Textural Changes by Mastication and Proper Food Texture for Patients with Oropharyngeal Dysphagia
Abstract
1. Introduction
2. Masticatory Function and Assessment of Its Components
2.1. Masticatory Behavior and Its Paradigmatic Model
2.1.1. Process Model of Feeding
2.1.2. Kinematic Linkage in Mastication
2.1.3. Stage II Transport
2.1.4. Factors Influencing Masticatory Performance
2.1.5. Factors Initiating the Pharyngeal Swallow
2.2. Assessment of Oral Function
2.2.1. Oral Hypofunction in Older Adults
2.2.2. Assessment of Oral Function
3. Proper Modification of Food Texture for Oropharyngeal Dysphagia
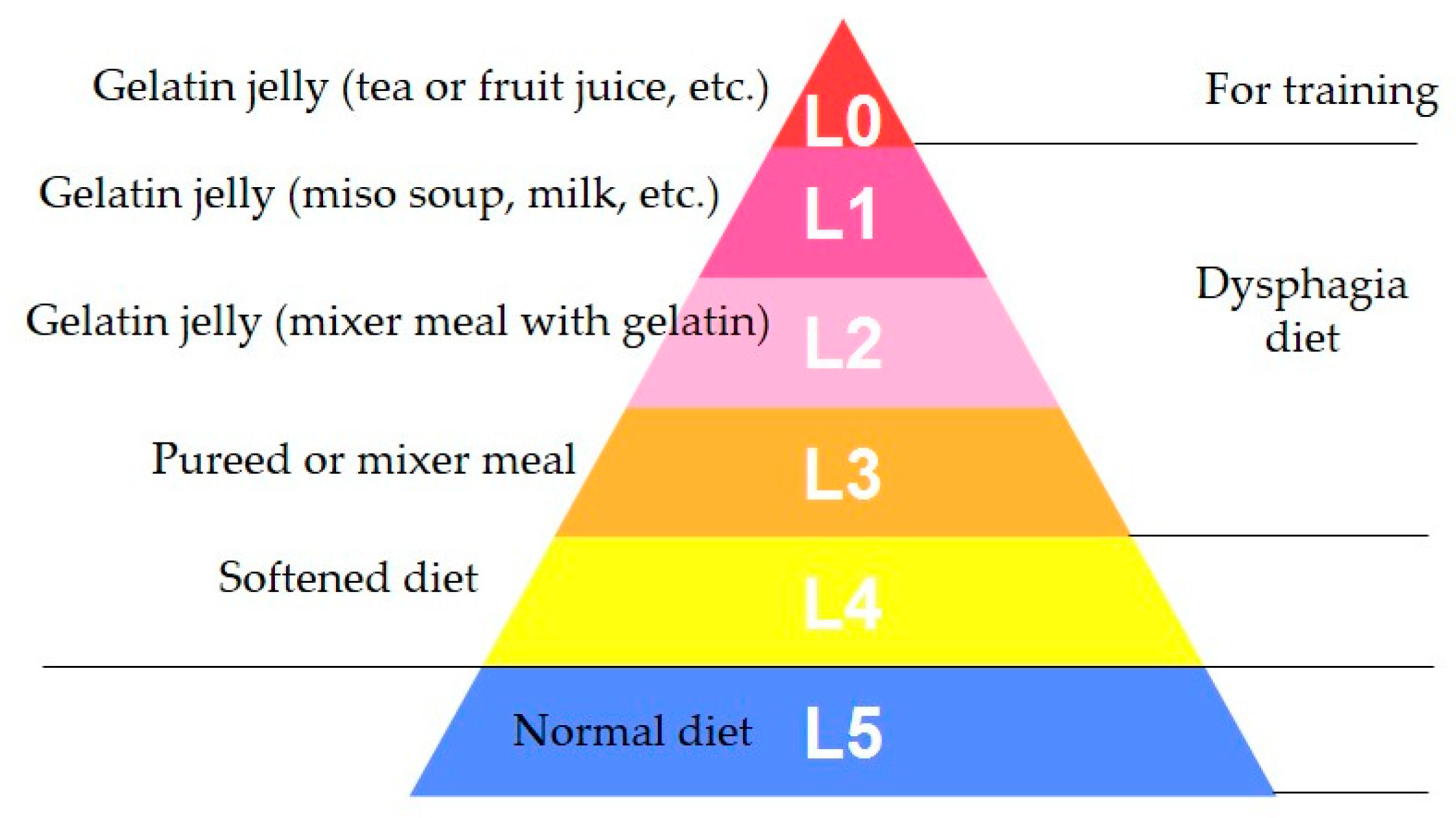
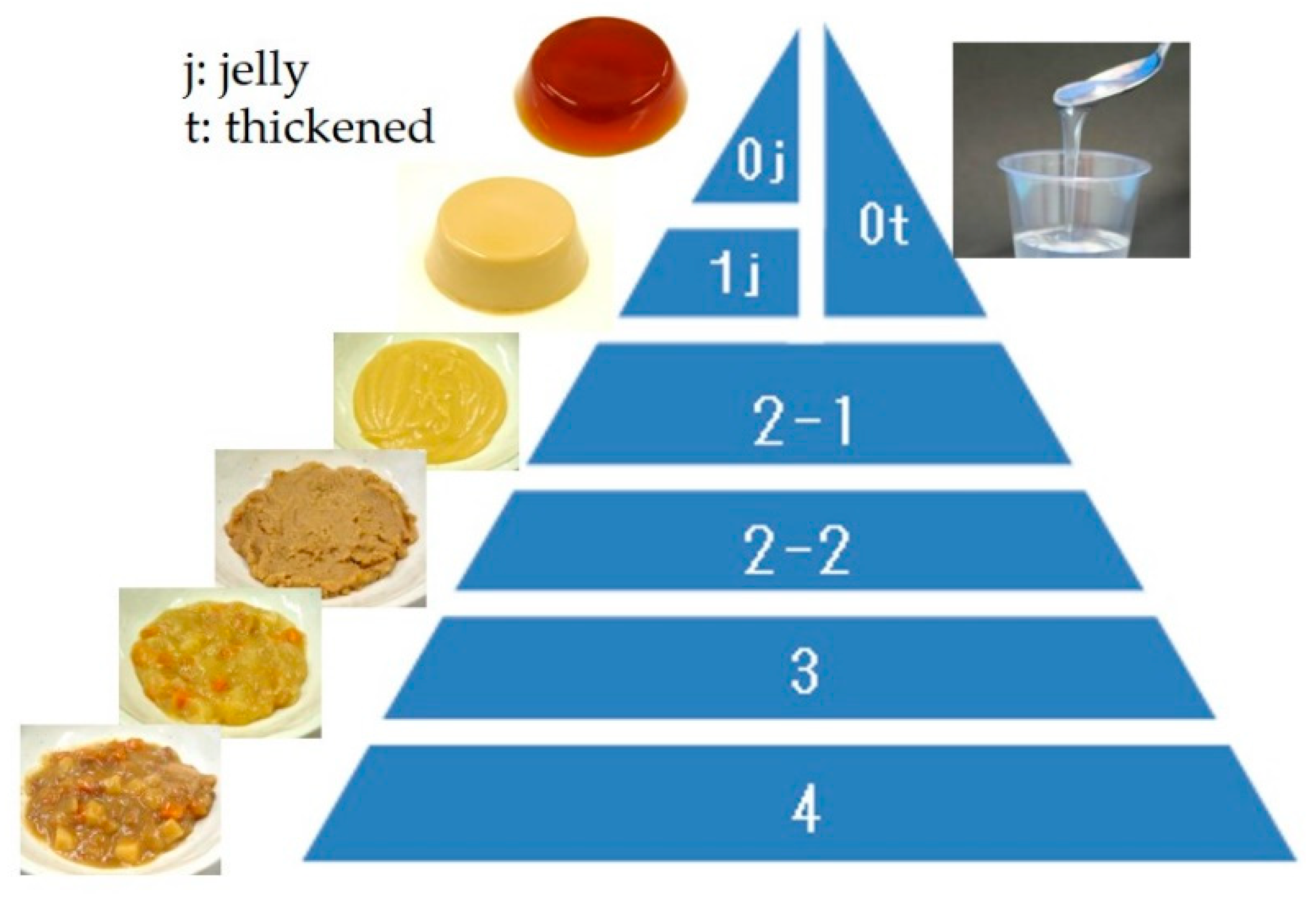
3.1. Standardized Texture Levels
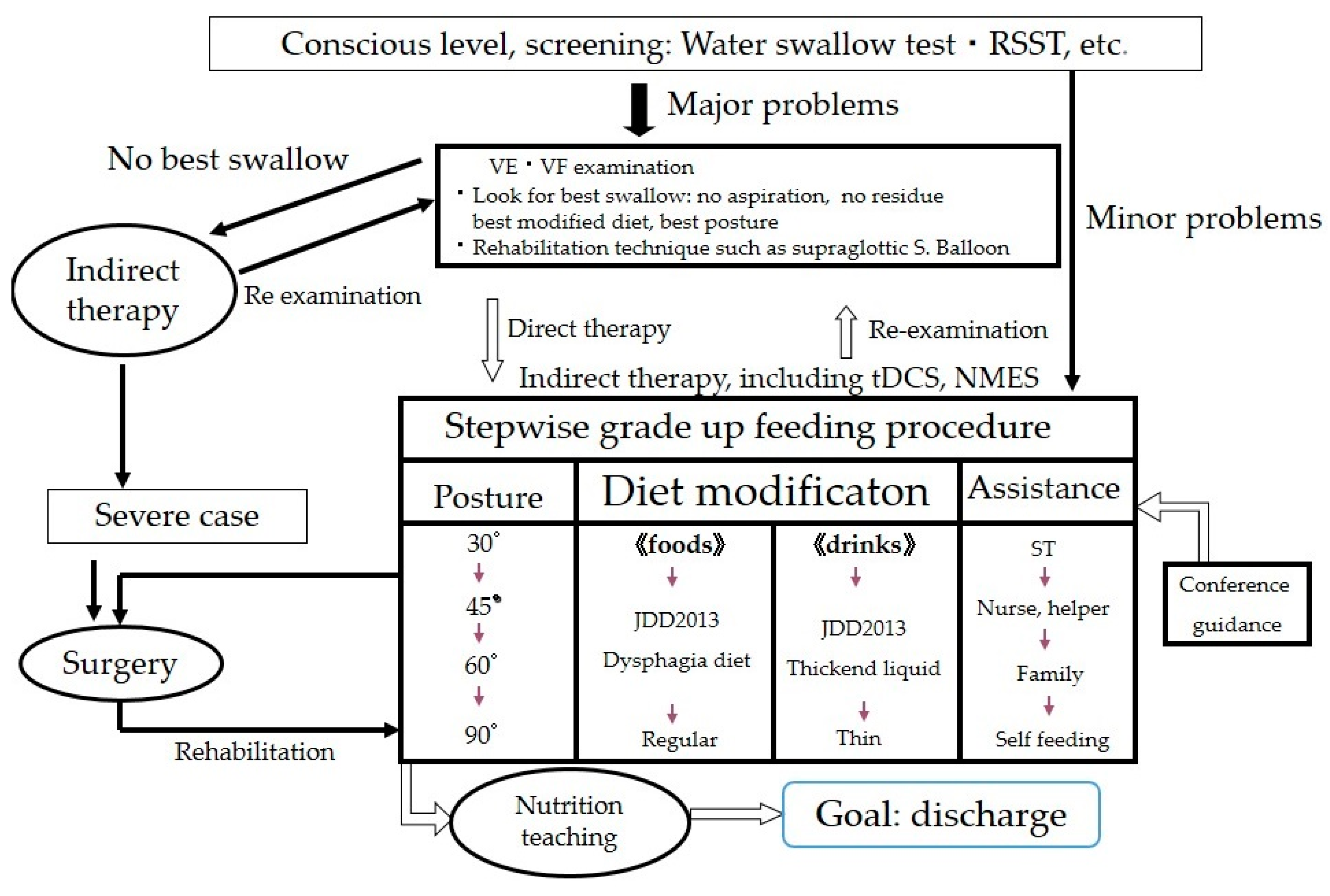
3.2. Assessment of Proper Texture for Patients with Oral Dysphagia
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cichero, J.A.; Steele, C.; Duivestein, J.; Clave, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, P.; et al. The Need for International Terminology and Definitions for Texture-Modified Foods and Thickened Liquids Used in Dysphagia Management: Foundations of a Global Initiative. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 280–291. [Google Scholar] [CrossRef]
- Cichero, J.A.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J.; et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, I. Rehabilitation for Swallowing Disorders Associated with Stroke; Ishiyaku Publishers, Inc.: Tokyo, Japan, 1993; pp. 81–86. [Google Scholar]
