IKONOS Image-Based Extraction of the Distribution Area of Stellera chamaejasme L. in Qilian County of Qinghai Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area

2.2. Field Spectra Measurements
2.3. Remote Sensing Data
| Bands | Spectral Interval (nm) | Spatial Resolution (m) | Grayscale of Data (bits) |
|---|---|---|---|
| Blue band | 450–525 | 4 | 11 |
| Green band | 510–600 | 4 | 11 |
| Red band | 630–700 | 4 | 11 |
| Near-infrared band | 760–850 | 4 | 11 |
| Panchromatic band | 450–900 | 1 | 11 |
2.4. Methodology
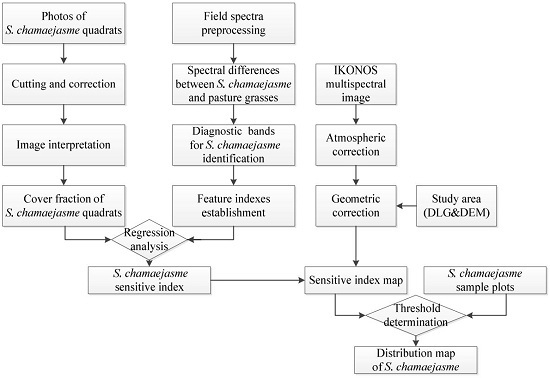
2.4.1. Overview of Methodology
2.4.2. Coverage Interpretation of S. Chamaejasme Quadrats

2.4.3. Spectra Data Preprocessing
2.4.4. Remote Sensing Data Preprocessing
2.4.5. Feature Indexes Construction Based on IKONOS Image
3. Results and Discussion
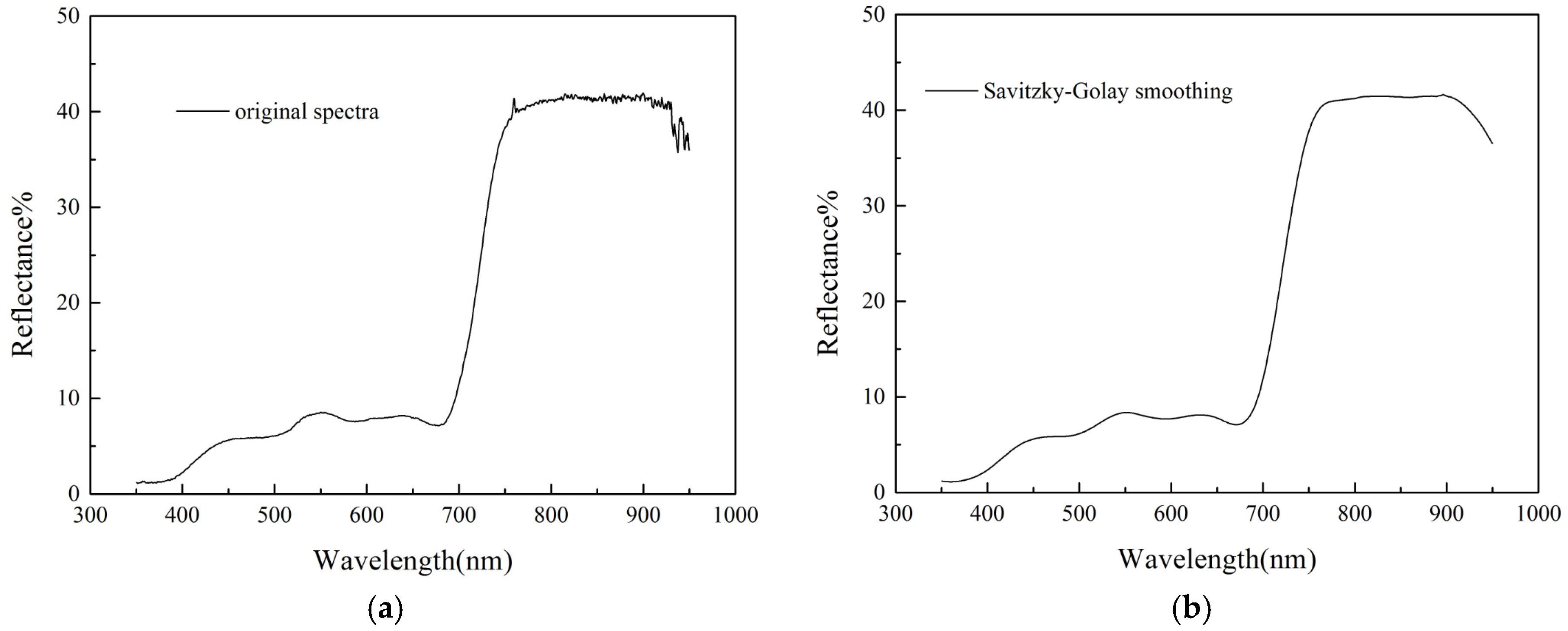
3.1. Spectral Differences between S. Chamaejasme and Surrounding Pasture Grasses

| Spectral Feature Parameters | Definitions |
|---|---|
| Blue edge amplitude | The maximum first-order differential reflectance of blue edge band between 490 and 530 nm |
| Yellow edge amplitude | The maximum first-order differential reflectance of yellow edge band between 560 and 640 nm |
| Red edge amplitude | The maximum first-order differential reflectance of red edge band between 680 and 760 nm |
| Blue valley | The minimum reflectance of blue band between 450 and 515 nm |
| Green peak | The maximum reflectance of green band between 510 and 560 nm |
| Red valley | The minimum reflectance of red band between 640 and 680 nm |
| Near-infrared peak | The maximum reflectance of near-infrared band between 780 and 900 nm |
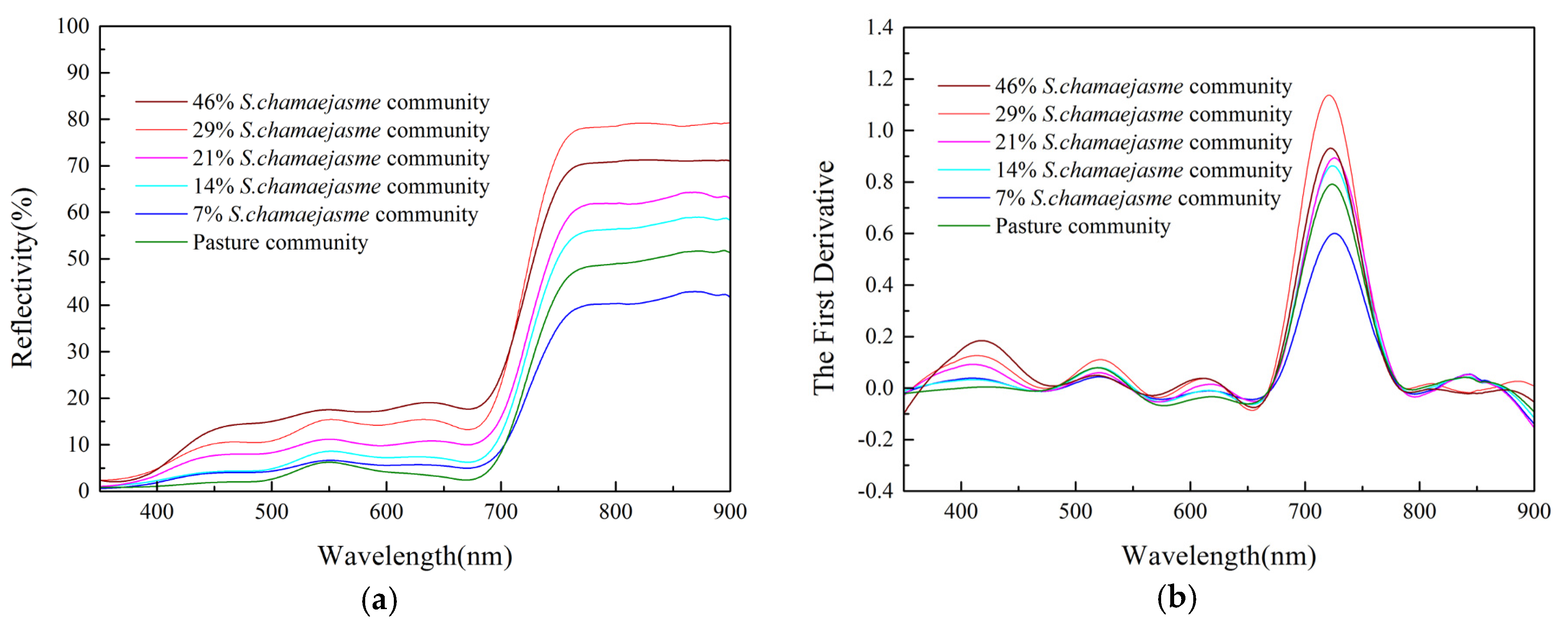
3.2. Sensitive Index Analysis
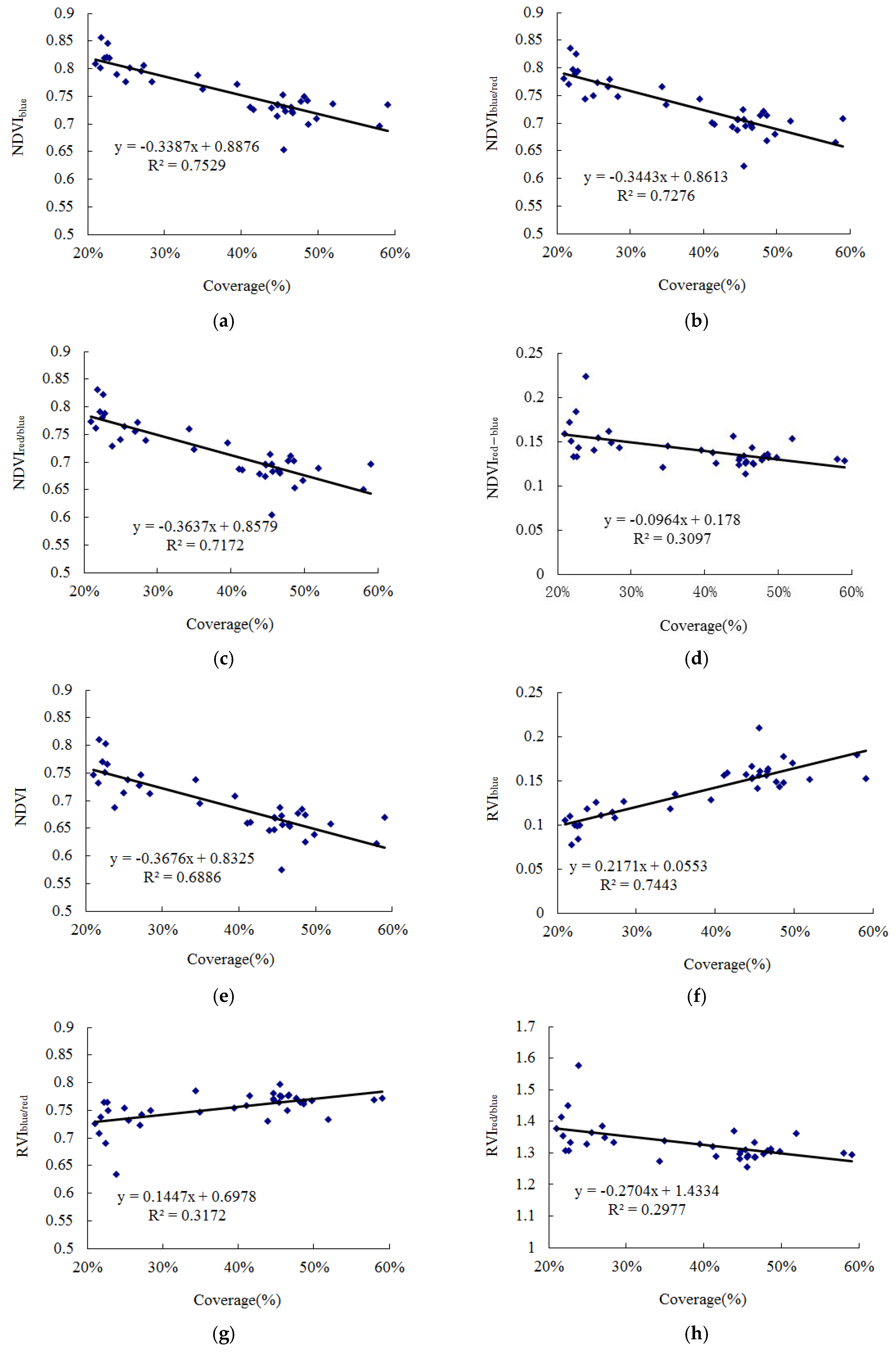
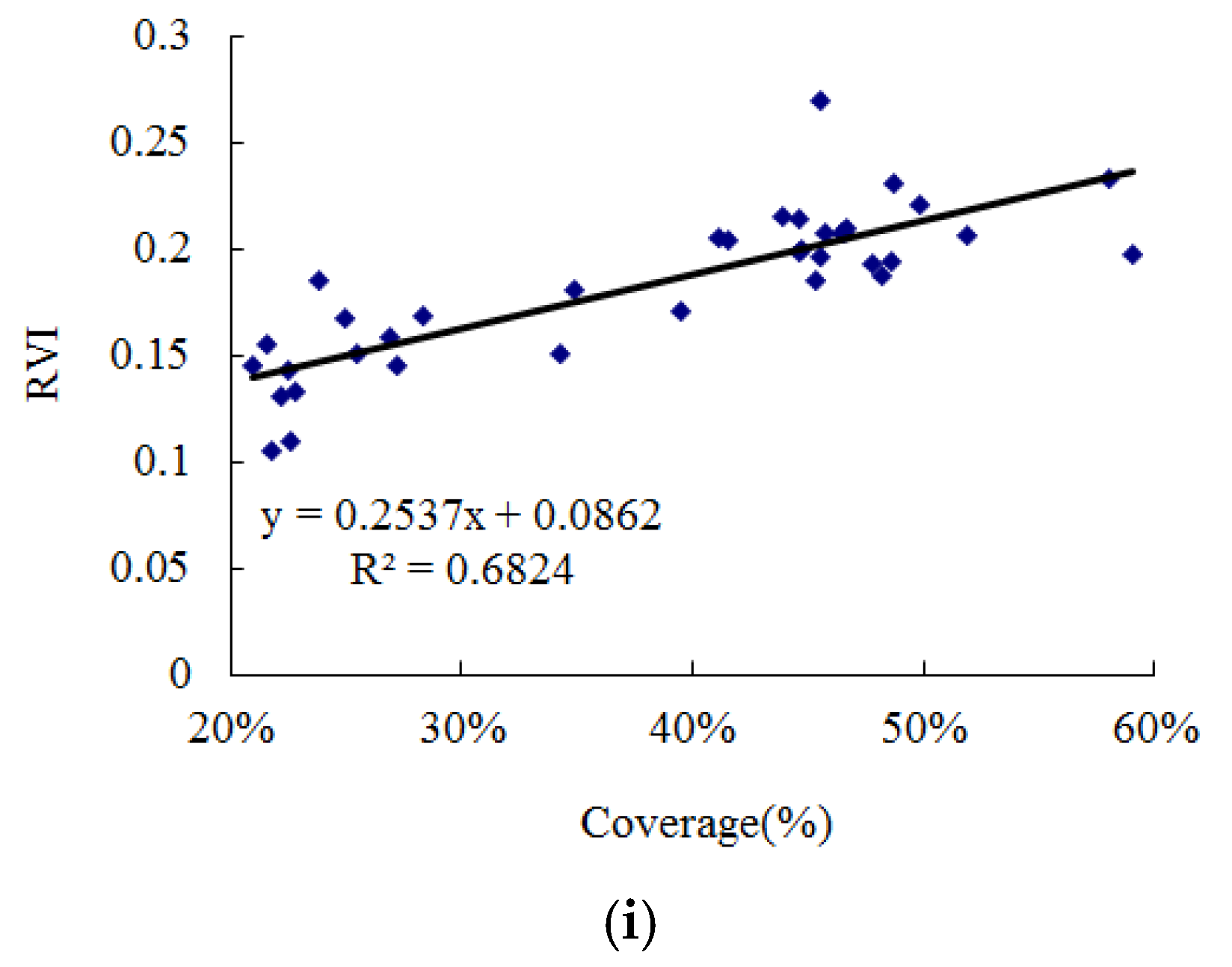
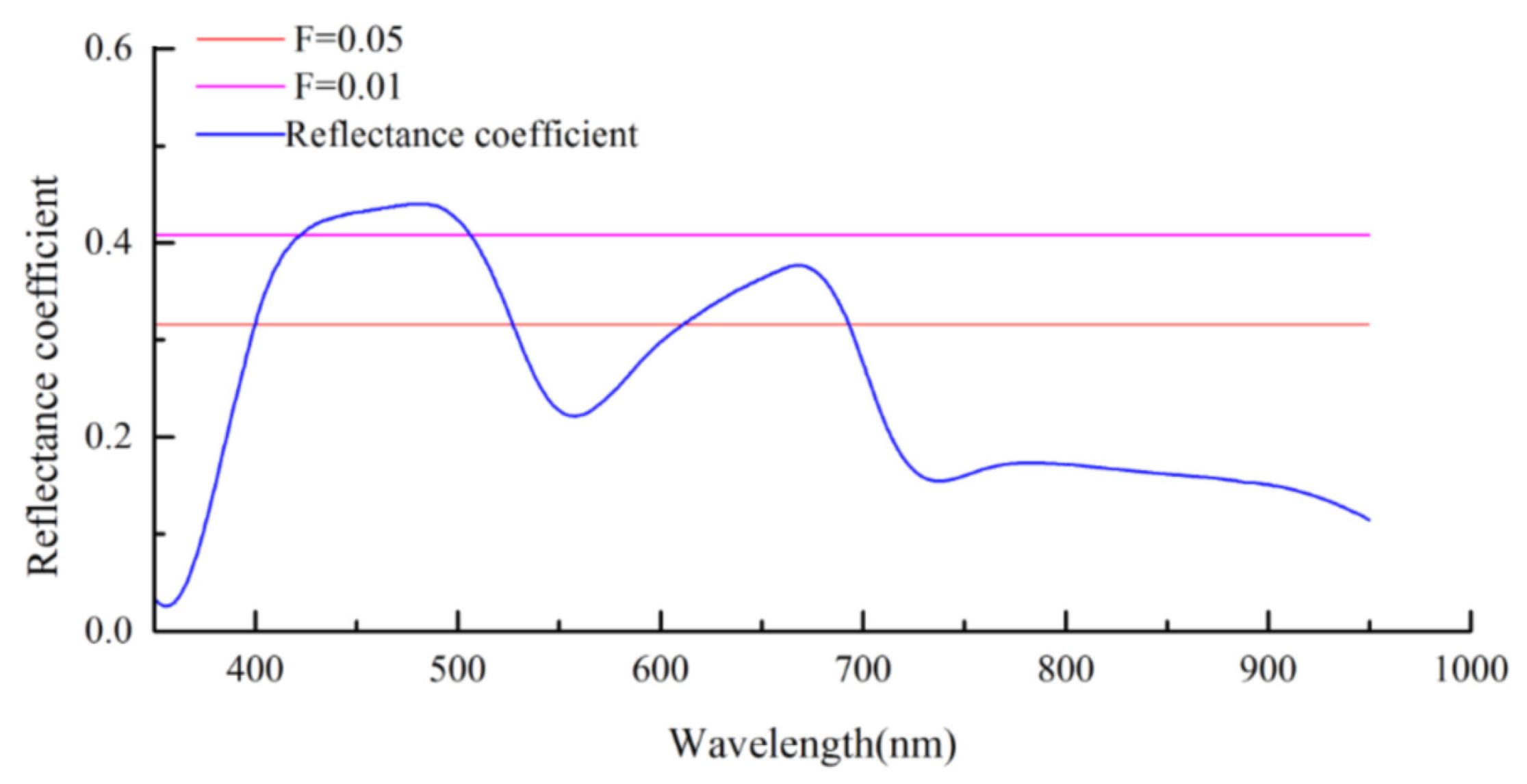
3.3. Distribution Extraction of S. chamaejasme

3.4. Validation
3.4.1. Statistical Data Validation
3.4.2. Field Data Validation
| I | Field Tested Results | ||||
|---|---|---|---|---|---|
