Long-Term Spatiotemporal Pattern and Temporal Dynamic Simulation of Pine Wilt Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
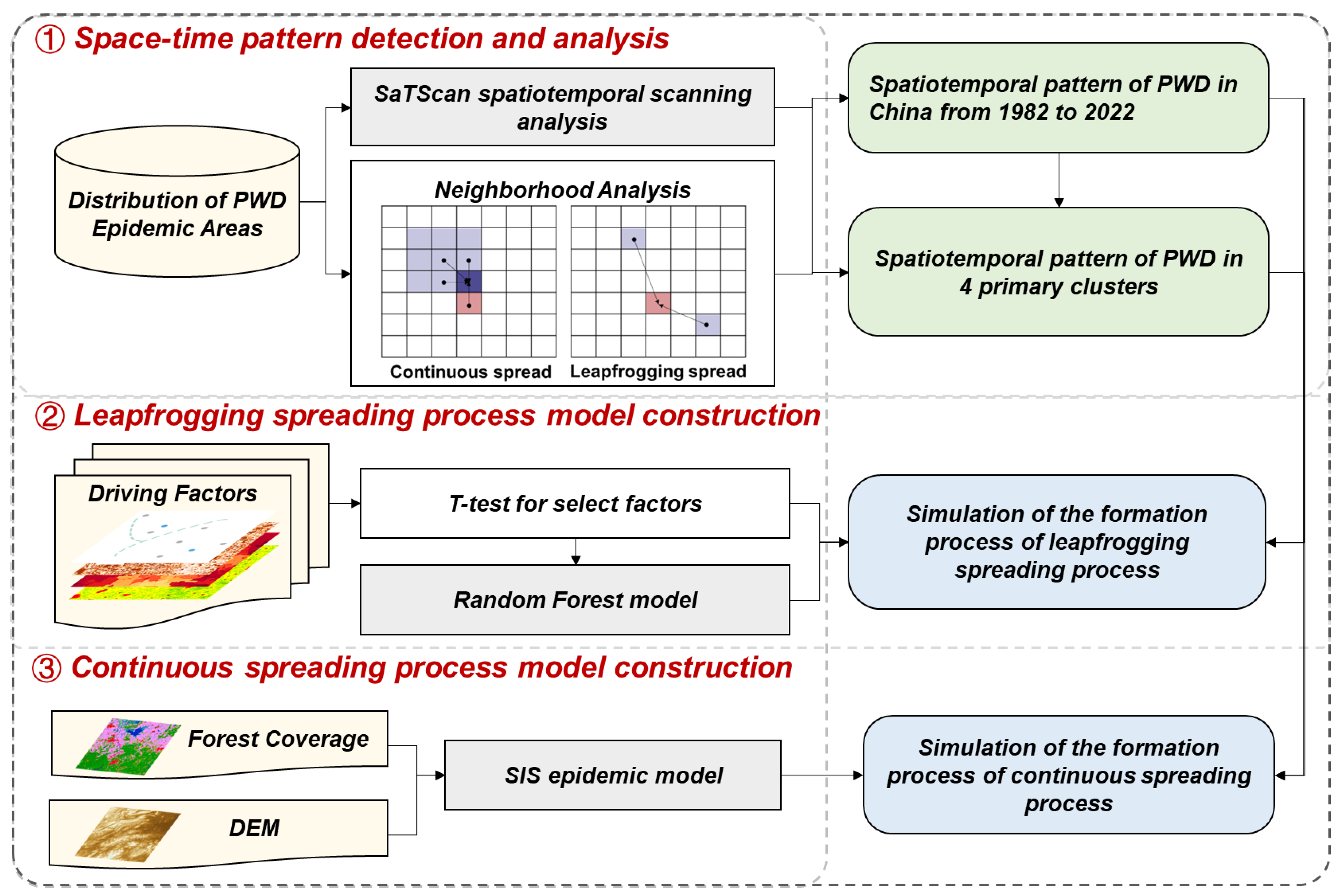
2.2. Study Method and Model
2.2.1. SaTScan Model
2.2.2. Leapfrogging Spreading Process
- (1)
- Driving Factors
- (2)
- Random Forest model
2.2.3. Continuous Spreading Process
2.3. Data Source
2.3.1. PWD Data
2.3.2. Host Data
2.3.3. Other Data
3. Results
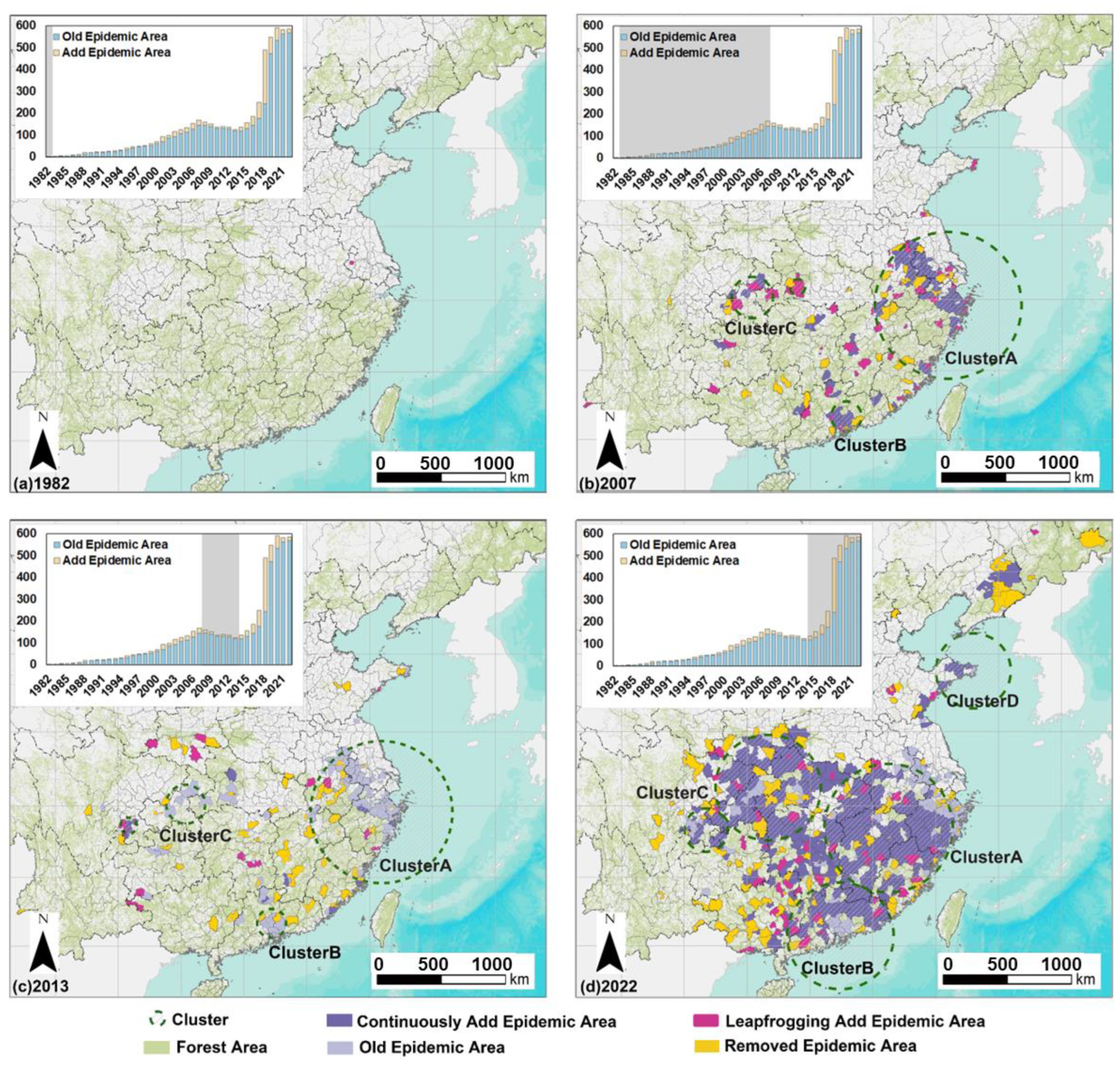
3.1. Spatiotemporal Pattern of PWD in China from 1982 to 2022
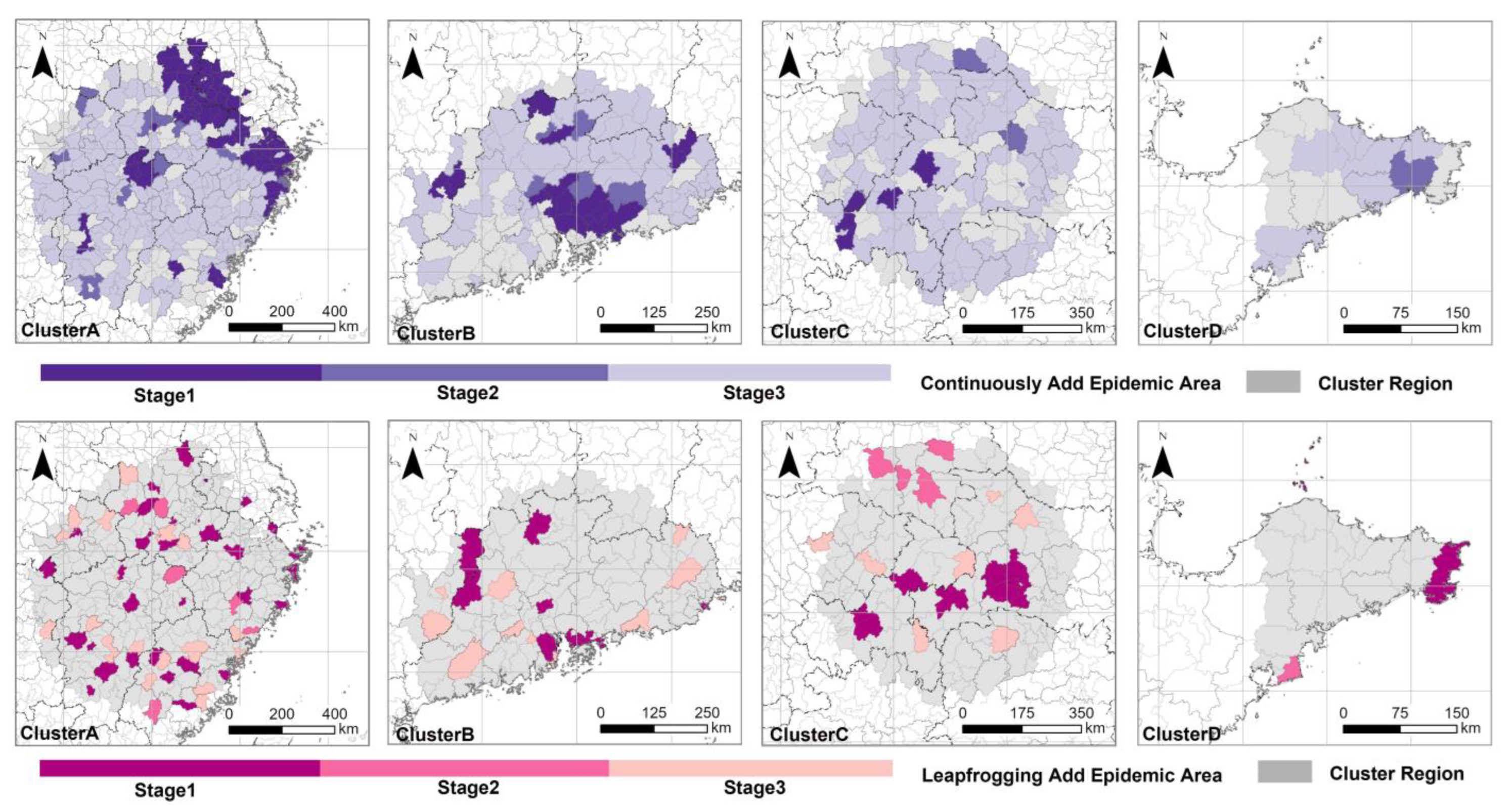
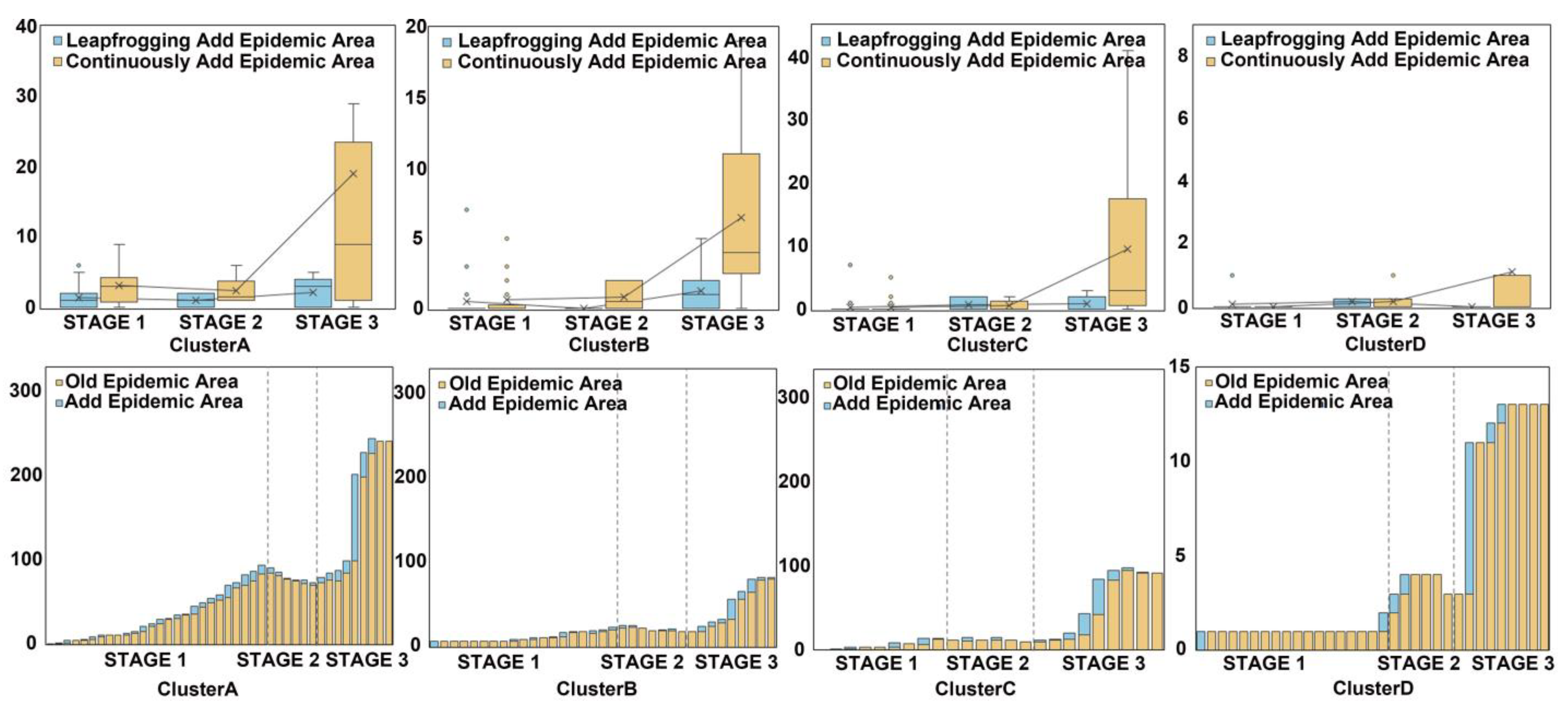
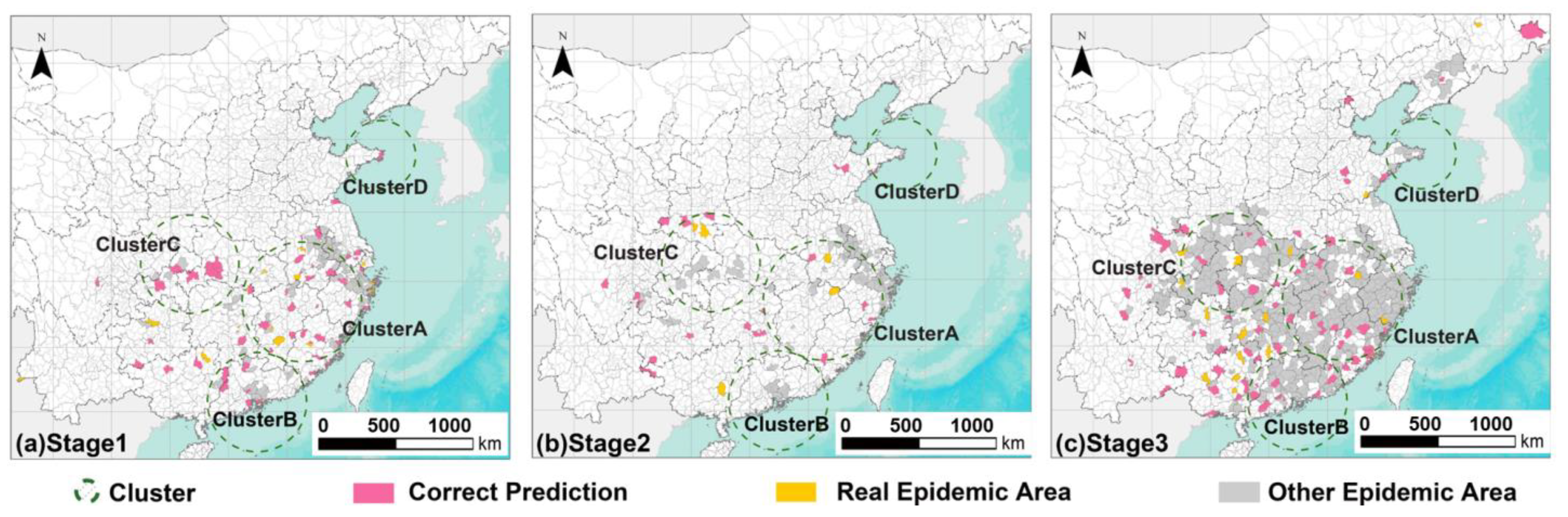
3.2. Spatiotemporal Pattern of PWD in Four Primary Clusters
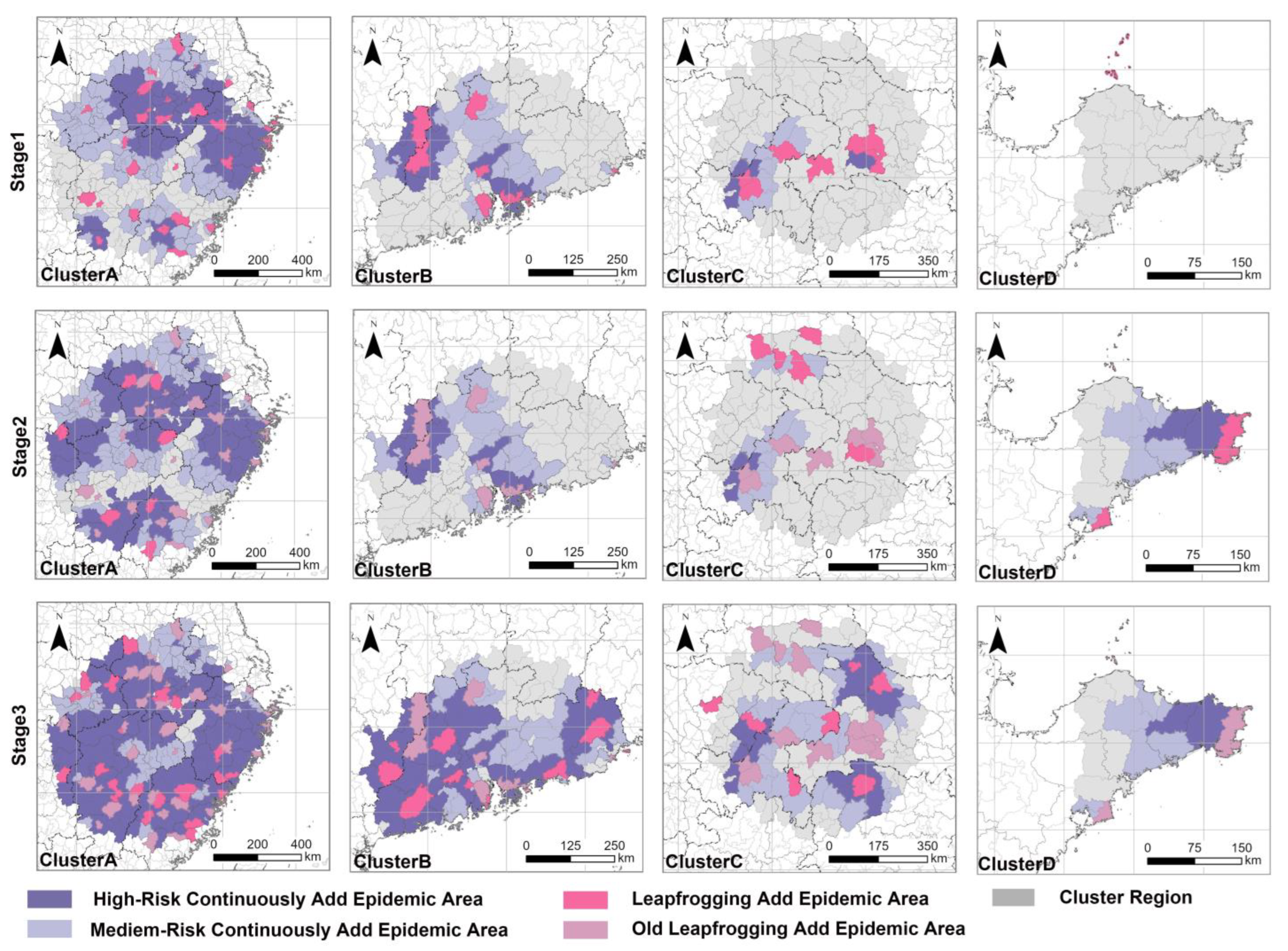
3.3. A Simulation of the Formation Process of the Leapfrogging Spreading Process
3.4. A Simulation of the Formation Process of the Continuous Spreading Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Espada, M.; Filipiak, A.; Li, H.; Shinya, R.; Vicente, C.S.L. Editorial: Global occurrence of pine wilt disease: Biological interactions and integrated management. Front. Plant Sci. 2022, 13, 993482. [Google Scholar] [CrossRef] [PubMed]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt Disease; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Mamiya, Y.; Kiyohara, T. Description of Bursaphelenchus lignicolus n. sp.(Nematoda: Aphelenchoididae) from pine wood and histopathology of nematode-infested trees. Nematologica 1972, 18, 120–124. [Google Scholar] [CrossRef]