- Matsuo, K.; Palmer, J.B. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Metani, H.; Mays, K.A.; Palmer, J.B. Effects of respiration on soft palate movement in feeding. J. Dent. Res. 2010, 89, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Palmer, J.B. Kinematic linkage of the tongue, jaw, and hyoid during eating and speech. Arch. Oral. Biol. 2010, 55, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Hiiemae, K.M.; Palmer, J.B. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 1999, 14, 31–42. [Google Scholar] [CrossRef]
- Matsuo, K.; Hiiemae, K.M.; Palmer, J.B. Cyclic motion of the soft palate in feeding. J. Dent. Res. 2005, 84, 39–42. [Google Scholar] [CrossRef]
- Hodgson, M.; Linforth, R.S.; Taylor, A.J. Simultaneous real-time measurements of mastication, swallowing, nasal airflow, and aroma release. J. Agric. Food Chem. 2003, 51, 5052–5057. [Google Scholar] [CrossRef]
- Palmer, J.B.; Hiiemae, K.M.; Liu, J. Tongue-jaw linkages in human feeding: A preliminary videofluorographic study. Arch. Oral. Biol. 1997, 42, 429–441. [Google Scholar] [CrossRef]
- Mioche, L.; Hiiemae, K.M.; Palmer, J.B. A postero-anterior videofluorographic study of the intra-oral management of food in man. Arch. Oral. Biol. 2002, 47, 267–280. [Google Scholar] [CrossRef]
- Taniguchi, H.; Matsuo, K.; Okazaki, H.; Yoda, M.; Inokuchi, H.; Gonzalez-Fernandez, M.; Inoue, M.; Palmer, J.B. Fluoroscopic evaluation of tongue and jaw movements during mastication in healthy humans. Dysphagia 2013, 28, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Metani, H.; Mays, K.A.; Palmer, J.B. Tempospatial Linkage of soft palate and jaw movements in feeding. Jpn. J. Dysphagia Rehabil. 2008, 12, 20–30. (In Japanese) [Google Scholar]