| J | S. chamaejasme | Farmland/Grassland | Total | ||
| Classified results | S. chamaejasme | 81 | 10 | 91 | |
| Farmland/grassland | 3 | 46 | 49 | ||
| Total | 84 | 55 | 140 | ||
| Categories | Mapping Accuracy (%) | User Accuracy (%) |
|---|---|---|
| S. chamaejasme | 96.43 | 89.01 |
| Farmland/grassland | 82.14 | 93.88 |
| Overall accuracy = 90.71% | Kappa coefficient = 0.80 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scurlock, J.M.O.; Johnson, K.; Olson, R.J. Estimating net primary productivity from grassland biomass dynamics measurements. Glob. Change Biol. 2002, 8, 736–753. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Liu, X.; Ma, F.; Cao, D.; Yang, X.; Wang, S.; Geng, P.; Lu, H.; Zhao, B. Pathogenesis and preventive treatment for animal disease due to locoweed poisoning. Environ. Toxicol. Pharmacol. 2014, 37, 336–347. [Google Scholar]
- Zhao, B.; Liu, Z.; Lu, H.; Wang, Z.; Sun, L.; Wan, X.; Guo, X.; Zhao, Y.; Wang, J.; Shi, Z. Damage and control of poisonous weeds in western grassland of China. Agric. Sci. China 2010, 10, 1512–1521. [Google Scholar] [CrossRef]
- Wen, L.; Dong, S.K.; Zhu, L.; Li, X.Y.; Shi, J.J.; Wang, Y.L.; Ma, Y.S. The construction of grassland degradation index for alpine meadow in Qinghai-Tibetan Plateau. Procedia Environ. Sci. 2010, 2, 1966–1969. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhao, X.Q.; Zhou, X.M. Strategy and countermeasure for sustainable development of animal husbandry in Qinghai. J. Nat. Resour. 2000, 4, 328–334. (In Chinese) [Google Scholar]
- Wei, Y.; Wang, L.; Shi, Y.; Li, L. Net productivity of grassland resources monitoring based on remote sensing data in Qinghai Province. Sci. Geogr. Sin. 2012, 5, 621–627. (In Chinese) [Google Scholar]
- Harris, R.B. Rangeland degradation on the Qinghai-Tibetan plateau: A review of the evidence of its magnitude and causes. J. Arid Environ. 2010, 74, 1–12. [Google Scholar] [CrossRef]
- Wen, L.; Dong, S.K.; Li, Y.Y. The effects of biotic and abiotic factors on the spatial heterogeneity of alpine grassland vegetation at a small scale on the Qinghai-Tihet Plateau, China. Environ. Monit. Assess. 2013, 10, 8051–8064. [Google Scholar] [CrossRef] [PubMed]
