Relationship between Selected Trace Elements and Hematological Parameters among Japanese Community Dwellers
Abstract
1. Introduction
2. Methods
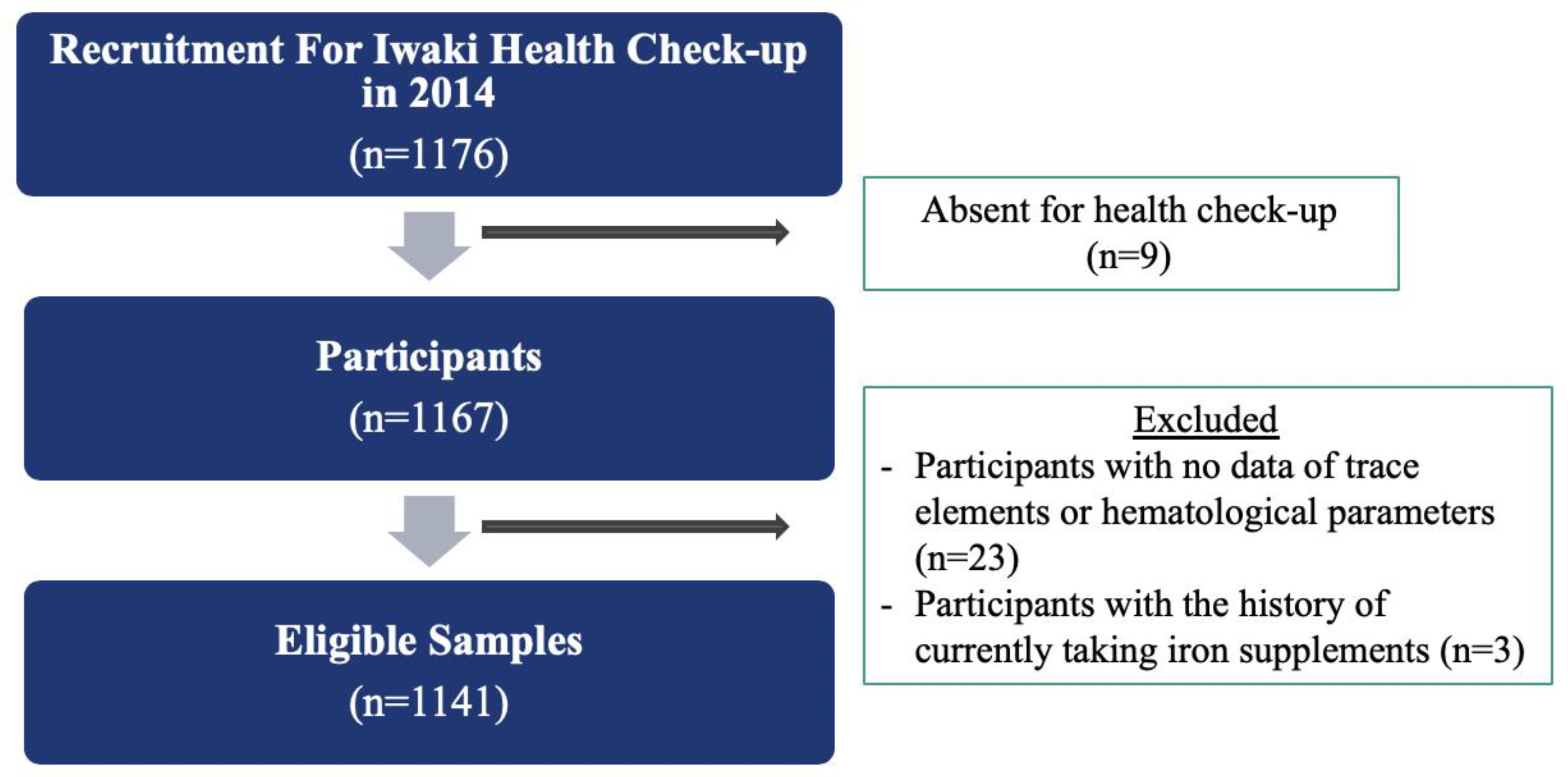
2.1. Study Design and Participants
2.2. Hematological Parameters and Serum Trace Elements Measurement
2.3. Covariates
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garnica, A.D. Trace metals and hemoglobin metabolism. Ann. Clin. Lab Sci. 1981, 11, 220–228. [Google Scholar] [PubMed]
- Janicka, M.; Binkowski, Ł.; Błaszczyk, M.; Paluch, J.; Wojtaś, W.; Massanyi, P.; Stawarz, R. Cadmium, lead and mercury concentrations and their influence on morphological parameters in blood donors from different age groups from southern Poland. J. Trace Elem. Med. Biol. 2015, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.O.; Ray, A.L.; Xue, Q.L.; Guralnik, J.M.; Fried, L.P. Low Serum Selenium Is Associated with Anemia among Older Women Living in the Community: The Women’s Health and Aging Studies I and II. Biol. Trace Elem. Res. 2006, 112, 97–107. [Google Scholar] [CrossRef]
- Chan, S.; Gerson, B.; Subramaniam, S. The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin. Lab Med. 1998, 18, 673–685. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68 (Suppl. 2), 442S–446S. [Google Scholar] [CrossRef]
- Gürgöze, M.K.; Olçücü, A.; Aygün, A.D.; Taskin, E.; Kiliç, M. Serum and hair levels of zinc, selenium, iron, and copper in children with iron-deficiency anemia. Biol. Trace Elem. Res. 2006, 111, 23–29. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Cobalt; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004.
- Angelova, M.G.; Petkova-Marinova, T.V.; Pogorielov, M.V.; Loboda, A.N.; Nedkova-Kolarova, V.N.; Bozhinova, A.N. Trace Element Status (Iron, Zinc, Copper, Chromium, Cobalt, and Nickel) in Iron-Deficiency Anaemia of Children under 3 Years. Anemia 2014, 2014, 718089. [Google Scholar] [CrossRef]
- Arredondo, M.; Núñez, M.T. Iron and copper metabolism. Mol. Aspects Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper deficiency anemia: Review article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Bárány, E.; Bergdahl, I.A.; Bratteby, L.E.; Lundh, T.; Samuelson, G.; Skerfving, S.; Oskarsson, A. Iron status influences trace element levels in human blood and serum. Environ. Res. 2005, 98, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.M.; Alexander, J.; Brantsæter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Thomassen, Y.; Holmen, J.; Ydersbond, T.A. The impact of iron status and smoking on blood divalent metal concentrations in Norwegian women in the HUNT2 Study. J. Trace Elem. Med. Biol. 2016, 38, 165–173. