1. Introduction
The nursery industry significantly contributes to the United States economy, providing substantial economic value and employment opportunities. Behe et al. (2008) reported that nursery production contributed USD 18.1 billion to the US economy in 2002 and created nearly 2,000,000 jobs [
1]. This economic impact underscores the importance of the nursery sector in supporting livelihoods and driving economic growth. Additionally, Hall et al. (2020) [
2] highlighted that nursery and greenhouse production firms accounted for a substantial number of jobs and contributed significantly to the GDP, emphasizing the industry’s economic significance. In the context of nursery production, water stress in ornamental plants poses a critical challenge. Toscano et al. (2019) [
3] discussed the responses of Mediterranean ornamental plants to drought stress, which include alterations in root-to-shoot ratio, growth reduction, changes in leaf anatomy, and reductions in leaf size and total leaf area. The biophysical alterations collectively minimize water loss to ensure optimal photosynthesis and subsequent carbon assimilation. Understanding these responses is crucial for nursery operators to implement effective water management strategies and mitigate the impact of water stress, plant health and growth. Majsztrik et al. (2017) [
4] highlighted the reliance of specialty crop producers, including nursery operations, on high-quality fresh water from surface and groundwater sources for containerized production. Water availability and quality are essential considerations in nursery production as inadequate water supply or poor water management practices can exacerbate water stress in ornamental plants. Álvarez and Sánchez-Blanco (2013) [
5] emphasized the importance of accurately scheduling irrigation to match water loss through evapotranspiration from the crop canopy and soil, underscoring the need for precise irrigation management in container ornamental nurseries with little water storage within the substrate to address water stress effectively. Efforts to address water stress in ornamental plants in nursery production involve various strategies including adopting advanced technologies and practices. Cameron et al. [
6] controlled water stress to manage shoot growth in container-grown Rhododendron cv. Hoppy, demonstrating that regulated water stress can reduce excessive growth and subsequent need to prone, leading to more compact and marketable plants. Similarly, Scagel et al. [
7] explored irrigation frequency and nutrient uptake in container-grown Rhododendron species, demonstrating that careful water management can minimize stress while supporting plant growth. These studies underscore the potential of water management techniques to optimize water use and plant health in nurseries through both stress-based and technology-driven solutions.
The nursery industry significantly contributes to the U.S. economy, providing substantial economic value and employment opportunities. However, water stress in ornamental plants poses a significant challenge for nursery operators, necessitating the adoption of effective water management strategies and innovative solutions to mitigate the impact of drought stress on plant health and productivity [
8]. By integrating advanced technologies, precise irrigation practices, and biological interventions, nurseries can enhance water use efficiency, optimize plant responses to water stress, and sustainably manage water resources in ornamental plant production.
Hyperspectral cameras have gained significant attention in agriculture due to their ability to provide detailed spectral information beyond the capabilities of traditional RGB cameras [
9]. These advanced imaging devices capture a wide range of wavelengths across the electromagnetic spectrum, enabling the detection of subtle changes in plant health and stress levels [
8]. They have been widely applied in plant phenotyping, disease detection, and monitoring stress factors such as water stress [
10,
11]. By analyzing the reflected spectral signatures of plants, hyperspectral imaging offers valuable insights into the early onset of diseases and stress factors, facilitating timely interventions to mitigate potential crop losses [
12,
13]. Furthermore, hyperspectral imaging has been instrumental in exploring natural plant diversity to improve tolerance to stressors like excess salt, contributing to sustainable agricultural practices [
14].
Hyperspectral cameras have shown great promise in the specific context of discriminating water stress levels in crops. By analyzing hyperspectral imagery and utilizing machine learning algorithms, researchers have been able to estimate water stress in mono-cultured crops (i.e., potato) with a high degree of accuracy [
13]. The spectral information captured by hyperspectral cameras allows for the development of spectral indices that can indicate the water status of plants, enabling farmers to make informed decisions regarding irrigation and water management strategies [
15]. This technology has the potential to revolutionize how water stress is monitored in agricultural settings leading to more efficient water usage and improved crop yields. Moreover, hyperspectral imaging has been used to discriminate between different types of stress factors affecting plants, including abiotic and biotic stresses. By analyzing hyperspectral data, researchers have been able to differentiate between drought stress caused by environmental factors and stress induced by pests or diseases [
16]. This capability is crucial for farmers to accurately diagnose the underlying causes of stress in their crops and implement targeted interventions to address specific stressors effectively. Furthermore, the combination of hyperspectral imaging with advanced technologies such as artificial neural networks and deep learning has enhanced the detection and identification of plant diseases. By training algorithms on hyperspectral images, researchers have developed models capable of identifying specific pathogens affecting plants, such as viruses and pests [
17]. This approach not only aids in early disease detection but also enables researchers to track the spread of diseases and assess their impact on crop health. In the realm of precision agriculture, hyperspectral cameras have proven to be valuable tools for monitoring crop health and optimizing agricultural practices. By providing detailed information about plant physiological processes, hyperspectral imaging can assist farmers in making data-driven decisions to enhance crop productivity and sustainability [
18]. Using hyperspectral data to assess biomass, nitrogen content, and other key indicators of plant health enables farmers to implement targeted interventions and maximize yields while minimizing resource inputs. Virnodkar et al. emphasized the critical role of machine learning in analyzing hyperspectral data for various crops, underscoring its potential to enhance water stress determination [