- Goslee, S.C.; Peters, D.P.C.; Beck, K.G. Modeling invasive weeds in grasslands: The role of allelopathy in Acroptilon repens invasion. Ecol. Model. 2001, 139, 21–45. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, X.; Wang, J.; He, X.; Han, B. A review of the most economically important poisonous plants to the livestock industry on temperate grasslands of China. J. Appl. Toxicol. 2013, 33, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guo, H.; Yang, J.; Liu, Q.; Jin, H.; Xu, R.; Cui, H.; Qin, B. Phytotoxic flavonoids from roots of S. chamaejasme L. (Thymelaeaceae). Phytochemistry 2014, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.C. The Poisonous Plants of Chinese Grassland, 1st ed.; China Agriculture Press: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Sun, G.; Luo, P.; Qiu, P.F.; Gao, Y.H.; Chen, H.; Shi, F.S. S. chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern Tibetan Plateau of China. Soil Biol. Biochem. 2009, 41, 86–91. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Volis, S.; Sun, H. Chloroplast phylogeny and phylogeography of S. chamaejasme on the Qinghai-Tibet Plateau and in adjacent regions. Mol. Phylogenet. Evol. 2010, 57, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Chen, Y.L.; Wang, Y.W.; Xiu, H.H. S. chamaejasme L. an pesticide plant of Thymelaeaceae. J. Cent. China Norm. Univ. 2000, 3, 326–330. (In Chinese) [Google Scholar]
- Lu, H.; Wang, S.S.; Zhou, Q.W.; Zhao, Y.N.; Zhao, B.Y. Damage and control of major poisonous plants in the western grasslands of China—A review. Rangel. J. 2012, 34, 329–339. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, C.; Shi, F.; Sheng, Y.; Ren, H.; He, G. Spatial pattern of S. chamaejasme population in degraded alpine grassland in northern slope of Qilian Mountains, China. Chin. J. Ecol. 2011, 6, 1312–1316. [Google Scholar]