- Saitoh, E.; Shibata, S.; Matsuo, K.; Baba, M.; Fujii, W.; Palmer, J.B. Chewing and food consistency: Effects on bolus transport and swallow initiation. Dysphagia 2007, 22, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.B. Bolus aggregation in the oropharynx does not depend on gravity. Arch. Phys. Med. Rehabil. 1998, 79, 691–696. [Google Scholar] [CrossRef]
- Peyron, M.A.; Woda, A.; Bourdiol, P.; Hennequin, M. Age-related changes in mastication. J. Oral. Rehabil. 2017, 44, 299–312. [Google Scholar] [CrossRef]
- Fontijn-Tekamp, F.A.; van der Bilt, A.; Abbink, J.H.; Bosman, F. Swallowing threshold and masticatory performance in dentate adults. Physiol. Behav. 2004, 83, 431–436. [Google Scholar] [CrossRef]
- Van der Bilt, A.; Olthoff, L.W.; Bosman, F.; Oosterhaven, S.P. The effect of missing postcanine teeth on chewing performance in man. Arch. Oral. Biol. 1993, 38, 423–429. [Google Scholar] [CrossRef]
- Hatch, J.P.; Shinkai, R.S.; Sakai, S.; Rugh, J.D.; Paunovich, E.D. Determinants of masticatory performance in dentate adults. Arch. Oral. Biol. 2001, 46, 641–648. [Google Scholar] [CrossRef]
- Wayler, A.H.; Muench, M.E.; Kapur, K.K.; Chauncey, H.H. Masticatory performance and food acceptability in persons with removable partial dentures, full dentures and intact natural dentition. J. Gerontol. 1984, 39, 284–289. [Google Scholar] [CrossRef]
- Yven, C.; Bonnet, L.; Cormier, D.; Monier, S.; Mioche, L. Impaired mastication modifies the dynamics of bolus formation. Eur. J. Oral. Sci. 2006, 114, 184–190. [Google Scholar] [CrossRef]
- Bourdiol, P.; Mioche, L. Correlations between functional and occlusal tooth-surface areas and food texture during natural chewing sequences in humans. Arch. Oral. Biol. 2000, 45, 691–699. [Google Scholar] [CrossRef]