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Gallagher, C.M.; Chen, J.J.; Kovach, J.S. The relationship between body iron stores and blood and urine cadmium concentrations in US never-smoking, non-pregnant women aged 20–49 years. Environ. Res. 2011, 111, 702–707. [Google Scholar] [CrossRef]
- Turgut, S.; Polat, A.; Inan, M.; Turgut, G.; Emmungil, G.; Bican, M.; Karakus, T.Y.; Genc, O. Interaction between anemia and blood levels of iron, zinc, copper, cadmium and lead in children. Indian J. Pediatr. 2007, 74, 827–830. [Google Scholar] [CrossRef]
- Shah, F.; Kazi, T.G.; Afridi, H.I.; Kazi, N.; Baig, J.A.; Shah, A.Q.; Khan, S.; Kolachi, N.F.; Wadhwa, S.K. Evaluation of status of trace and toxic metals in biological samples (scalp hair, blood, and urine) of normal and anemic children of two age groups. Biol. Trace Elem. Res. 2011, 141, 131–149. [Google Scholar] [CrossRef]
- McLean, E.; McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Milman, N. Anemia—Still a major health problem in many parts of the world! Ann. Hematol. 2011, 90, 369–377. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrional Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar]
- Hegazy, A.A.; Zaher, M.M.; Abd El-Hafez, M.A.; Morsy, A.A.; Saleh, R.A. Relation between anemia and blood levels of lead, copper, zinc and iron among children. BMC Res. Notes 2010, 3, 133. [Google Scholar] [CrossRef]
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.M.; Brantsaeter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Grimalt, J.O.; Casas, M.; Sunyer, J. Interdependence between urinary cobalt concentrations and hemoglobin levels in pregnant women. Environ. Res. 2015, 136, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. Biometals 2003, 16, 41–54. [Google Scholar] [CrossRef]
- Semba, R.D.; Ricks, M.O.; Ferrucci, L.; Xue, Q.L.; Guralnik, J.M.; Fried, L.P. Low serum selenium is associated with anemia among older adults in the United States. Eur. J. Clin. Nutr. 2009, 63, 93–99. [Google Scholar] [CrossRef]