- Lawrence, R.L.; Wood, S.D.; Sheley, R.L. Mapping invasive plants using hyperspectral imagery and Breiman Cutler classifications (RandomForest). Remote Sens. Environ. 2006, 100, 356–362. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ma, M.; Wang, X. The spectral characteristics of S. chamaejasme L. with varied coverage in Qilian of China. Proc. SPIE 2009. [Google Scholar] [CrossRef]
- Gausman, H.W.; Menges, R.M.; Escobar, D.E.; Everitt, J.H.; Bowen, R.L. Pubescence affects spectra and imagery of Silverleaf Sunflower (Helianthus argophyllus). Weed Sci. 1977, 25, 437–440. [Google Scholar]
- Everitt, J.H.; Anderson, G.L.; Escobar, D.E.; Davis, M.R.; Spencer, N.R.; Andrascik, R.J. Use of remote sensing for detecting and mapping leafy spurge (Euphorbia esula). Weed Technol. 1995, 3, 599–609. [Google Scholar]
- Underwood, E.; Ustin, S.; DiPietro, D. Mapping nonnative plants using hyperspectral imagery. Remote Sens. Environ. 2003, 86, 150–161. [Google Scholar] [CrossRef]
- Miao, X.; Gong, P.; Swope, S.; Pu, R.; Carruthers, R.; Anderson, G.L.; Heaton, J.S.; Tracy, C.R. Estimation of yellow starthistle abundance through CASI-2 hyperspectral imagery using linear spectral mixture models. Remote Sens. Environ. 2006, 101, 329–341. [Google Scholar] [CrossRef]
- Lass, L.W.; Thill, D.C.; Shafii, B.; and Prather, T.S. Detecting spotted knapweed (Centaurea maculosa) with hyperspectral remote sensing technology. Weed Technol. 2002, 16, 426–432. [Google Scholar] [CrossRef]
- Mirik, M.; Ansley, R.J.; Steddom, K.; Jones, D.C.; Rush, C.M.; Michels, G.J.; Elliott, N.C. Remote distinction of a noxious weed (musk thistle: Carduus nutans) using airborne hyperspectral imagery and the support vector machine classifier. Remote Sens. 2013, 5, 612–630. [Google Scholar] [CrossRef]
- Williams, A.P.; Hunt, E.R. Estimation of leafy spurge cover from hyperspectral imagery using mixture tuned matched filtering. Remote Sens. Environ. 2002, 82, 446–456. [Google Scholar] [CrossRef]
- Lass, L.W.; Prather, T.S. Detecting the locations of Brazilian pepper trees in the everglades with a hyperspectral sensor. Weed Technol. 2004, 18, 437–442. [Google Scholar] [CrossRef]