- Engelen, L.; van den Keybus, P.A.; de Wijk, R.A.; Veerman, E.C.; Amerongen, A.V.; Bosman, F.; Prinz, J.F.; van der Bilt, A. The effect of saliva composition on texture perception of semi-solids. Arch. Oral. Biol. 2007, 52, 518–525. [Google Scholar] [CrossRef]
- Ship, J.A.; Pillemer, S.R.; Baum, B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002, 50, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Woda, A.; Foster, K.; Mishellany, A.; Peyron, M.A. Adaptation of healthy mastication to factors pertaining to the individual or to the food. Physiol. Behav. 2006, 89, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Prinz, J.F.; Lucas, P.W. An optimization model for mastication and swallowing in mammals. Proc. R. Soc. Lond. B Biol. Sci. 1997, 264, 1715–1721. [Google Scholar] [CrossRef]
- Matsuo, K.; Kawase, S.; Wakimoto, N.; Iwatani, K.; Masuda, Y.; Ogasawara, T. Effect of viscosity on food transport and swallow initiation during eating of two-phase food in normal young adults: A pilot study. Dysphagia 2013, 28, 63–68. [Google Scholar] [CrossRef]
- Minakuchi, S.; Tsuga, K.; Ikebe, K.; Ueda, T.; Tamura, F.; Nagao, K.; Furuya, J.; Matsuo, K.; Yamamoto, K.; Kanazawa, M.; et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology 2018, 35, 317–324. [Google Scholar] [CrossRef]
- Hamada, R.; Suehiro, J.; Nakano, M.; Kikutani, T.; Konishi, K. Development of rapid oral bacteria detection apparatus based on dielectrophoretic impedance measurement method. IET Nanobiotechnol. 2011, 5, 25–31. [Google Scholar] [CrossRef]
- Fukushima, Y.; Yoda, T.; Kokabu, S.; Araki, R.; Murata, T.; Kitagawa, Y.; Omura, K.; Toya, S.; Ito, K.; Funayama, S. Evaluation of an oral moisture-checking device for screening dry mouth. Open J. Stomatol. 2013, 3, 440. [Google Scholar] [CrossRef]
