Selenium Deficiency Is Widespread and Spatially Dependent in Ethiopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Analysis
2.2.1. Socio-Demography
2.2.2. Collection, Processing and Analysis of Serum Se
2.2.3. Data Analysis
2.3. Ethical Approval
3. Results
Characteristics of Study Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Senevirante, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.P.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 1, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Gashu, D.; Stocker, B.J. Selenium and Cognition: Mechanism and Evidence. In Handbook of Famine, Starvation, and Nutrient Deprivation; Preedy, V., Patel, V., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar]
- Joy, E.J.M.; Ander, E.L.; Young, S.D.; Black, C.R.; Watts, M.J.; Chilimba, A.D.C.; Chilima, B.; Siyame, E.W.P.; Kalimbira, A.A.; Hurst, R.; et al. Dietary mineral supplies in Africa. Physiol. Plant. 2014, 151, 208–229. [Google Scholar] [CrossRef]
- Ligowe, I.S.; Phiri, F.P.; Ander, E.L.; Bailey, E.H.; Chilimba, A.D.C.; Gashu, D.; Joy, E.J.M.; Lark, R.M.; Kabambe, V.; Kalimbira, A.A.; et al. Selenium (Se) deficiency risks in sub-Saharan African food systems and their geospatial linkages. Proc. Nutr. Soc. 2020. [Google Scholar] [CrossRef]
- Phiri, F.P.; Ander, E.L.; Bailey, E.H.; Chilima, B.; Chilimba, A.D.C.; Joy, E.J.M.; Kalimbira, A.A.; Kumssa, D.B.; Lark, R.M.; Phuka, J.C.; et al. The risk of selenium deficiency in Malawi is large and varies over multiple spatial scales. Sci. Rep. 2019, 9, 6566. [Google Scholar] [CrossRef]
- Hurst, R.; Siyame, E.W.P.; Young, S.D.; Chilimba, A.D.C.; Joy, E.J.M.; Black, C.R.; Ander, E.L.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. Soil-type influences human selenium status and underlies widespread selenium deficiency risks in Malawi. Sci. Rep. 2013, 3, 1425. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium geochemistry and health. AMBIO 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Rogerson, K.B.; Ander, E.L.; Watts, M.J.; Lammel, J.; Broadley, M.R. Maize grain and soil surveys revael suboptimal dietrary selenium intake is widespread in Malawi. Sci. Rep. 2011, 1, 72. [Google Scholar] [CrossRef]
- Gashu, D.; Stoecker, B.J.; Adish, A.; Haki, G.D.; Bougma, K.; Aboud, F.E.; Marquis, G.S. Association of serum selenium with thyroxin in severely iodine-deficient young children from the Amhara region of Ethiopia. Eur. J. Clin. Nutr. 2016, 70, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Tekeste, Z.; Amare, B.; Asfaw, F.; Fantahun, B.; van Nguyen, N.V.; Nishikawa, T.; Yabutani, T.; Okayasu, T.; Ota, F.; Kassu, A. Determination of trace elements in Ethiopian, Vietnamese, and Japanese women using high-resolution IC-PMS. Nutrition 2015, 31, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Amare, B.; Moges, B.; Fantahun, B.; Tafess, K.; Woldeyohannes, D.; Yismaw, G.; Ayane, T.; Yabutani, T.; Mulu, A.; Ota, F.; et al. Micronutrient levels and nutritional status of school children living in Northwest Ethiopia. Nutr. J. 2012, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; World Bank; World Food Program; USAID ENGIN. Ethiopian National Micronutrient Survey Report. 2016. Available online: https://www.ephi.gov.et/images/pictures/download2009/National_MNS_report.pdf (accessed on 22 April 2019).
- Tessema, M.; De Groote, H.; Brouwer, I.D.; Feskens, E.J.M.; Belachew, T.; Zerfu, D.; Belay, A.; Demelash, Y.; Gunaratna, N.S. Soil zinc is associated with serum zinc but not with linear growth of children in Ethiopia. Nutrients 2019, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Population Census Commission. Summary and Statistical Report of the 2007 Population and Housing Census. Population Size by Age and Sex; Population Census Commission: Addis Ababa, Ethiopia, 2008.
- WHO. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; WHO: Geneva, Switzerland, 2010.
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Lark, R.M. A comparison of some robust estimators of the variogram for use in soil survey. Eur. J. Soil Sci. 2000, 51, 137–157. [Google Scholar] [CrossRef]
- Hijmans, R.J. Geosphere: Spherical Trigonometry. R Package Version 1.5-7. 2017. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 22 April 2020).