- Lass, L.W.; Prather, T.S.; Glenn, N.F.; Weber, K.T.; Mundt, J.T.; Pettingill, J. A review of remote sensing of invasive weeds and example of the early detection of spotted knapweed (Centaurea maculosa) and babysbreath (Gypsophila paniculata) with a hyperspectral sensor. Weed Sci. 2005, 53, 242–251. [Google Scholar] [CrossRef]
- Narumalani, S.; Mishra, D.R.; Burkholder, J.; Merani, P.B.T. A comparative evaluation of ISODATA and spectral angle mapping for the detection of saltcedar using airborne hyperspectral imagery. Geocarto Int. 2006, 2, 59–66. [Google Scholar] [CrossRef]
- Miao, X.; Gong, P.; Swope, S.; Pu, R.; Carruthers, R.; Anderson, G.L. Detection of yellow starthistle through band selection and feature extraction from hyperspectral imagery. Photogramm. Eng. Remote Sens. 2007, 9, 1005–1015. [Google Scholar]
- Yang, C.; Everitt, J.H. Comparison of hyperspectral imagery with aerial photography and multispectral imagery for mapping broom snakeweed. Int. J. Remote Sens. 2010, 20, 5423–5438. [Google Scholar] [CrossRef]
- Evangelista, P.H.; Stohlgren, T.J.; Morisette, J.T.; Kumar, S. Mapping invasive tamarisk (Tamarix): A comparison of single-scene and time-series analyses of remotely sensed data. Remote Sens. 2009, 1, 519–533. [Google Scholar] [CrossRef]
- Root, R.; Tejada, P.Z.; Pinilla, C.; Ustin, S.; Kokaly, R.; Anderson, G.; Hager, S. Identification, Classification, and Mapping of Invasive Leafy Spurge Using Hyperion, AVIRIS, and CASI. Available online: http://eo1.gsfc.nasa.gov/new/validationReport/Technology/ Documents/ Tech.Val.Report/Science_Summary_Root.pdf (accessed on 25 October 2015).
- Wang, L.; Sousa, W.S.; Gong, P.; Biging, G.S. Comparison of IKONOS and QuickBird images for mapping mangrove species on the Caribbean coast of Panama. Remote Sens. Environ. 2004, 91, 432–440. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Paramananda, S.; Ramnarayan, M. Per-pixel and object-oriented classification methods for mapping urban features using Ikonos satellite data. Appl. Geogr. 2010, 30, 650–665. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, H.; Huang, Q. Landscape and water quality change detection in urban wetland: A Post-classification comparison method with IKONOS data. Procedia Environ. Sci. 2011, 10, 1726–1731. [Google Scholar]