- Cheng, G.; Lu, Q.; Feng, Y.M.; Li, Y.X.; Wang, Y.L.; Zhang, X.Y. Temporal and Spatial Dynamic Pattern of Pine Wilt Disease Distribution in China Predicted under Climate Change Scenario. Sci. Silvae Sin. 2015, 51, 119–126. [Google Scholar] [CrossRef]
- Tóth, Á. Bursaphelenchus xylophilus, the pinewood nematode: Its significance and a historical review. Acta Biol. Szeged. 2011, 55, 213–217. [Google Scholar]
- Ye, J.R. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef]
- Mota, M.M.; Vieira, P.R. Pine wilt disease: A worldwide threat to forest ecosystems. Nematology 2009, 11, 727–734. [Google Scholar] [CrossRef]
- Cheng, R.; Lin, M.; Li, W.; Fang, Z. Pine Wilt disease caused by pinewood nematode on black pine in Nanjing. For. Pest Dis. 1983, 4, 1–5. [Google Scholar]
- Hao, Z.; Huang, J.; Li, X.; Sun, H.; Fang, G. A multi-point aggregation trend of the outbreak of pine wilt disease in China over the past 20 years. For. Ecol. Manag. 2022, 505, 119890. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, P.; Shi, Y.; Wu, H.; Yu, H.; Jiang, S. Difference analysis on pine wilt disease between Liaoning Province of northeastern China and other epidemic areas in China. J. Beijing For. Univ. 2021, 43, 155–160. [Google Scholar] [CrossRef]
- Smith, T.M.; York, P.H.; Broitman, B.R.; Thiel, M.; Hays, G.C.; van Sebille, E.; Putman, N.F.; Macreadie, P.I.; Sherman, C.D.H. Rare long-distance dispersal of a marine angiosperm across the Pacific Ocean. Glob. Ecol. Biogeogr. 2018, 27, 487–496. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Nathan, R.; Perry, G.; Richardson, D.M. The importance of long-distance dispersal in biodiversity conservation. Divers. Distrib. 2005, 11, 173–181. [Google Scholar] [CrossRef]