- Gneiting, T. Strictly and non-strictly positive definite functions on spheres. Bernoulli 2013, 19, 1327–1349. [Google Scholar] [CrossRef]
- Matheron, G. Traité de Géostatistique Appliqué, Tome 1. Memoires du Bureau de Recherches Géologiques et Minières; Technip: Paris, France, 1962. [Google Scholar]
- Dowd, P.A. The variogram and kriging: Robust and resistant estimators. In Geostatistics for Natural Resources Characterization; Verly, G., David, M., Journel, A.G., Marechal, A., Eds.; Reidel: Dordrecht, The Netherlands, 1984; pp. 91–106. [Google Scholar]
- Cressie, N.; Hawkins, D.M. Robust estimation of the variogram. Math. Geol. 1980, 12, 115–125. [Google Scholar] [CrossRef]
- Oliver, M.A.; Webster, R. Basic Steps in Geostatistics: The Variogram and Kriging; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Tadayon, V.; Torabi, M. Spatial models for non-Gaussian data with covariate measurement error. Environmetrics 2018, 30, e2513. [Google Scholar] [CrossRef]
- Gashu, D.; Lark, R.M.; Milne, A.E.; Amede, T.; Bailey, E.H.; Chagumaira, C.; Dunham, S.J.; Gameda, S.; Kumssa, D.B.; Mossa, A.W.; et al. Spatial prediction of the concentration of selenium (Se) in grain across part of Amhara Region, Ethiopia. Sci. Total Environ. 2020, 733, 139231. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistics for Natural Resource Evaluation; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Mastrandrea, M.D.; Field, C.B.; Stocker, T.F.; Edenhofer, O.; Ebi, K.L.; Frame, D.J.; Held, H.; Kriegler, E.; Mach, K.J.; Matschoss, P.R.; et al. Guidance Note for Lead Authors of the IPCC Fifth Assessment Report on Consistent Treatment of Uncertainties; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2010. Available online: https://www.ipcc.ch/site/assets/uploads/2017/08/AR5_Uncertainty_Guidance_Note (accessed on 20 May 2020).
- Lark, R.M.; Ander, E.L.; Cave, M.R.; Knights, K.V.; Glennon, M.M.; Scanlon, R.P. Mapping trace element deficiency by cokriging from regional geochemical soil data: A case study on cobalt for grazing sheep in Ireland. Geoderma 2014, 226, 64–78. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Gashu, D.; Marquis, G.S.; Bougma, K.; Stoecker, B.J. Spatial variation of human selenium in Ethiopia. Biol. Trace Elem. Res. 2019, 189, 354–360. [Google Scholar] [CrossRef]
- Maehira, F.; Luyo, G.A.; Miyagi, I.; Oshiro, M.; Yamane, N.; Kuba, M.; Nakazato, Y. Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin. Chim. Acta. 2002, 316, 137–146. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Chenal, S.; Arnault, N.; Favier, A.; Roussel, A.M. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Central Statistical Agency of Ethiopia. Household Consumption and Expenditure Survey 2010/11: Analytical Report. Statistical Bulletin 563; Central Statistical Agency of Ethiopia: Addis Ababa, Ethiopia, 2012.
- Floor, G.H.; Román-Ross, G. Selenium in volcanic environments: A review. Appl. Geochem. 2012, 27, 517–531. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop. Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef]
- Kafai, M.R.; Ganji, V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: Third National Health and Nutrition Examination Survey, 1988–1994. J. Trace Elem. Med. Bio. 2003, 17, 13–18. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Schomburg, L.; Buentzel, J.; Kisters, K.; Micke, O.; German Working Group Trace Elements and Electrolytes in Oncology-AKTE. Selenium or no selenium-that is the question in tumor patients: A new controversy. Integr. Cancer Ther. 2010, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, F.A.; Talwar, D.; O’Reilly, D.S.; Dickinson, N.; Atkinson, J.; Hursthouse, A.S.; Rankin, J.; Duncan, A. Erythrocyte selenium concentration as a marker of selenium status. Clin. Nutr. 2013, 32, 837–842. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Watts, M.J.; Young, S.D.; Chilimba, A.D.C.; Ander, E.L. Dietary mineral supplies in Malawi: Spatial and socioeconomic assessment. BMC Nutr. 2015, 1, 42. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Kumssa, D.B.; Joy, E.J.M.; Young, S.D.; Odee, D.W.; Ander, E.L.; Broadley, M.R. Variation in the mineral element concentration of Moringa oleifera Lam. and M. stenopetala (Bak. f.) Cuf.: Role in human nutrition. PLoS ONE 2017, 12, e0175503. [Google Scholar] [CrossRef]