- Delm, A.V.; Gulinck, H. Classification and quantification of green in the expanding urban and semi-urban complex: Application of detailed field data and IKONOS-imagery. Ecol. Indic. 2011, 11, 52–60. [Google Scholar] [CrossRef]
- Pu, R.; Landry, S. A comparative analysis of high spatial resolution IKONOS and WorldView-2 imagery for mapping urban tree species. Remote Sens. Environ. 2012, 124, 516–533. [Google Scholar] [CrossRef]
- Laet, V.D.; Paulissen, E.; Waelkens, M. Methods for the extraction of archaeological features from very high-resolution Ikonos-2 remote sensing imagery, Hisar (southwest Turkey). J. Archaeol. Sci. 2007, 34, 830–841. [Google Scholar] [CrossRef]
- Malhi, Y.; María, R.; Cuesta, R. Analysis of lacunarity and scales of spatial homogeneity in IKONOS images of Amazonian tropical forest canopies. Remote Sens. Environ. 2008, 112, 2074–2087. [Google Scholar] [CrossRef]
- Asner, G.P.; Warner, A.S. Canopy shadow in IKONOS satellite observations of tropical forests and savannas. Remote Sens. Environ. 2003, 87, 521–533. [Google Scholar] [CrossRef]
- Kayitakire, F.; Hamel, C.; Defourny, P. Retrieving forest structure variables based on image texture analysis and IKONOS-2 imagery. Remote Sens. Environ. 2006, 102, 390–401. [Google Scholar] [CrossRef]
- Casady, G.M.; Hanley, R.S.; Seelan, S.K. Detection of Leafy Spurge using multidate high-resoultion satellite imagery. Weed Technol. 2005, 19, 462–467. [Google Scholar] [CrossRef]
- Qilian Grassland Station. Control Plan for the Toxic Grass of Qilian County in 2011. A government work report, unpublished. 2011. (In Chinese) [Google Scholar]
- Hamzeh, S.; Naseri, A.A.; Alavipanah, S.K.; Mojaradi, B.; Bartholomeus, H.M.; Clevers, J.G.P.W.; Behzad, M. Estimating salinity stress in sugarcane fields with spaceborne hyperspectral vegetation indices. Int. J. Appl. Earth Obs. Geoinform. 2013, 21, 282–290. [Google Scholar] [CrossRef]