- Bates, C.J.; Thane, C.W.; Prentice, A.; Delves, H.T. Selenium status and its correlates in a British national diet and nutrition survey: People aged 65 years and over. J. Trace Elem. Med. Biol. 2002, 16, 1–8. [Google Scholar] [CrossRef]
- Mostert, V.; Hill, K.E.; Burk, R.F. Loss of activity of the selenoenzyme thioredoxin reductase causes induction of hepatic heme oxygenase-1. FEBS Lett. 2003, 541, 85–88. [Google Scholar] [CrossRef]
- Park, J.D.; Cherrington, N.J.; Klaassen, C.D. Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol. Sci. 2002, 68, 288–294. [Google Scholar] [CrossRef]
- Lindeman, R.D.; Clark, M.L.; Colmore, J.P. Influence of age and sex on plasma and red-cell zinc concentrations. J. Gerontol. 1971, 26, 358–363. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Lieberman, H.R.; Fulgoni, V.L.; McClung, J.P. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef]
- Chen, B.; Lamberts, L.V.; Behets, G.J.; Zhao, T.; Zhou, M.; Liu, G.; Hou, X.; Guan, G.; D’Haese, P.C. Selenium, lead, and cadmium levels in renal failure patients in China. Biol. Trace Elem. Res. 2009, 131, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.M.; Poulsen, O.M.; Thomsen, M. A short-term cross-over study on oral administration of soluble and insoluble cobalt compounds: Sex differences in biological levels. Int. Arch. Occup. Environ. Health 1993, 65, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Unice, K.M.; Monnot, A.D.; Gaffney, S.H.; Tvermoes, B.E.; Thuett, K.A.; Paustenbach, D.J.; Finley, B.L. Inorganic cobalt supplementation: Prediction of cobalt levels in whole blood and urine using a biokinetic model. Food Chem. Toxicol. 2012, 50, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Farooq, D.M.; Alamri, A.F.; Alwhahabi, B.K.; Metwally, A.M.; Kareem, K.A. The status of zinc in type 2 diabetic patients and its association with glycemic control. J. Family Community Med. 2020, 27, 29–36. [Google Scholar] [PubMed]
- Mladenka, P.; Zatloukalova, L.; Filipsky, T.; Vavrova, J.; Holeckova, M.; Palicka, V.; Hrdina, R. Common Biomarkers of Oxidative Stress Do Not Reflect Cardiovascular Dys/Function in Rats. Biomed. Pap. 2013, 157, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, S.; Saberi-Karimian, M.; Tayefi, M.; Tayefi, B.; Khashyarmanesh, Z.; Fereydouni, N.; Haghighi, H.M.; Mahmoudi, A.A.; Kharazmi-Khorassani, J.; Gonoodi, K.; et al. Association between Hypertension in Healthy Participants and Zinc and Copper Status: A Population-Based Study. Biol. Trace Elem. Res. 2019, 190, 38–44. [Google Scholar] [CrossRef] [PubMed]