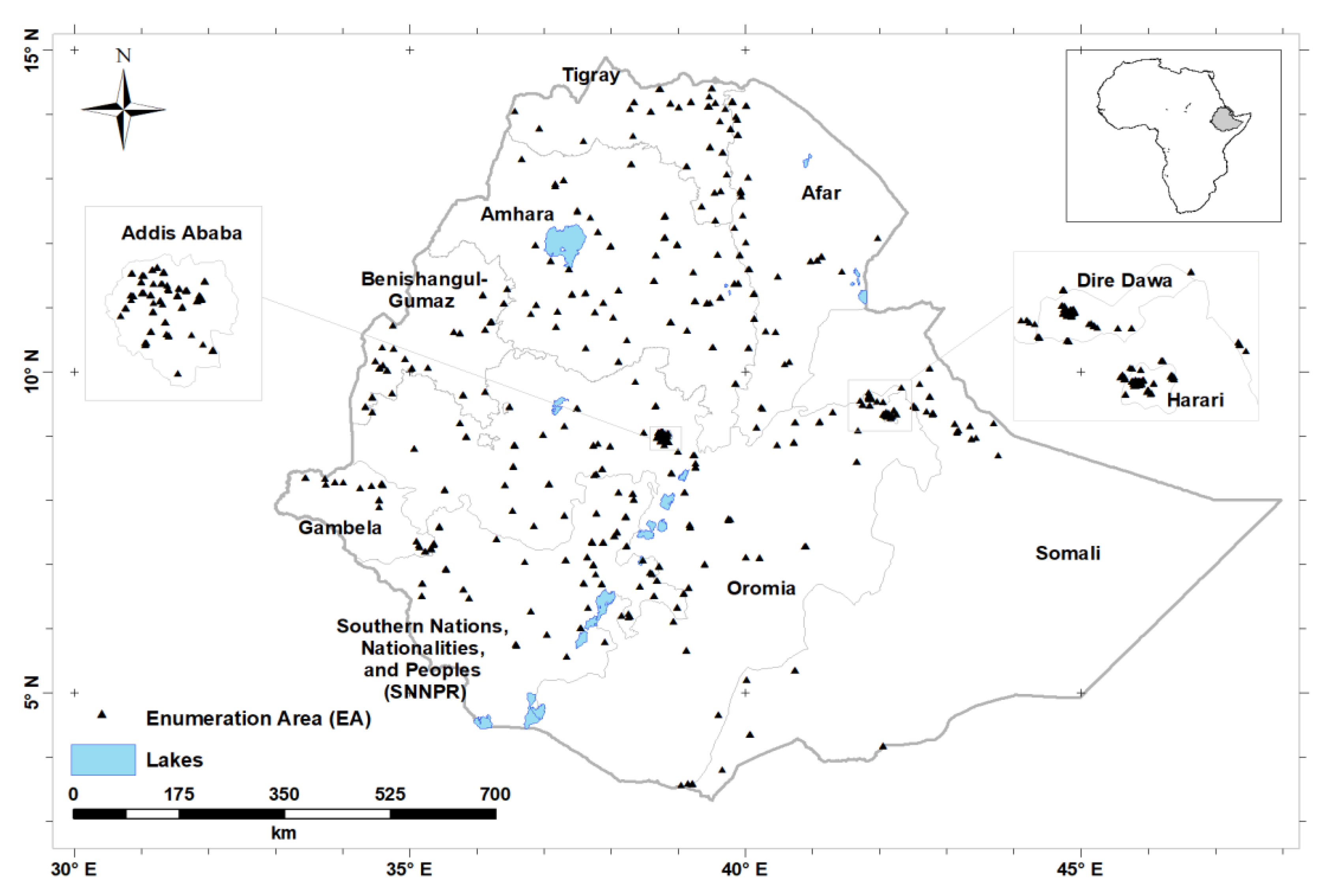
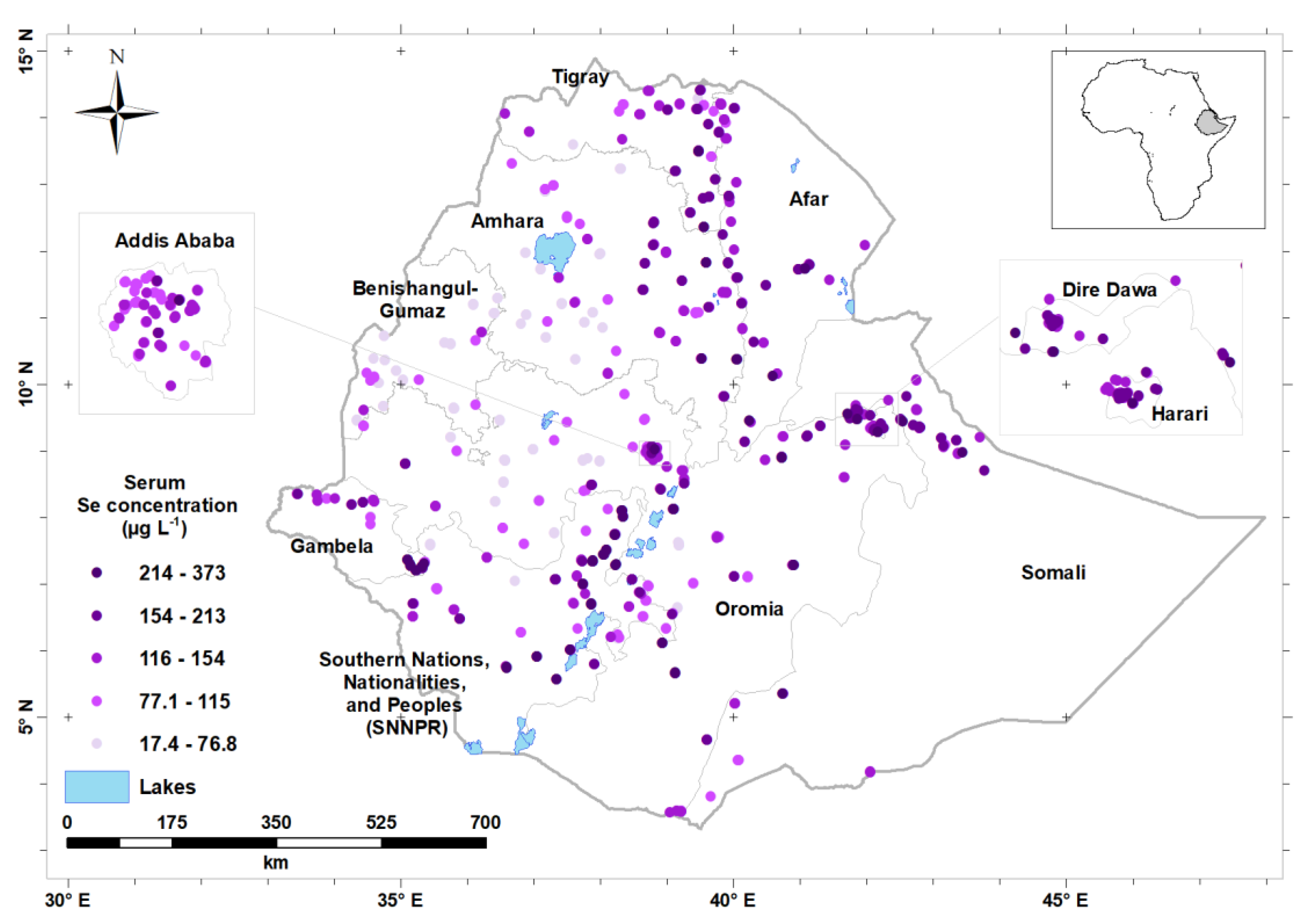
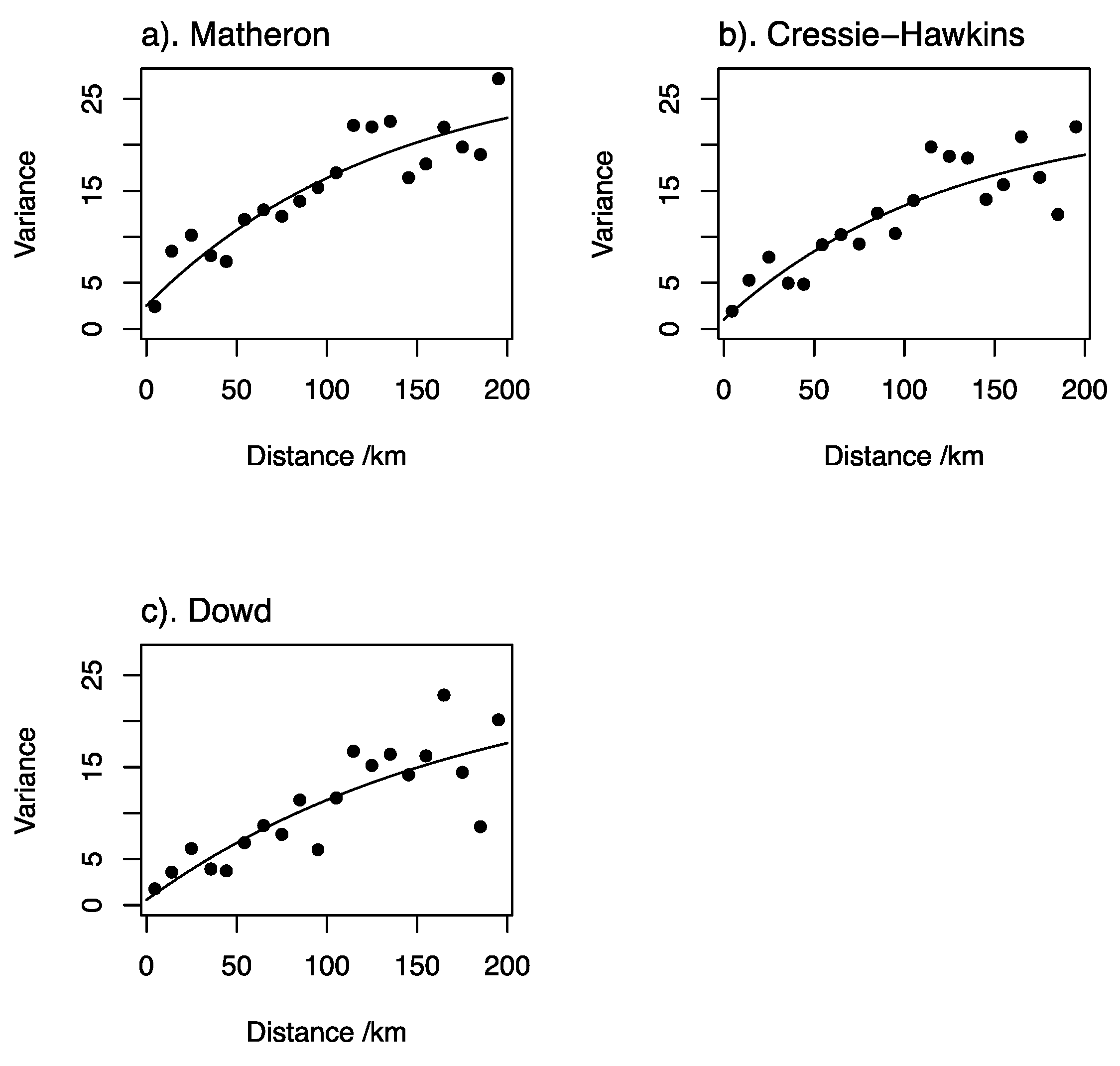
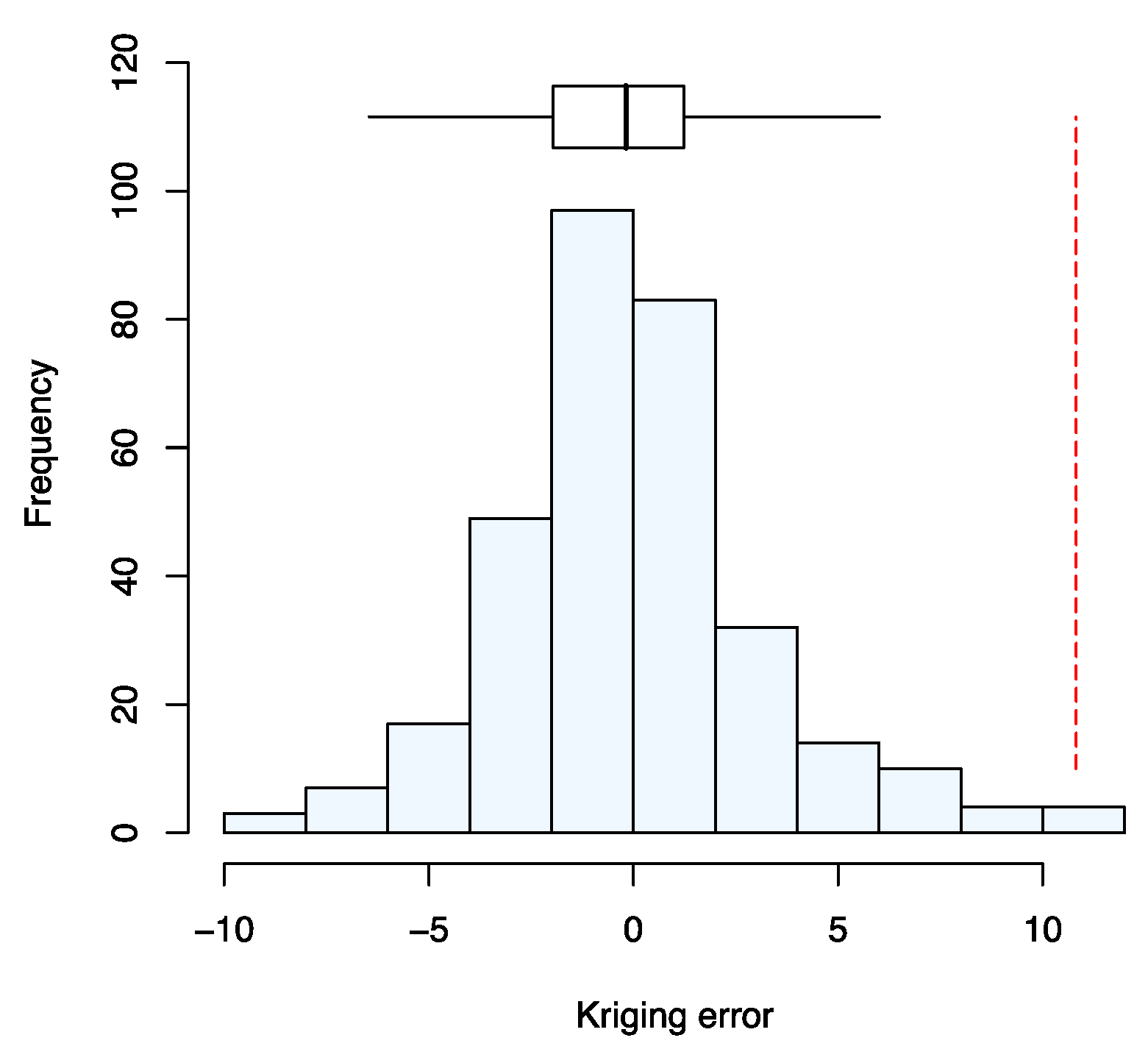
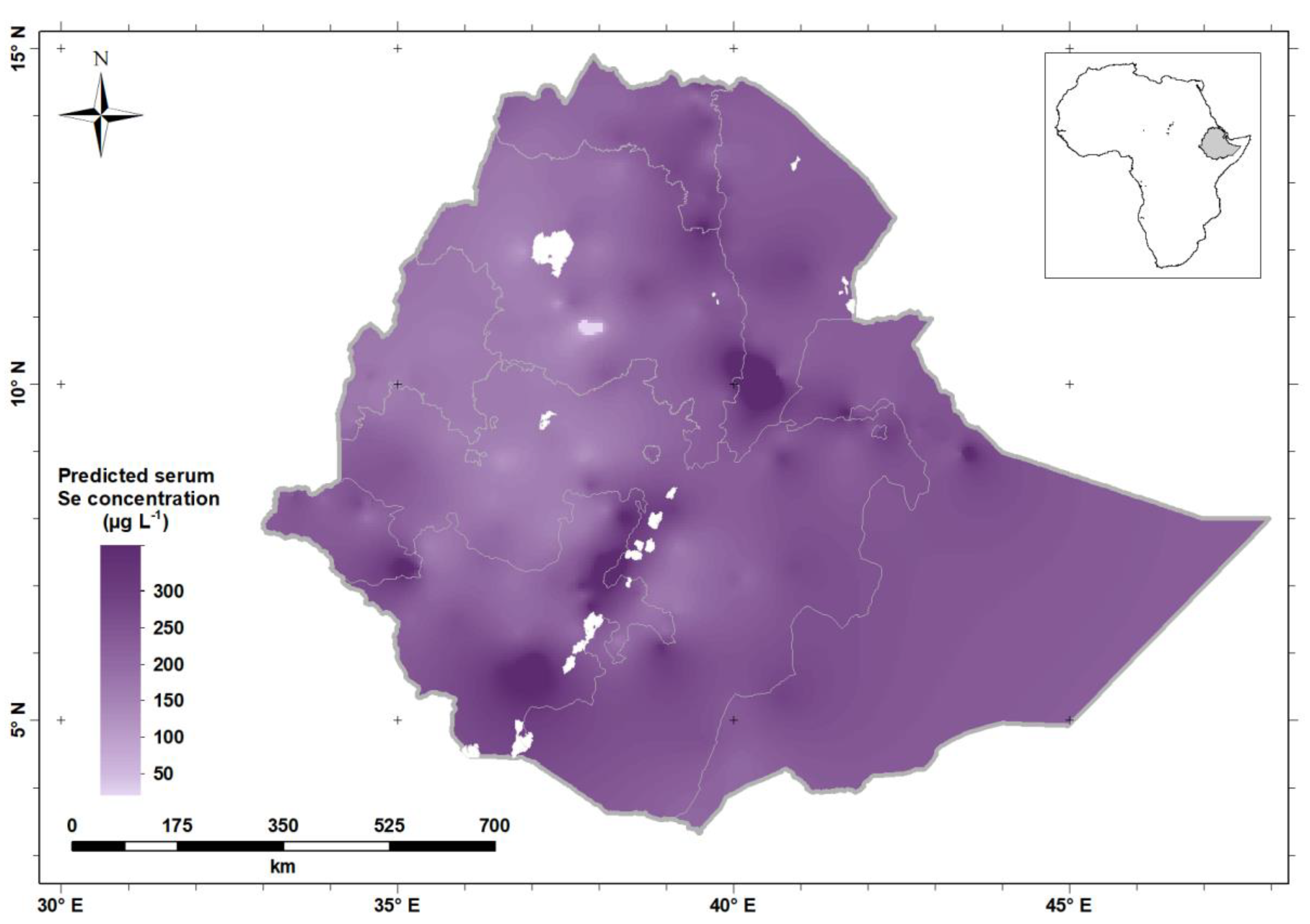
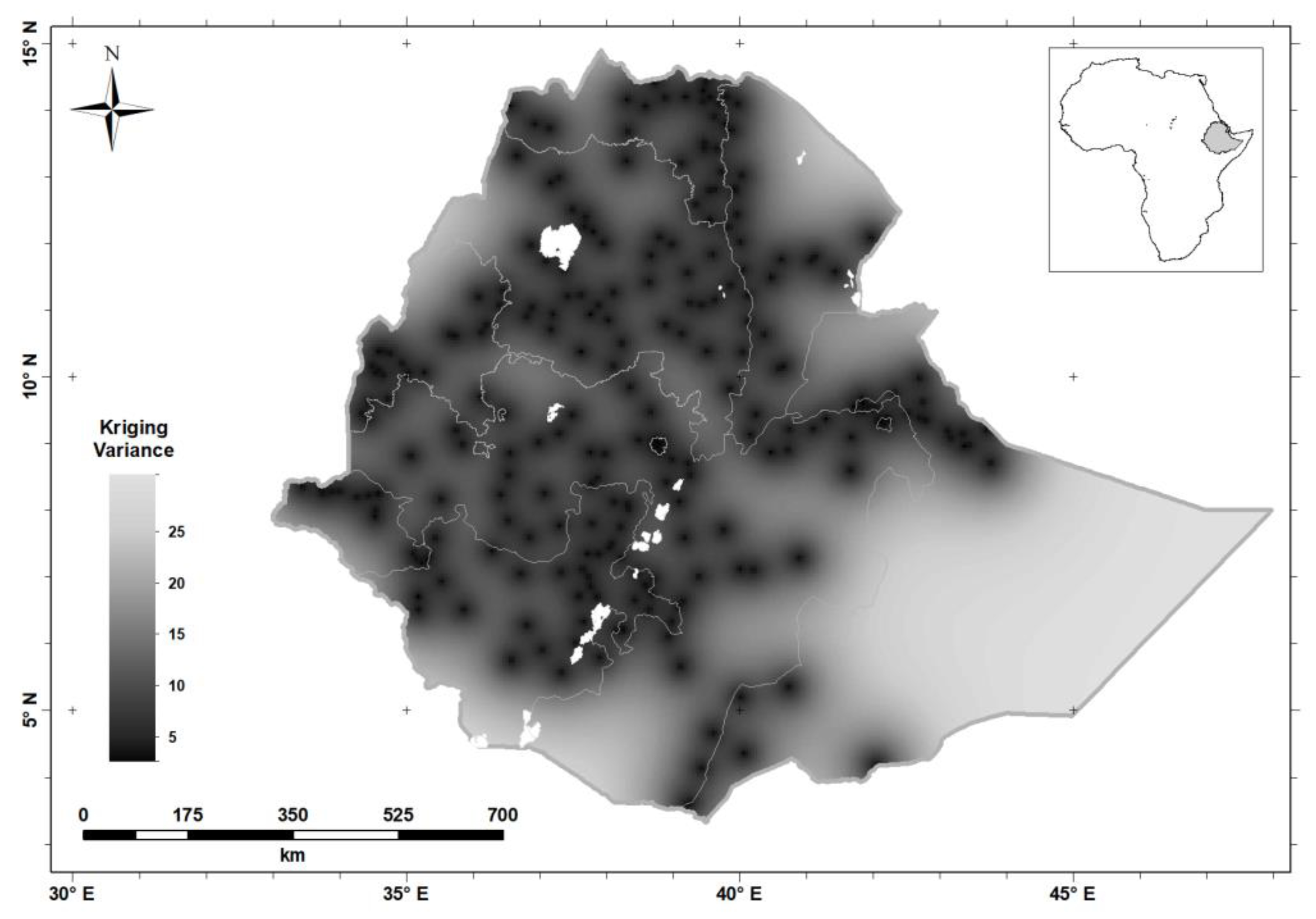
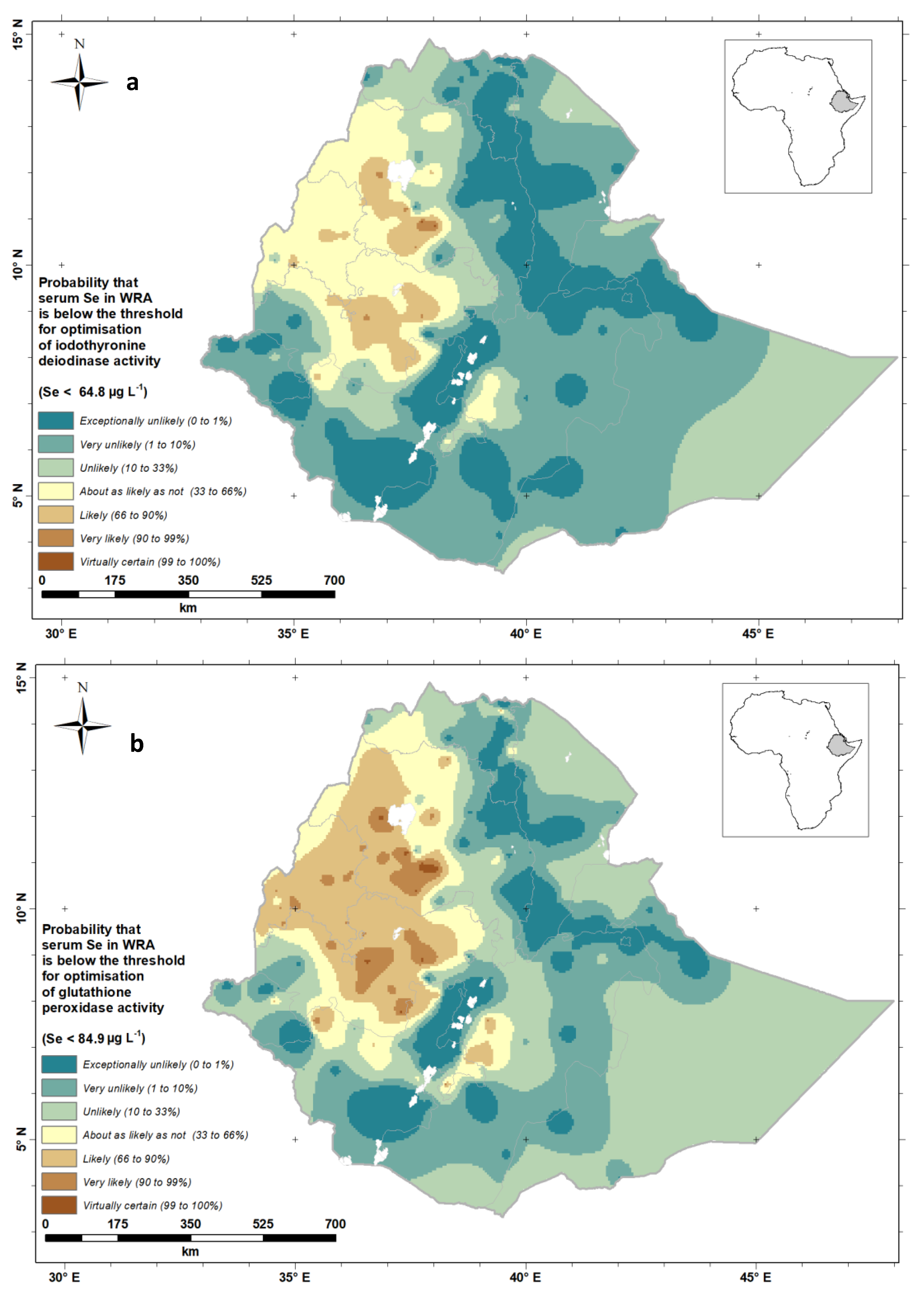
| Characteristics | n | Percentage | ||
|---|---|---|---|---|
| Region | Addis Ababa | 243 | 7.4 | |
| Afar | 254 | 7.8 | ||
| Amhara | 492 | 15.1 | ||
| Benishangul-Gumuz | 213 | 6.5 | ||
| Dire Dawa | 152 | 4.7 | ||
| Gambela | 199 | 6.1 | ||
| Harari | 243 | 7.4 | ||
| Oromia | 523 | 16.0 | ||
| SNNP | 365 | 11.1 | ||
| Somali | 204 | 6.3 | ||
| Tigray | 381 | 11.6 | ||
| National | 3269 | 100.0 | ||
| Demographic group | Young children | Male | 284 | 15.9 |
| Female | 237 | |||
| School age children | ||||
| Male | 451 | 30.8 | ||
| Female | 556 | |||
| Adult Men | 414 | 12.7 | ||
| Women of reproductive age | 1327 | 40.6 | ||
| Residence | Urban | 841 | 25.7 | |
| Rural | 2428 | 74.3 | ||
| Gender | Male | 1149 | 35.1 | |