- Lee, S.; Cho, H.; Choi, Y.; Choi, W.I.; Chung, H.I.; Lim, N.O.; Nam, Y.; Jeon, S. Path-finding algorithm as a dispersal assessment method for invasive species with human-vectored long-distance dispersal event. Divers. Distrib. 2022, 28, 1214–1226. [Google Scholar] [CrossRef]
- Travis, J.M.; Dytham, C. Dispersal evolution during invasions. Evol. Ecol. Res. 2002, 4, 1119–1129. [Google Scholar] [CrossRef]
- Ding, X.L.; Wang, Q.T.; Lin, S.; Zhao, R.W.; Zhang, Y.; Ye, J.R. Analysis of Genetic Variations of Bursaphelenchus xylophilus Populations between Guangdong and Jiangsu Provinces with SN Marker. Sci. Silvae Sin. 2022, 58, 1–9. [Google Scholar] [CrossRef]
- Bullock, J.M.; Bonte, D.; Pufal, G.; Carvalho, C.d.S.; Chapman, D.S.; García, C.; García, D.; Matthysen, E.; Delgado, M.M. Human-mediated dispersal and the rewiring of spatial networks. Trends Ecol. Evol. 2018, 33, 958–970. [Google Scholar] [CrossRef]
- Haran, J.; Roques, A.; Bernard, A.; Robinet, C.; Roux, G. Altitudinal Barrier to the Spread of an Invasive Species: Could the Pyrenean Chain Slow the Natural Spread of the Pinewood Nematode? PLoS ONE 2015, 10, e0134126. [Google Scholar] [CrossRef]
- Evans, H.F.; McNAMARA, D.G.; Braasch, H.; Chadoeuf, J.; Magnusson, C. Pest Risk Analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating landscape resistance to movement: A review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Schmitt, T. Biogeographical and evolutionary importance of the European high mountain systems. Front. Zool. 2009, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Robinet, C.; Van Opstal, N.; Baker, R.; Roques, A. Applying a spread model to identify the entry points from which the pine wood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol. Invasions 2011, 13, 2981–2995. [Google Scholar] [CrossRef]