- Suzuki, T.; Kumagai, H.; Watanabe, T.; Uchida, T.; Nagao, M. Evaluation of complete denture occlusal contacts using pressure-sensitive sheets. Int. J. Prosthodont. 1997, 10, 386–391. [Google Scholar]
- Yamada, A.; Kanazawa, M.; Komagamine, Y.; Minakuchi, S. Association between tongue and lip functions and masticatory performance in young dentate adults. J. Oral Rehabil. 2015, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Utanohara, Y.; Hayashi, R.; Yoshikawa, M.; Yoshida, M.; Tsuga, K.; Akagawa, Y. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia 2008, 23, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, H.; Shiga, H. Relationship between masticatory performance using a gummy jelly and masticatory movement. J. Prosthodont. Res. 2017, 61, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, M.; Takagi, D.; Katagiri, N.; Fujishima, I.; Ohno, T. Penetration/Aspiration and Pharyngeal Residues among Different Types of Food Evaluated via a Videofluoroscopic Examination of Swallowing. Deglutition 2017, 6, 196–201. (In Japanese) [Google Scholar]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- The Dysphagia Diet Committee of the Japanese Society of Dysphagia Rehabilitation. The Japanese Dysphagia diet 2013. Jpn. J. Dysphagia Rehabil. 2013, 17, 255–267. (In Japanese) [Google Scholar]
- Sakai, M.; Egashira, F.; Kanaya, S.; Kayashita, J. Staged physical property assessment of dysphagia diet. Nihon Byoutai Eiyo Gakkaishi (J. Metab. Clin. Nutr.) 2007, 10, 269–279. (In Japanese) [Google Scholar]
- Sakai, M.; Egashira, F.; Kanaya, S.; Kayashita, J. Comparison of food physical properties with reference to clinically effective stepwise swallowing foods. J. Jpn. Soc. Dysphagia Rehabil. 2006, 10, 239–248. (In Japanese) [Google Scholar]
