [Google Scholar] [CrossRef]
- Oliveira, M.N.G.; Rosa, G.; Mansure, J.J.; Ramos, V.W.; Godoy, P.H.J.I.J.f.V.; Research, N. Anthropometric Profile, Serum Leptin, Antioxidant Vitamins, LEPR Q223R Polymorphism and Cardiovascular Risk Factors in Adolescents. Int. J. Vitam. Nutr. Res. 2019. [Google Scholar] [CrossRef]
- Godala, M.; Materek-Kusmierkiewicz, I.; Moczulski, D.; Rutkowski, M.; Szatko, F.; Gaszynska, E.; Tokarski, S.; Kowalski, J. The risk of plasma vitamin A, C, E and D deficiency in patients with metabolic syndrome: A case-control study. Adv. Clin. Exp. Med. 2017, 26, 581–586. [Google Scholar] [CrossRef]
- Bousvaros, A.; Zurakowski, D.; Duggan, C.; Law, T.; Rifai, N.; Goldberg, N.E.; Leichtner, A.M. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: Effect of disease activity. J. Pediatric Gastroenterol. Nutr. 1998, 26, 129–135. [Google Scholar] [CrossRef]
- Leo, M.A.; Lieber, C.S. Hepatic vitamin A depletion in alcoholic liver injury. N. Engl. J. Med. 1982, 307, 597–601. [Google Scholar] [CrossRef]
- Lindqvist, A.; Sharvill, J.; Sharvill, D.E.; Andersson, S. Loss-of-function mutation in carotenoid 15,15’-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J. Nutr. 2007, 137, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.C.; Paiva Ade, A.; de Queiroz, D.; Araujo Franca Costa, R.; Lins da Cunha, M.A.; Figueroa Pedraza, D. Nutritional status of iron in children from 6 to 59 months of age and its relation to vitamin A deficiency. Nutr. Hosp. 2013, 28, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef]
- Bloomberg, R.D.; Fleishman, A.; Nalle, J.E.; Herron, D.M.; Kini, S. Nutritional deficiencies following bariatric surgery: What have we learned? Obes Surg. 2005, 15, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.P.; Petry, N.; Tanumihardjo, S.A.; Rogers, L.M.; McLean, E.; Greig, A.; Garrett, G.S.; Klemm, R.D.; Rohner, F. Vitamin A Supplementation Programs and Country-Level Evidence of Vitamin A Deficiency. Nutrients 2017, 9, 190. [Google Scholar] [CrossRef]
- Hammouda, S.A.; Abd Al-Halim, O.A.; Mohamadin, A.M. Serum levels of some micronutrients and congenital malformations: A prospective cohort study in healthy saudi-arabian first-trimester pregnant women. Int. J. Vitam. Nutr. Res. 2013, 83, 346–354. [Google Scholar] [CrossRef]
- Mills, J.P.; Tanumihardjo, S.A. Vitamin A toxicity in wild-caught African green vervet monkeys (Chlorocebus aethiops) after 2 years in captivity. Comp. Med. 2006, 56, 421–425. [Google Scholar]
- Crandall, C. Vitamin A intake and osteoporosis: A clinical review. J. Womens Health (Larchmt) 2004, 13, 939–953. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Kloti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef]
- Watkins, J.L.; Pogson, B.J. Prospects for Carotenoid Biofortification Targeting Retention and Catabolism. Trends Plant. Sci. 2020. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of beta-Carotene and Phytoene in the Alga Dunaliella bardawil. Plant. Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Alpha-Tocopherol, B.C.C.P.S.G. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Kamari, Y.; Gonen, A.; Gerber, Y.; Ben-Amotz, A.; Shaish, A. A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J. Nutr. 2008, 138, 1923–1930. [Google Scholar] [CrossRef]
- Relevy, N.Z.; Rühl, R.; Harari, A.; Grosskopf, I.; Barshack, I.; Ben-Amotz, A.; Nir, U.; Gottlieb, H.; Kamari, Y.; Harats, D.J.F.F.i.H.; et al. 9-cis β-Carotene inhibits atherosclerosis development in female LDLR-/-mice. Funct. Foods Health Dis. 2015, 5, 67–79. [Google Scholar] [CrossRef]
- Harari, A.; Abecassis, R.; Relevi, N.; Levi, Z.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. Prevention of atherosclerosis progression by 9-cis-beta-carotene rich alga Dunaliella in apoE-deficient mice. Biomed. Res. Int. 2013, 2013, 169517. [Google Scholar] [CrossRef]
- Relevy, N.Z.; Harats, D.; Harari, A.; Ben-Amotz, A.; Bitzur, R.; Ruhl, R.; Shaish, A. Vitamin A-deficient diet accelerated atherogenesis in apolipoprotein E(-/-) mice and dietary beta-carotene prevents this consequence. Biomed. Res. Int. 2015, 2015, 758723. [Google Scholar] [CrossRef]