- Zhao, Z.; Reddy, G.V.P.; Chen, L.; Qin, Y.; Li, Z. The synergy between climate change and transportation activities drives the propagation of an invasive fruit fly. J. Pest Sci. 2020, 93, 615–625. [Google Scholar] [CrossRef]
- Skarpaas, O.; Økland, B. Timber import and the risk of forest pest introductions. J. Appl. Ecol. 2009, 46, 55–63. [Google Scholar] [CrossRef]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Xia, C.; Chon, T.-S.; Takasu, F.; Choi, W.I.; Park, Y.-S. Simulating Pine Wilt disease dispersal with an Individual-Based Model incorporating individual movement patterns of Vector Beetles. Front. Plant Sci. 2022, 13, 886867. [Google Scholar] [CrossRef]
- Zhang, K. Research Advances of Pine Wood Nematode Disease in China. World For. Res. 2010, 23, 59–63. [Google Scholar]
- Ye, J.; Wu, X. Research progress of pine wilt disease. For. Pest Dis. 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Jiang, S.W.; Wu, H.; Li, D.B.; Luo, Z.Q.; He, S.; Song, Y.S. Analysis on disaster characteristics of pine wood nematode in Northeast China. For. Pest Dis. 2022, 41, 9–15. [Google Scholar] [CrossRef]
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assunção, R.; Mostashari, F. A space-time permutation scan statistic for disease outbreak detection. PLoS Med. 2005, 2, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Olshen, R.; Stone, C. Cart. In Classification and Regression Trees; CRC: Boca Raton, FL, USA, 1984. [Google Scholar]