- Omodanisi, E.O.; Salami, A.T. An Assessment of the spectra characteristics of vegetation in south western Nigeria. IERI Procedia 2014, 9, 26–32. [Google Scholar] [CrossRef]
- Yang, G.; Pu, R.; Zhao, C.; Xue, X. Estimating high spatiotemporal resolution evapotranspiration over a winter wheat field using an IKONOS image based complementary relationship and Lysimeter observations. Agric. Water Manag. 2014, 133, 34–43. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Zhu, X.; Tang, Y. Indicator of flower status derived from in situ hyperspectral measurement in an alpine meadow on the Tibetan Plateau. Ecol. Indic. 2009, 9, 818–823. [Google Scholar] [CrossRef]
- Daniel, G.F.; David, E.H.; Antonio, R.C.; Julian, C.; Jose, M.M.M. A digital image processing based method for determining the crop coefficient of lettuce crops in the southeast of Spain. Biosyst. Eng. 2014, 117, 23–34. [Google Scholar]
- Tsai, F.; Philpot, W. Derivative analysis of hyperspectral data. Remote Sens. Environ. 1998, 66, 41–51. [Google Scholar]
- Hanan, N.P.; Prince, S.D.; Holben, B.N. Atmospheric correction of AVHRR data for biophysical remote sensing of the Sahel. Remote Sens. Environ. 1995, 51, 306–316. [Google Scholar] [CrossRef]
- Magendran, T.; Sanjeevi, S. Hyperion image analysis and linear spectral unmixing to evaluate the grades of iron ores in parts of Noamundi, Eastern India. Int. J. Appl. Earth Obs. Geoinform. 2014, 26, 413–426. [Google Scholar] [CrossRef]
- Law, K.H.; Nichol, J. Topographic correction for differential illumination effects on IKONOS satellite imagery. Int. Arch. Photogramm. Remote Sens. Spat. Inform. Sci. 2004, 35, 641–646. [Google Scholar]
- Wang, J.; Ge, Y.; Heuvelink, G.B.M.; Zhou, C.; Brus, D. Effect of the sampling design of ground control points on the geometric correction of remotely sensed imagery. Int. J. Appl. Earth Obs. Geoinform. 2012, 18, 91–100. [Google Scholar] [CrossRef]
- Rocchini, D.; Rita, A.D. Relief effects on aerial photos geometric correction. Appl. Geogr. 2005, 25, 159–168. [Google Scholar] [CrossRef]