- Dua, P.N.; Day, E.J.; Tipton, H.C.; Hill, J.E. Influence of dietary vitamin A on carotenoid utilization, nitrogen retention and energy utilization by the chick. J. Nutr. 1966, 90, 117–122. [Google Scholar] [CrossRef]
- Knight, T.; Death, A.; Muir, P.; Ridland, M.; Wyeth, T.J.N.Z.j.o.a.r. Effect of dietary vitamin A on plasma and liver carotenoid concentrations and fat colour in Angus and Angus crossbred cattle. N. Zealand J. Agric. Res. 1996, 39, 281–292. [Google Scholar] [CrossRef]
- Hickenbottom, S.J.; Follett, J.R.; Lin, Y.; Dueker, S.R.; Burri, B.J.; Neidlinger, T.R.; Clifford, A.J. Variability in conversion of beta-carotene to vitamin A in men as measured by using a double-tracer study design. Am. J. Clin. Nutr. 2002, 75, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Krinsky, N.I.; Benotti, P.N.; Russell, R.M. Biosynthesis of 9-cis-retinoic acid from 9-cis-beta-carotene in human intestinal mucosa in vitro. Arch. Biochem. Biophys. 1994, 313, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Axel, D.I.; Frigge, A.; Dittmann, J.; Runge, H.; Spyridopoulos, I.; Riessen, R.; Viebahn, R.; Karsch, K.R. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc. Res. 2001, 49, 851–862. [Google Scholar] [CrossRef]
- Zhou, B.; Pan, Y.; Hu, Z.; Wang, X.; Han, J.; Zhou, Q.; Zhai, Z.; Wang, Y. All-trans-retinoic acid ameliorated high fat diet-induced atherosclerosis in rabbits by inhibiting platelet activation and inflammation. J. Biomed. Biotechnol. 2012, 2012, 259693. [Google Scholar] [CrossRef] [PubMed]
- Claudel, T.; Leibowitz, M.D.; Fievet, C.; Tailleux, A.; Wagner, B.; Repa, J.J.; Torpier, G.; Lobaccaro, J.M.; Paterniti, J.R.; Mangelsdorf, D.J.; et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Pelton, P.D.; Patel, M.; Demarest, K.T. Nuclear receptors as potential targets for modulating reverse cholesterol transport. Curr. Top. Med. Chem. 2005, 5, 265–282. [Google Scholar] [CrossRef]
- Zolberg Relevy, N.; Bechor, S.; Harari, A.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. The inhibition of macrophage foam cell formation by 9-cis beta-carotene is driven by BCMO1 activity. PLoS ONE 2015, 10, e0115272. [Google Scholar] [CrossRef]
- Oliveros, L.B.; Domeniconi, M.A.; Vega, V.A.; Gatica, L.V.; Brigada, A.M.; Gimenez, M.S. Vitamin A deficiency modifies lipid metabolism in rat liver. Br. J. Nutr. 2007, 97, 263–272. [Google Scholar] [CrossRef]
- Gatica, L.V.; Vega, V.A.; Zirulnik, F.; Oliveros, L.B.; Gimenez, M.S. Alterations in the lipid metabolism of rat aorta: Effects of vitamin a deficiency. J. Vasc. Res. 2006, 43, 602–610. [Google Scholar] [CrossRef]
- Yeum, K.J.; Booth, S.L.; Sadowski, J.A.; Liu, C.; Tang, G.; Krinsky, N.I.; Russell, R.M. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am. J. Clin. Nutr. 1996, 64, 594–602. [Google Scholar] [CrossRef]
- Shaish, A.; George, J.; Gilburd, B.; Keren, P.; Levkovitz, H.; Harats, D. Dietary beta-carotene and alpha-tocopherol combination does not inhibit atherogenesis in an ApoE-deficient mouse model. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1470–1475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melendez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Topletz, A.R.; Thatcher, J.E.; Zelter, A.; Lutz, J.D.; Tay, S.; Nelson, W.L.; Isoherranen, N. Comparison of the function and expression of CYP26A1 and CYP26B1, the two retinoic acid hydroxylases. Biochem. Pharmacol. 2012, 83, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Andreola, F.; Calvisi, D.F.; Elizondo, G.; Jakowlew, S.B.; Mariano, J.; Gonzalez, F.J.; De Luca, L.M. Reversal of liver fibrosis in aryl hydrocarbon receptor null mice by dietary vitamin A depletion. Hepatology 2004, 39, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B.; Blount, S.R.; Schoeb, T.R.; Park, J.Y. Vitamin A deficiency impairs some aspects of the host response to influenza A virus infection in BALB/c mice. J. Nutr. 1993, 123, 823–833. [Google Scholar] [CrossRef]
- Olson, C.R.; Mello, C.V. Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 2010, 54, 489–495. [Google Scholar] [CrossRef]
- Laskowska-Klita, T.; Chelchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Klemarczyk, W. The effect of vegetarian diet on selected essential nutrients in children. Med. Wieku. Rozwoj. 2011, 15, 318–325. [Google Scholar]