- Watanabe, E.; Yamagata, Y.; Fujitani, J.; Fujishima, I.; Takahashi, K.; Uyama, R.; Ogoshi, H.; Kojo, A.; Maeda, H.; Ueda, K.; et al. The Criteria of Thickened Liquid for Dysphagia Management in Japan. Dysphagia 2018, 33, 26–32. [Google Scholar] [CrossRef]
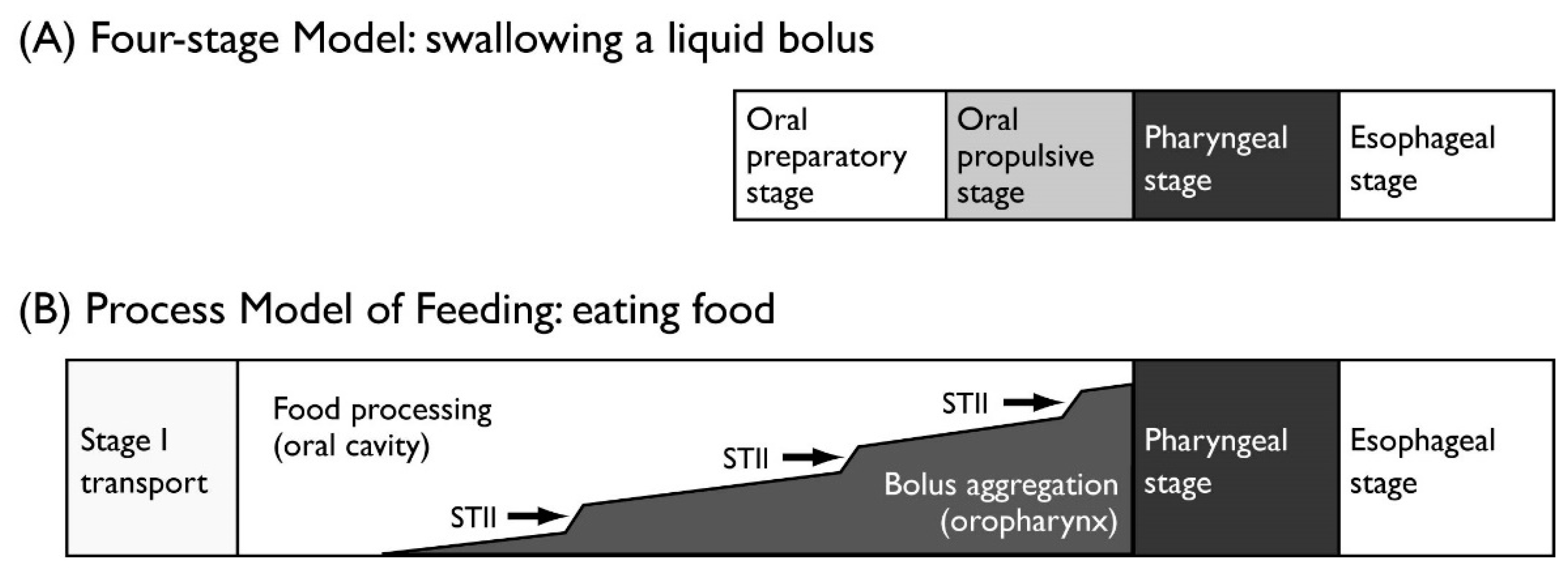
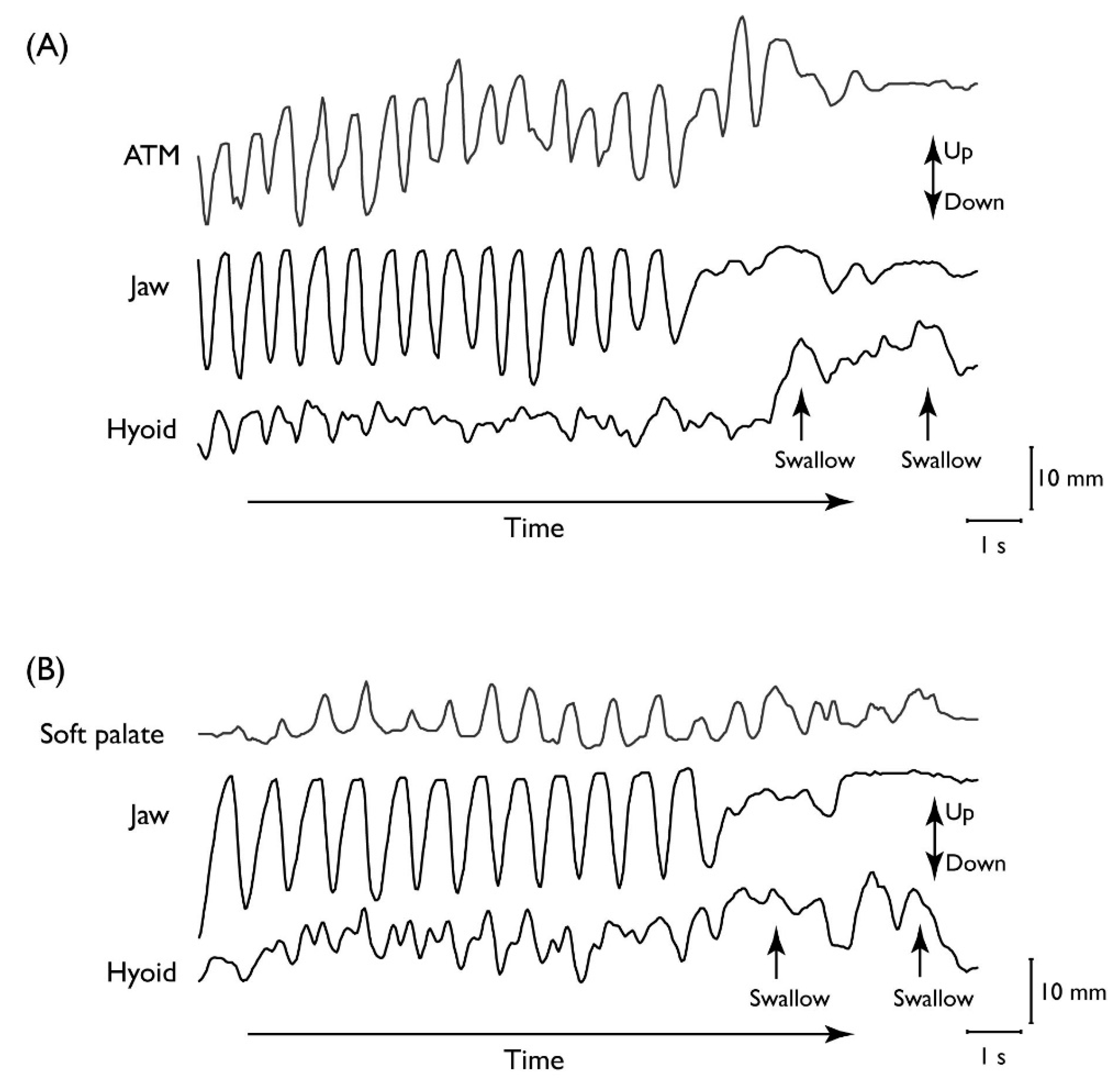
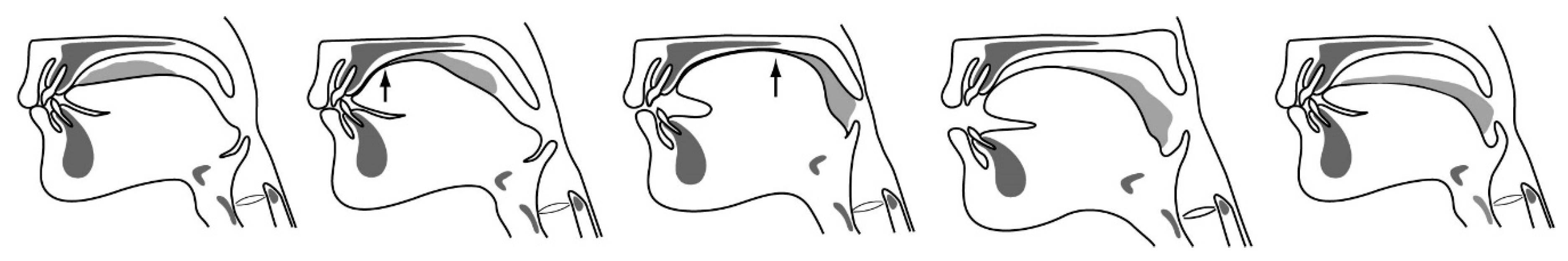




| Stage | Description | |
|---|---|---|
| Four-stage Model for liquids | ||
| Oral preparatory stage | A liquid bolus is held in the mouth. | |
| Oral propulsive stage | The bolus is propelled to the pharynx. | |
| Pharyngeal stage | The bolus is transited to the esophagus through the UES. | |
| Esophageal stage | The bolus is transported in the esophagus by peristalsis. | |
| Process Model of Feeding for solid food | ||
| Stage I transport | Ingested food is transported to the molar region. | |
| Processing | The food is reduced in size and a bolus is formed with saliva. | |
| Stage II transport | The formed bolus is transported to the oropharynx and collected until swallow onset. | |
| Swallowing | The bolus is transited to the esophagus through the UES. | |
| Influence Factor | Effect on Masticatory Function |
|---|---|
| Internal factors | |
| Loss of post-canine teeth, | Increased number of chewing strokes. |
| reduced area of functional | Larger particle size of the swallowed bolus. |
| occlusal contact, or | |
| use of dentures | |
| Age or gender | Little effect on masticatory performance. |
| External factors | |
| Hardness of food | Increased number of chewing strokes. |
| Longer duration of chewing and pharyngeal aggregation. | |
| Dryness of food | Increased chewing duration, more saliva. |