- Gai, Y.; Fan, W.; Xu, X.; Yan, B.; Wang, H.; Liu, Y. Flower species identification and coverage estimation based on hyperspectral remote sensing data in Hulunbeier grassland. Spectrosc. Spectr. Anal. 2011, 10, 2778–2783. (In Chinese) [Google Scholar]
- Elvidge, C.D.; Chen, Z.K. Comparison of broad-band and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ. 1995, 54, 38–48. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Heald, R.C. Analysis of in situ hyperspectral data for nutrient estimation of giant sequoia. Int. J. Remote Sens. 2002, 23, 1827–1850. [Google Scholar] [CrossRef]
- Pu, R.; Foschi, L.; Gong, P. Spectral feature analysis for assessment of water status and health level in coast live oak (Quercus agrifolia) leaves. Int. J. Remote Sens. 2004, 25, 4267–4286. [Google Scholar] [CrossRef]
- Monserud, R.A.; Leemans, R. Comparing global vegetation maps with the Kappa statistic. Ecol. Model. 1992, 62, 275–293. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, Y.; Mo, C.; Wang, L.; Pang, G.; Cao, M. IKONOS Image-Based Extraction of the Distribution Area of Stellera chamaejasme L. in Qilian County of Qinghai Province, China. Remote Sens. 2016, 8, 148. https://doi.org/10.3390/rs8020148
Li J, Liu Y, Mo C, Wang L, Pang G, Cao M. IKONOS Image-Based Extraction of the Distribution Area of Stellera chamaejasme L. in Qilian County of Qinghai Province, China. Remote Sensing. 2016; 8(2):148. https://doi.org/10.3390/rs8020148
Chicago/Turabian StyleLi, Jingzhong, Yongmei Liu, Chonghui Mo, Lei Wang, Guowei Pang, and Mingming Cao. 2016. "IKONOS Image-Based Extraction of the Distribution Area of Stellera chamaejasme L. in Qilian County of Qinghai Province, China" Remote Sensing 8, no. 2: 148. https://doi.org/10.3390/rs8020148
APA StyleLi, J., Liu, Y., Mo, C., Wang, L., Pang, G., & Cao, M. (2016). IKONOS Image-Based Extraction of the Distribution Area of Stellera chamaejasme L. in Qilian County of Qinghai Province, China. Remote Sensing, 8(2), 148. https://doi.org/10.3390/rs8020148