- Thane, C.W.; Bates, C.J. Dietary intakes and nutrient status of vegetarian preschool children from a British national survey. J. Hum. Nutr. Diet. 2000, 13, 149–162. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef]
- Wassef, L.; Shete, V.; Costabile, B.; Rodas, R.; Quadro, L. High Preformed Vitamin A Intake during Pregnancy Prevents Embryonic Accumulation of Intact beta-Carotene from the Maternal Circulation in Mice. J. Nutr. 2015, 145, 1408–1414. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, J.; Chen, H.; Wang, J.; Liu, Y.; Xia, M.J.B.J.o.N. Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in apoE-deficient mice. Br. J. Nutr. 2015, 114, 509–518. [Google Scholar] [CrossRef]
- Zhu, L.; Bisgaier, C.L.; Aviram, M.; Newton, R.S.J.A.; Biology, V. 9-cis retinoic acid induces monocyte chemoattractant protein-1 secretion in human monocytic THP-1 cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2105–2111. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular Health Benefits of Specific Vegetable Types: A Narrative Review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef]
- Miller, A.P.; Coronel, J.; Amengual, J.J.B.e.B.A.-M.; Lipids, C.B.o. The role of β-carotene and vitamin A in atherogenesis: Evidences from pre-clinical and clinical studies. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 158635. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T.J.N.r. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Kabagambe, E.K.; Furtado, J.; Baylin, A.; Campos, H. Some dietary and adipose tissue carotenoids are associated with the risk of nonfatal acute myocardial infarction in Costa Rica. J. Nutr. 2005, 135, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, S.; Harats, D.; Salameh, F.; Lubish, T.; Harari, A.; Trau, H.; Shaish, A. 9-cis-rich beta-carotene powder of the alga Dunaliella reduces the severity of chronic plaque psoriasis: A randomized, double-blind, placebo-controlled clinical trial. J. Am. Coll Nutr. 2012, 31, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Shaish, A.; Harari, A.; Hananshvili, L.; Cohen, H.; Bitzur, R.; Luvish, T.; Ulman, E.; Golan, M.; Ben-Amotz, A.; Gavish, D.; et al. 9-cis beta-carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis 2006, 189, 215–221. [Google Scholar] [CrossRef]
- Rotenstreich, Y.; Belkin, M.; Sadetzki, S.; Chetrit, A.; Ferman-Attar, G.; Sher, I.; Harari, A.; Shaish, A.; Harats, D. Treatment with 9-cis beta-carotene-rich powder in patients with retinitis pigmentosa: A randomized crossover trial. JAMA Ophthalmol. 2013, 131, 985–992. [Google Scholar] [CrossRef]
- Tavridou, A.; Unwin, N.C.; Laker, M.F.; White, M.; Alberti, K.G. Serum concentrations of vitamins A and E in impaired glucose tolerance. Clin. Chim. Acta 1997, 266, 129–140. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J. Nutr. 2011, 141, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Abahusain, M.A.; Wright, J.; Dickerson, J.W.; de Vol, E.B. Retinol, alpha-tocopherol and carotenoids in diabetes. Eur. J. Clin. Nutr. 1999, 53, 630–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harari, A.; Melnikov, N.; Kandel Kfir, M.; Kamari, Y.; Mahler, L.; Ben-Amotz, A.; Harats, D.; Cohen, H.; Shaish, A. Dietary β-Carotene Rescues Vitamin A Deficiency and Inhibits Atherogenesis in Apolipoprotein E-Deficient Mice. Nutrients 2020, 12, 1625. https://doi.org/10.3390/nu12061625
Harari A, Melnikov N, Kandel Kfir M, Kamari Y, Mahler L, Ben-Amotz A, Harats D, Cohen H, Shaish A. Dietary β-Carotene Rescues Vitamin A Deficiency and Inhibits Atherogenesis in Apolipoprotein E-Deficient Mice. Nutrients. 2020; 12(6):1625. https://doi.org/10.3390/nu12061625
Chicago/Turabian StyleHarari, Ayelet, Nir Melnikov, Michal Kandel Kfir, Yehuda Kamari, Lidor Mahler, Ami Ben-Amotz, Dror Harats, Hofit Cohen, and Aviv Shaish. 2020. "Dietary β-Carotene Rescues Vitamin A Deficiency and Inhibits Atherogenesis in Apolipoprotein E-Deficient Mice" Nutrients 12, no. 6: 1625. https://doi.org/10.3390/nu12061625
APA StyleHarari, A., Melnikov, N., Kandel Kfir, M., Kamari, Y., Mahler, L., Ben-Amotz, A., Harats, D., Cohen, H., & Shaish, A. (2020). Dietary β-Carotene Rescues Vitamin A Deficiency and Inhibits Atherogenesis in Apolipoprotein E-Deficient Mice. Nutrients, 12(6), 1625. https://doi.org/10.3390/nu12061625