- Allen, L.J.S. Some discrete-time SI, SIR, and SIS epidemic models. Math. Biosci. 1994, 124, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Takasu, F. Equilibrium properties of the spatial SIS model as a point pattern dynamics-How is infection distributed over space? J. Theor. Biol. 2019, 468, 12–26. [Google Scholar] [CrossRef]
- Shang, Y. Distribution dynamics for SIS model on random networks. J. Biol. Syst. 2012, 20, 213–220. [Google Scholar] [CrossRef]
- Li, L.Y.; Gao, L.; Wen, Y.L.; Shen, Y.Q. Advances in Research on Bursaphelenchus xylophilus. J. Zhejiang For. Sci. Technol. 2006, 5, 74–80. [Google Scholar] [CrossRef]
- Huang, H.H.; Liu, C.Y.; Hu, L.L.; Huang, Y.; Huang, H.; Zhao, D. Continuous control technology of pine wilt disease in Guangdong province. For. Pest Dis. 2022, 41, 36–42. [Google Scholar] [CrossRef]
- Hao, Z.; Huang, J.; Zhou, Y.; Fang, G. Spatiotemporal Pattern of Pine Wilt Disease in the Yangtze River Basin. Forests 2021, 12, 731. [Google Scholar] [CrossRef]
- Wang, C. Study on the Harm of Pine Wood Nematode Disease and Comprehensive Control Countermeasures. Anhui Agric. Sci. Bull. 2015, 21, 108–109. [Google Scholar]
- Liebhold, A.M.; Tobin, P.C. Population Ecology of Insect Invasions and Their Management. Annu. Rev. Entomol. 2008, 53, 387–408. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, J.; Xu, H.; Yu, Z.; Yuan, L.; Chen, Y.; Huang, H. Evaluating the potential of airborne hyperspectral LiDAR for assessing forest insects and diseases with 3D Radiative Transfer Modeling. Remote Sens. Environ. 2023, 297, 113759. [Google Scholar] [CrossRef]
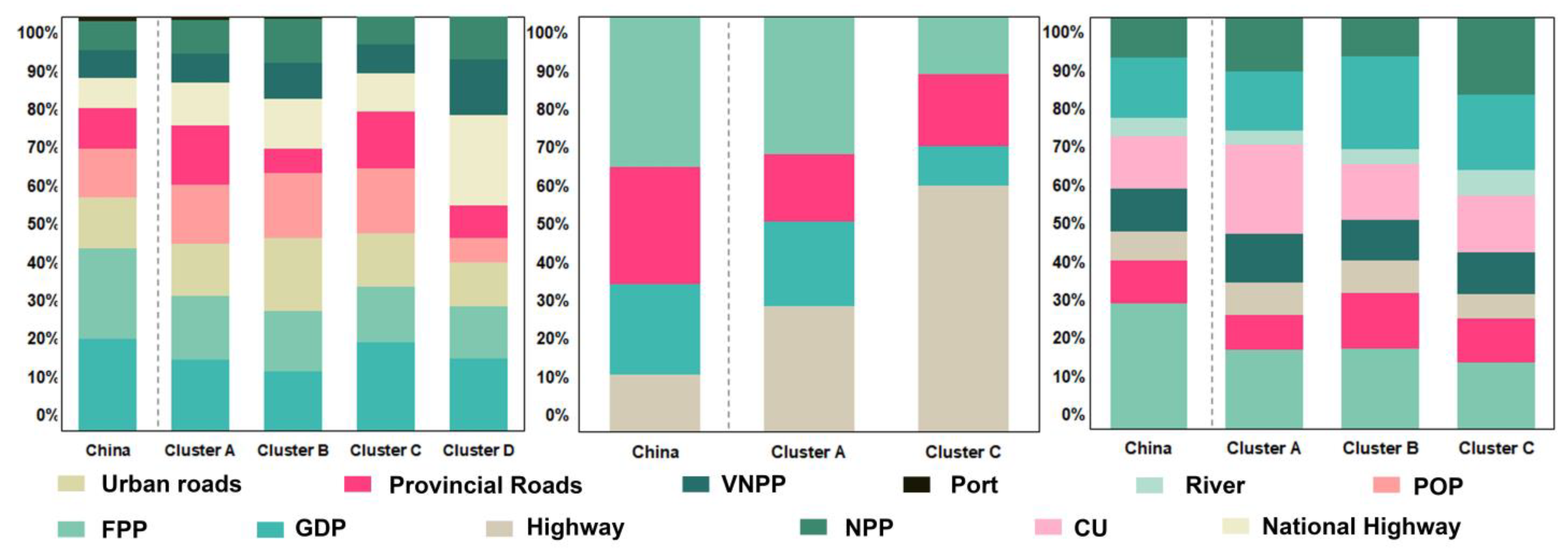
| Type | Factor Name (Meaning) | Description |