| Oral Sub-Symptom | Cut-Off Criterion |
|---|---|
| Oral hygiene | Total number of bacteria > 106.5 CFU/mL. |
| Oral dryness | Measured value with a moisture checker < 27.0. |
| Occlusal force | Occlusal force < 200 N. |
| Tongue-lip motor function | Utterance count of /pa/, /ta/, or /ka/ < 6/s. |
| Tongue pressure | Maximum tongue pressure < 30 kPa. |
| Masticatory function | Glucose concentration in chewing test < 100 mg/dL. |
| Swallowing function | Total Eating Assessment Tool (EAT-10) score ≥ 3. |
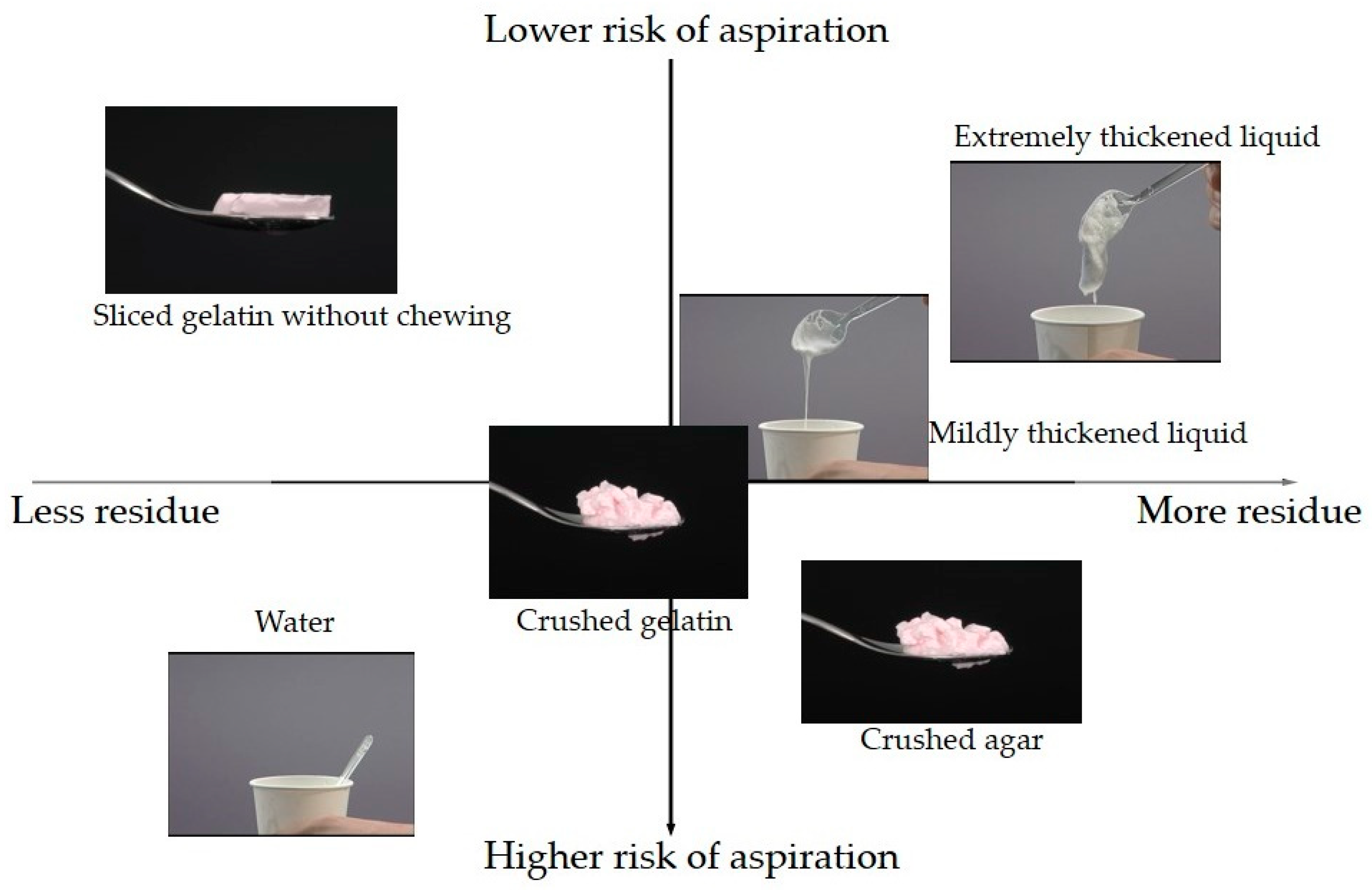
| Object 1 | Object 2 | N | p-Value | Frequent Object |
|---|---|---|---|---|
| Sliced gelatin without chewing | Crushed gelatin | 37 | 0.000 ** | Crushed gelatin |
| Extremely thickened liquid | 85 | 0.201 | ||
| Mildly thickened liquid | 69 | 0.700 | ||
| Water | 39 | 0.000 ** | Water | |
| Crushed agar | 63 | 0.019 * | Crushed agar | |
| Crushed gelatin | Extremely thickened liquid | 62 | 0.004 * | Crushed gelatin |
| Mildly thickened liquid | 54 | 0.267 | ||
| Water | 33 | 0.001 ** | Water | |
| Crushed agar | 48 | 0.805 | ||
| Extremely thickened liquid | Mildly thickened liquid | 89 | 0.008 * | Mildly thickened liquid |
| Water | 52 | 0.000 ** | Water | |
| Crushed agar | 80 | 0.001 ** | Crushed agar | |
| Mildly thickened liquid | Water | 51 | 0.000 ** | Water |
| Crushed agar | 77 | 0.138 | ||
| Water | Crushed agar | 50 | 0.000 ** | Water |
| Object 1 | Object 2 | N | p-Value | Less Object |
|---|---|---|---|---|
| Sliced gelatin without chewing | Crushed gelatin | 37 | 0.104 | |
| Extremely thickened liquid | 85 | 0.002 ** | Sliced gelatin without chewing | |
| Mildly thickened liquid | 69 | 0.011 * | Sliced gelatin without chewing | |