| Variables | Number | Frequency (%) | Median | [IQR] |
|---|---|---|---|---|
| Age (years) | 1141 | 57 | [42–67] | |
| Sex | 1141 | |||
| Male | 433 | 37.9 | ||
| Female | 708 | 62.1 | ||
| Smoking Status | 1139 | |||
| Non-smokers | 728 | 63.9 | ||
| Current Smokers | 195 | 17.2 | ||
| Past Smokers | 216 | 18.9 | ||
| Alcohol Drinking | 1139 | |||
| Non-drinkers | 604 | 53.1 | ||
| Current Drinkers | 482 | 42.3 | ||
| Past Drinkers | 53 | 4.6 | ||
| Blood Pressure (mmHg) | 1141 | |||
| Systolic Blood Pressure | 128 | [115–143] | ||
| Diastolic Blood Pressure | 77 | [70–86] | ||
| Body Mass Index (kg/m2) | 1141 | 22.4 | [20.3–24.6] | |
| Fasting Blood Sugar (mg/dL) | 1141 | 79.0 | [74.0–87.0] |
| Variables | Male (n = 433) | Female (n = 708) | p-Value a |
|---|---|---|---|
| Serum Trace Elements Concentration (μg/L) | |||
| Cadmium | 0.06 | 0.07 | <0.001 |
| Zinc | 900.1 | 871.5 | 0.001 |
| Cobalt | 0.32 | 0.42 | <0.001 |
| Copper | 965.31 | 1061.69 | <0.001 |
| Selenium | 152.1 | 149.9 | 0.144 |
| Iron | 1096.1 | 946.8 | <0.001 |
| Anemia | <0.001 | ||
| No | 416 (36.5) | 587 (51.5) | |
| Yes | 17 (1.5) | 121 (10.6) | |
| Hematological Parameters | |||
| Hemoglobin (g/dL) | 14.9 | 12.8 | <0.001 |
| PCV (%) | 46.6 | 41.1 | <0.001 |
| MCV (fL) | 97.3 | 95.2 | <0.001 |
| MCH (pg) | 31.1 | 29.6 | <0.001 |
| RBCs Count (×104 μg/L) | 480.8 | 432.9 | <0.001 |
| Serum Trace Elements Concentration (μg/L) | Cadmium | Cobalt | Copper | Selenium | Zinc | Iron |
|---|---|---|---|---|---|---|
| Cadmium | 1 | |||||
| Cobalt | 0.137 *** | 1 | ||||
| Copper | 0.216 *** | 0.120 *** | 1 | |||
| Selenium | 0.023 | 0.130 *** | 0.288 *** | 1 | ||
| Zinc | 0.064 * | 0.123 *** | 0.157 *** | 0.396 *** | 1 | |
| Iron | −0.078 ** | −0.169 *** | −0.085 ** | 0.079 ** | 0.086 ** | 1 |
| Serum Trace Elements Concentrations (Log-Transformed) | Male (n = 433) | Female (n = 708) | All (n = 1141) | |||
|---|---|---|---|---|---|---|
| Beta † | (95% CI) | Beta † | (95% CI) | Beta ‡ | (95% CI) | |
| Cadmium | ||||||
| Hemoglobin (g%) | −0.02 | (−0.22, 0.19) | 0.10 | (−0.07, 0.27) | 0.05 | (−0.08, 0.18) |
| PCV (%) | −0.05 | (−0.65, 0.55) | 0.38 | (−0.10, 0.86) | 0.21 | (−0.17, 0.58) |
| MCV (fL) | 0.36 | (−0.49, 1.22) | 0.16 | (−0.66, 0.98) | 0.26 | (−0.35, 0.88) |