|---|---|---|
| Source of spread | NPP (Number of Processing Plants) | The number of timber processing plants within the county and the increments in timber processing plants across different time periods. |
| VNPP (Variation in Number of Processing Plants) | ||
| Pathway | FPP (Forest Proximate People) | The maximum population within one kilometer of forests within the county, derived from host data and population data. |
| Highway | The number of different types of roads in each county. | |
| National highway | ||
| Provincial roads | ||
| Urban roads | ||
| River | Major rivers in China (with ports distributed along them). | |
| Port | Counties with ports identified using WPI data. | |
| Introduction event | CU (Changes of Urbanized) | Urbanization data are used to extract the area CU within each county, reflecting the timber transport volume through project implementation. |
| GDP (Gross Domestic Product) | The total GDP value within the county. | |
| POP (Change of Population) | Population changes within the county across different time periods, representing population mobility. |
| Name | Source | Type | Temporal Type |
|---|---|---|---|
| Elevation | The SRTM 30 m DEM dataset. | Tiff | Non-temporal |
| Forest coverage | This study employed the China National Land Use and Land Cover Dataset (CNLUCC). | Tiff | Temporal |
| Population data | GHS-POP R2023A—GHS multi-temporal population grid dataset (1975–2030), with a spatial resolution of 100 m. | Tiff | Temporal |
| GDP data | The spatial distribution dataset of China’s GDP on a kilometer grid scale is sourced from the Science Data Bank for Resources and Environmental Sciences, with a spatial resolution of 1000 m. | Tiff | Temporal |