| Water | 39 | 0.631 | ||
| Crushed agar | 63 | 0.000 ** | Sliced gelatin without chewing | |
| Crushed gelatin | Extremely thickened liquid | 62 | 0.850 | |
| Mildly thickened liquid | 54 | 0.850 | ||
| Water | 33 | 0.044 * | Water | |
| Crushed agar | 48 | 0.157 | ||
| Extremely thickened liquid | Mildly thickened liquid | 89 | 0.758 | |
| Water | 52 | 0.002 ** | Water | |
| Crushed agar | 80 | 0.050 | ||
| Mildly thickened liquid | Water | 51 | 0.017 * | Water |
| Crushed agar | 77 | 0.021 * | Mildly thickened liquid | |
| Water | Crushed agar | 50 | 0.000 ** | Water |
| Dysphagia Pyramid: Level | 0 | 1,2 | 3 | 4 |
|---|---|---|---|---|
| JDD2013: Code | 0j | 1j | 2-1, 2-2 | 3, 4 |
| Hardness (N/m2) | 2000–7000 | 0–12,000 | ≤15,000 | ≤40,000 |
| Cohesiveness | 0.2–0.5 | 0.2–0.7 | 0.2–0.9 | 0–1.0 |
| Adhesiveness (J/m3) | ≤200 | ≤300 | ≤1000 | ≤1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, K.; Fujishima, I. Textural Changes by Mastication and Proper Food Texture for Patients with Oropharyngeal Dysphagia. Nutrients 2020, 12, 1613. https://doi.org/10.3390/nu12061613
Matsuo K, Fujishima I. Textural Changes by Mastication and Proper Food Texture for Patients with Oropharyngeal Dysphagia. Nutrients. 2020; 12(6):1613. https://doi.org/10.3390/nu12061613
Chicago/Turabian StyleMatsuo, Koichiro, and Ichiro Fujishima. 2020. "Textural Changes by Mastication and Proper Food Texture for Patients with Oropharyngeal Dysphagia" Nutrients 12, no. 6: 1613. https://doi.org/10.3390/nu12061613
APA StyleMatsuo, K., & Fujishima, I. (2020). Textural Changes by Mastication and Proper Food Texture for Patients with Oropharyngeal Dysphagia. Nutrients, 12(6), 1613. https://doi.org/10.3390/nu12061613