| Female | 2120 | 64.9 |
| Variables | n | Median Serum Se (µg L−1) † | Prevalence (%) of Se Deficiency (Serum Se <70 µg L−1) | |
|---|---|---|---|---|
| Region | Addis Ababa | 243 | 92.7 [80.2, 111.0] | 7.1 |
| Afar | 254 | 122.0 [105.0, 142.0] | 0.9 | |
| Amhara | 492 | 67.4 [44.7, 94.7] | 52.2 | |
| Benishangul-Gumuz | 213 | 54.6 [43.1, 66.3] | 81.2 | |
| Dire Dawa | 152 | 118.9 [106.0,137.0] | 2.8 | |
| Gambela | 199 | 114.8 [88.5,157.0] | 16.1 | |
| Harari | 243 | 92.2 [62.9,114.0] | 32.8 | |
| Oromia | 523 | 76.8 [48.8,114.0] | 44.4 | |
| SNNP | 365 | 122.0 [85.3,171.0] | 14.1 | |
| Somali | 204 | 118.0 [100,141.0] | 3.5 | |
| Tigray | 381 | 104.0 [82.3, 128.0] | 15.5 | |
| National | 3269 | 87.7 [56.7, 123.0] | 35.5 | |
| Demographic group | Young children | 521 | 67.2 [41.9, 94.7] | 52.3 |
| School age children | 1007 | 78.7 [53.4,107.8] | 40.6 | |
| Men | 414 | 94.8 [60.4, 128.0] | 30.8 | |