| Urbanization data | GHS-SMOD R2023A—GHS settlement layers, applying the Degree of Urbanization methodology (stage I) to GHS-POP R2023A and GHS-BUILT-S R2023A, covering multiple temporal points (1975–2030), with a spatial resolution of 1000 m. | Tiff | Temporal |
| Timber processing plant data | The data extraction process obtained attributes such as names, geographic coordinates (latitude and longitude), and establishment years (https://www.qcc.com/ (accessed on 23 October 2023)). | Shp | Temporal |
| Port data | The World Port Index. | Shp | Non-temporal |
| River | The Geographic Data Sharing Infrastructure at the College of Urban and Environmental Science, Peking University. | Shp | Non-temporal |
| Road network | The Geographic Data Sharing Infrastructure at the College of Urban and Environmental Science, Peking University. | Shp | Non-temporal |
| stage 1 | ||||||
| Factor | VNPP | NPP | FPP | POP | GDP | CU |
| p value | 0.0646 | 0.0501 | <0.0001 | <0.0001 | <0.0001 | 0.2829 |
| ns | ns | **** | **** | **** | ns | |
| Sig | Yes | Yes | Yes | Yes | Yes | No |
| Factor | River | Highway | National highway | Provincial roads | Urban roads | Port |
| p value | 0.9699 | 0.0015 | 0.0014 | 0.0014 | <0.0001 | <0.0001 |
| ns | ** | ** | ** | **** | **** | |
| Sig | No | Yes | Yes | Yes | Yes | Yes |
| stage 2 | ||||||
| Factor | VNPP | NPP | FPP | POP | GDP | CU |
| p value | 0.9851 | 0.1157 | 0.0002 | 0.4117 | 0.0787 | 0.2021 |
| ns | ns | *** | ns | ns | ns | |
| Sig | No | No | Yes | No | Yes | No |