| MCH (pg) | 0.14 | (−0.16, 0.43) | 0.01 | (−0.28, 0.32) | 0.07 | (−0.16, 0.29) |
| RBCs Count (×104 μg/L) | −2.19 | (−9.19, 4.80) | 3.85 | (−2.09, 9.81) | 1.30 | (−3.25, 5.86) |
| Cobalt | ||||||
| Hemoglobin (g%) | −0.20 | (−0.58, 0.19) | −0.53 | (−0.75, −0.31) *** | −0.60 | (−0.78, −0.41) *** |
| PCV (%) | −0.62 | (−1.75, 0.52) | −1.17 | (−1.79, −0.54) *** | −1.41 | (−1.95, −0.88) *** |
| MCV (fL) | −0.71 | (−2.51, 1.93) | −2.07 | (−3.13, −1.02) *** | −2.29 | (−3.15, −1.42) *** |
| MCH (pg) | −0.21 | (−0.77, 0.35) | −1.02 | (−1.39, −0.64) *** | −1.05 | (−1.36, −0.74) *** |
| RBCs Count (×104 μg/L) | −3.78 | (−17.01, 9.53) | −1.87 | (−10.42, 2.54) | −3.94 | (−10.42, −0.30) * |
| Copper | ||||||
| Hemoglobin (g%) | 0.27 | (−0.23, 0.77) | −0.10 | (−0.52, 0.33) | −0.09 | (−0.42, 0.24) |
| PCV (%) | 1.21 | (−0.23, 2.66) | 0.12 | (−1.05, 1.30) | 0.18 | (−0.76, 1.11) |
| MCV (fL) | 1.60 | (−0.52, 3.72) | −0.31 | (−2.29, 1.67) | −0.13 | (−1.62, 1.35) |
| MCH (pg) | 0.19 | (−0.55, 0.94) | −0.37 | (−1.12, 0.38) | −0.37 | (−0.93, 0.19) |
| RBCs Count (×104 μg/L) | 5.49 | (−11.21, 22.19) | 1.93 | (−11.47, 15.33) | 1.86 | (−8.63, 12.36) |
| Selenium | ||||||
| Hemoglobin (g%) | 0.07 | (−0.48, 0.55) | 0.03 | (−0.32, 0.39) | 0.01 | (−0.27, 0.33) |
| PCV (%) | 1.33 | (−0.05, 2.20) | 0.80 | (−0.22, 1.82) | 1.04 | (0.17, 1.92) * |
| MCV (fL) | 0.66 | (−1.32, 2.64) | 1.06 | (−0.66, 2.80) | 0.50 | (−0.83, 1.85) |
| MCH (pg) | −0.21 | (−0.90, 0.48) | −0.17 | (−0.80, 0.46) | −0.35 | (−0.85, 0.13) |
| RBCs Count (×104 μg/L) | 5.23 | (−11.02, 21.47) | 3.42 | (−9.23, 16.07) | 5.28 | (−4.67, 15.22) |
| Zinc | ||||||
| Hemoglobin (g%) | 0.69 | (−0.50, 0.64) | 0.58 | (0.13, 1.01) ** | 0.42 | (0.07, 0.77) ** |
| PCV (%) | 0.91 | (−0.75, 2.57) | 2.18 | (0.92, 3.44) *** | 1.79 | (0.78, 2.81) *** |
| MCV (fL) | −0.47 | (−2.85, 1.90) | −0.31 | (−2.46, 1.84) | −0.27 | (−1.92, 0.24) |
| MCH (pg) | −0.57 | (−1.40, 0.25) | −0.29 | (−1.08, 0.48) | −0.36 | (−0.96, 0.24) |
| RBCs Count (×104 μg/L) | 11.09 | (−8.37, 30.56) | 23.86 | (8.26, 39.46) ** | 19.50 | (7.31, 31.69) ** |
| Serum Trace Elements Concentration (μg/L) | Crude OR | (95% CI) | Adjusted OR † | (95% CI) |
|---|---|---|---|---|
| Cadmium | ||||
| Quartile 1 (≤0.046) | ref | ref | ||
| Quartile 2 (0.046–0.062) | 1.30 | (0.80, 2.34) | 1.15 | (0.61, 2.15) |
| Quartile 3 (0.062–0.081) | 1.22 | (0.71, 2.12) | 0.81 | (0.42, 1.55) |