| WRA | 1327 | 103.6 [68.2,143.0] | 26.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belay, A.; Joy, E.J.M.; Chagumaira, C.; Zerfu, D.; Ander, E.L.; Young, S.D.; Bailey, E.H.; Lark, R.M.; Broadley, M.R.; Gashu, D. Selenium Deficiency Is Widespread and Spatially Dependent in Ethiopia. Nutrients 2020, 12, 1565. https://doi.org/10.3390/nu12061565
Belay A, Joy EJM, Chagumaira C, Zerfu D, Ander EL, Young SD, Bailey EH, Lark RM, Broadley MR, Gashu D. Selenium Deficiency Is Widespread and Spatially Dependent in Ethiopia. Nutrients. 2020; 12(6):1565. https://doi.org/10.3390/nu12061565
Chicago/Turabian StyleBelay, Adamu, Edward J. M. Joy, Christopher Chagumaira, Dilnesaw Zerfu, E. Louise Ander, Scott D. Young, Elizabeth H. Bailey, R. Murray Lark, Martin R. Broadley, and Dawd Gashu. 2020. "Selenium Deficiency Is Widespread and Spatially Dependent in Ethiopia" Nutrients 12, no. 6: 1565. https://doi.org/10.3390/nu12061565
APA StyleBelay, A., Joy, E. J. M., Chagumaira, C., Zerfu, D., Ander, E. L., Young, S. D., Bailey, E. H., Lark, R. M., Broadley, M. R., & Gashu, D. (2020). Selenium Deficiency Is Widespread and Spatially Dependent in Ethiopia. Nutrients, 12(6), 1565. https://doi.org/10.3390/nu12061565