| Factor | River | Highway | National highway | Provincial roads | Urban roads | Port |
| p value | 0.195 | 0.0002 | 0.3249 | 0.0008 | 0.4256 | 0.6164 |
| ns | *** | ns | *** | ns | ns | |
| Sig | No | Yes | No | Yes | No | No |
| stage 3 | ||||||
| Factor | VNPP | NPP | FPP | POP | GDP | CU |
| p value | <0.0001 | 0.0003 | <0.0001 | 0.1522 | <0.0001 | <0.0001 |
| **** | *** | **** | ns | **** | **** | |
| Sig | Yes | Yes | Yes | No | Yes | Yes |
| Factor | River | Highway | National highway | Provincial roads | Urban roads | Port |
| p value | 0.0352 | 0.0454 | 0.1076 | 0.0093 | 0.1205 | 0.887 |
| * | * | ns | ** | ns | ||
| Sig | Yes | Yes | No | Yes | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Huang, W.; Zhang, B.; Chen, Y.; Fang, G.; Guo, J.; Zhang, Y. Long-Term Spatiotemporal Pattern and Temporal Dynamic Simulation of Pine Wilt Disease. Remote Sens. 2025, 17, 348. https://doi.org/10.3390/rs17030348
Hao Z, Huang W, Zhang B, Chen Y, Fang G, Guo J, Zhang Y. Long-Term Spatiotemporal Pattern and Temporal Dynamic Simulation of Pine Wilt Disease. Remote Sensing. 2025; 17(3):348. https://doi.org/10.3390/rs17030348
Chicago/Turabian StyleHao, Zhuoqing, Wenjiang Huang, Biyao Zhang, Yifan Chen, Guofei Fang, Jing Guo, and Yucong Zhang. 2025. "Long-Term Spatiotemporal Pattern and Temporal Dynamic Simulation of Pine Wilt Disease" Remote Sensing 17, no. 3: 348. https://doi.org/10.3390/rs17030348
APA StyleHao, Z., Huang, W., Zhang, B., Chen, Y., Fang, G., Guo, J., & Zhang, Y. (2025). Long-Term Spatiotemporal Pattern and Temporal Dynamic Simulation of Pine Wilt Disease. Remote Sensing, 17(3), 348. https://doi.org/10.3390/rs17030348