| Quartile 4 (≥0.081) | 1.86 | (1.12, 3.12) * | 1.05 | (0.55, 1.97) |
| Cobalt | ||||
| Quartile 1 (≤0.29) | ref | ref | ||
| Quartile 2 (0.29–0.34) | 1.11 | (0.58, 2.12) | 0.87 | (0.43, 1.73) |
| Quartile 3 (0.34–0.42) | 1.64 | (0.90, 2.98) | 1.12 | (0.59, 2.17) |
| Quartile 4 (≥0.42) | 4.46 | (2.60, 7.66) *** | 1.95 | (1.04, 3.67) * |
| Copper | ||||
| Quartile 1 (≤896.90) | ref | ref | ||
| Quartile 2 (896.90–1004.00) | 1.08 | (0.60, 1.92) | 0.83 | (0.45, 1.55) |
| Quartile 3 (1004.00–1131.53) | 2.18 | (1.29, 3.67) ** | 1.72 | (0.98, 3.03) |
| Quartile 4 (≥1131.53) | 1.89 | (1.10, 3.21) * | 1.19 | (0.66, 2.13) |
| Selenium | ||||
| Quartile 1 (≤129.86) | ref | ref | ||
| Quartile 2 (129.86–148.50) | 0.76 | (0.46, 1.27) | 0.78 | (0.44, 1.38) |
| Quartile 3 (148.50–170.68) | 0.97 | (0.59, 1.58) | 1.09 | (0.63, 1.91) |
| Quartile 4 (≥170.68) | 0.96 | (0.69, 1.61) | 1.17 | (0.67, 2.04) |
| Zinc | ||||
| Quartile 1 (≤788.87) | ref | ref | ||
| Quartile 2 (788.87–871.86) | 0.53 | (0.33, 0.86) * | 0.52 | (0.30, 0.89) * |
| Quartile 3 (871.86–965.82) | 0.65 | (0.41, 1.03) | 0.86 | (0.51, 1.46) |
| Quartile 4 (≥965.82) | 0.34 | (0.20, 0.58) *** | 0.42 | (0.23, 0.76) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wai, K.M.; Sawada, K.; Kumagai, M.; Itai, K.; Tokuda, I.; Murashita, K.; Nakaji, S.; Ihara, K. Relationship between Selected Trace Elements and Hematological Parameters among Japanese Community Dwellers. Nutrients 2020, 12, 1615. https://doi.org/10.3390/nu12061615
Wai KM, Sawada K, Kumagai M, Itai K, Tokuda I, Murashita K, Nakaji S, Ihara K. Relationship between Selected Trace Elements and Hematological Parameters among Japanese Community Dwellers. Nutrients. 2020; 12(6):1615. https://doi.org/10.3390/nu12061615
Chicago/Turabian StyleWai, Kyi Mar, Kaori Sawada, Mika Kumagai, Kazuyoshi Itai, Itoyo Tokuda, Koichi Murashita, Shigeyuki Nakaji, and Kazushige Ihara. 2020. "Relationship between Selected Trace Elements and Hematological Parameters among Japanese Community Dwellers" Nutrients 12, no. 6: 1615. https://doi.org/10.3390/nu12061615
APA StyleWai, K. M., Sawada, K., Kumagai, M., Itai, K., Tokuda, I., Murashita, K., Nakaji, S., & Ihara, K. (2020). Relationship between Selected Trace Elements and Hematological Parameters among Japanese Community Dwellers. Nutrients, 12(6), 1615. https://doi.org/10.3390/nu12061615