19]. The ability of these algorithms to process large datasets and identify subtle variations in spectral signatures is crucial for timely intervention in agricultural practices. Physiological indicators of water stress have also been a focal point in recent studies. Wong et al. utilized hyperspectral reflectance to phenotype the physiological drought response in common and tepary beans, measuring parameters such as stomatal conductance and leaf water potential (LWP) [
20]. These indicators are sensitive to changes in plant water content and stress, providing a direct link between hyperspectral data and plant health. Furthermore, Park et al. explored the relationship between canopy temperature and water stress, using UAV-borne thermal sensing alongside hyperspectral imaging to monitor plant water status effectively [
21]. This multi-faceted approach highlights the importance of integrating different sensing modalities to enhance the understanding of plant responses to water availability. Technological innovations in hyperspectral imaging systems have also contributed to advancements in water stress detection. The development of UAV-borne hyperspectral systems has made high-resolution data collection more accessible and efficient. Liu et al. designed a UAV-based hyperspectral imaging system specifically for water quality monitoring, showcasing the versatility of these technologies in agricultural applications [
22]. Additionally, the continuous improvement of hyperspectral sensors, which capture a wide range of spectral bands, allows for more precise assessments of plant health and stress levels [
23]. This capability is essential for precision agriculture, where timely and accurate data can significantly impact crop management decisions. Susič et al. demonstrated the ability of hyperspectral imaging to differentiate abiotic from biotic stress in tomato plants, emphasizing the role of spectral analysis in diagnosing and addressing complex stress scenarios [
16]. Similarly, Zeng et al. presented an improved hyperspectral index for monitoring water stress in real-time field conditions, highlighting the potential for integrating this technology into large-scale agricultural practices [
24]. These recent studies underscore the growing adoption of hyperspectral imaging in stress detection and its applicability to dynamic agricultural environments. Hyperspectral cameras have revolutionized the field of agriculture by offering a non-invasive and comprehensive way to monitor plant health, detect diseases, and assess stress factors such as water stress. The detailed spectral information provided by hyperspectral imaging allows for precise analysis of plant conditions, enabling farmers and researchers to make informed decisions to optimize crop production and sustainability. As technology advances, hyperspectral imaging is poised to play an increasingly vital role in shaping the future of agriculture by providing valuable insights into plant health and stress factors.
The objective of this work was to investigate and quantify the spectral responses of four ornamental plant taxa—Rosa hybrid (rose), Itea virginica (itea), Spiraea nipponica (spirea) and Weigela florida (weigela)—under varying levels of water stress. Utilizing hyperspectral imaging combined with principal component analysis (PCA), the study aims to identify key spectral bands associated with physiological changes induced by water deprivation. By analyzing how these spectral signatures evolve over time, the research seeks to develop reliable indicators of crop water stress spatially across taxa that can be used for early detection and monitoring of ornamental plant species, ultimately contributing to improved water management practices. These taxa were selected to represent a diverse range of morphological characteristics and drought tolerance levels, providing a spectrum of responses to water stress. Rosa hybrid, a widely cultivated ornamental species, was included for its economic importance and high sensitivity to drought conditions. Itea virginica and Spiraea nipponica, with moderate drought tolerance, were chosen as examples of shrubs that can endure varying levels of stress. Weigela florida, known for its robust morphology and higher drought tolerance, was included to assess the sensitivity of hyperspectral imaging in detecting subtle stress responses. This selection ensures that the findings are broadly applicable to ornamental plants with varying physiologies and drought adaptations.
2. Materials and Methods
The experiment was conducted at the Hampton Roads Agricultural Research and Extension Center, Virginia Beach, VA, USA. The nursery pad used for this experiment was prepared to accommodate a grid layout for plant placement and sensor analysis.
Figure 1 shows the four ornamental plant taxa used in the experiment to evaluate the effects of water stress: rose, itea, spirea, and weigela. These taxa were chosen to represent a range of morphological traits and drought tolerance levels, ensuring diverse responses to water stress for analysis. Rose, a widely cultivated and economically important species, is highly sensitive to drought. Itea and spirea were selected for their moderate drought tolerance, while weigela—known for its robust morphology—was included to evaluate hyperspectral imaging’s ability to detect subtle stress responses.
2.1. Morphological and Drought Response Characteristics of Four Ornamental Plant Taxa
Table 1 provides detailed descriptions and measurements for four ornamental plant taxa used in the study:
Weigela florida (“Czechmark Trilogy™”),
Itea virginica (“Scentlandia
®”),
Spiraea nipponica (“Wedding Cake
®”), and rose hybrid (“Julia Child™”). Each taxon was characterized by specific morphological traits, including average height, width, and substrate depth as well as qualitative descriptions of leaf structure, color, and overall plant form.
Following the collection of hyperspectral images, one plant was randomly selected from each water stress level for each plant taxa and photographed as shown in
Figure 2,
Figure 3,
Figure 4 and
Figure 5.
Weigela is noted for its round shape, coarse texture, and moderate drought resistance, displaying leaf curling under stress. Itea features an upright, vase-like form with medium- to light-green foliage and high drought resistance, showing leaf droop under severe water stress. Spirea presents as a fine-textured, spreading ground cover with poor drought resistance, characterized by blue-green foliage. Rose hybrid is a medium-textured, round shrub with dark-green, glossy leaves and ongoing flowering, showing resilience across different growth stages. These observations provide critical insights into the physical responses of these taxa under varying environmental conditions.
2.2. Plant Arrangement and Treatment
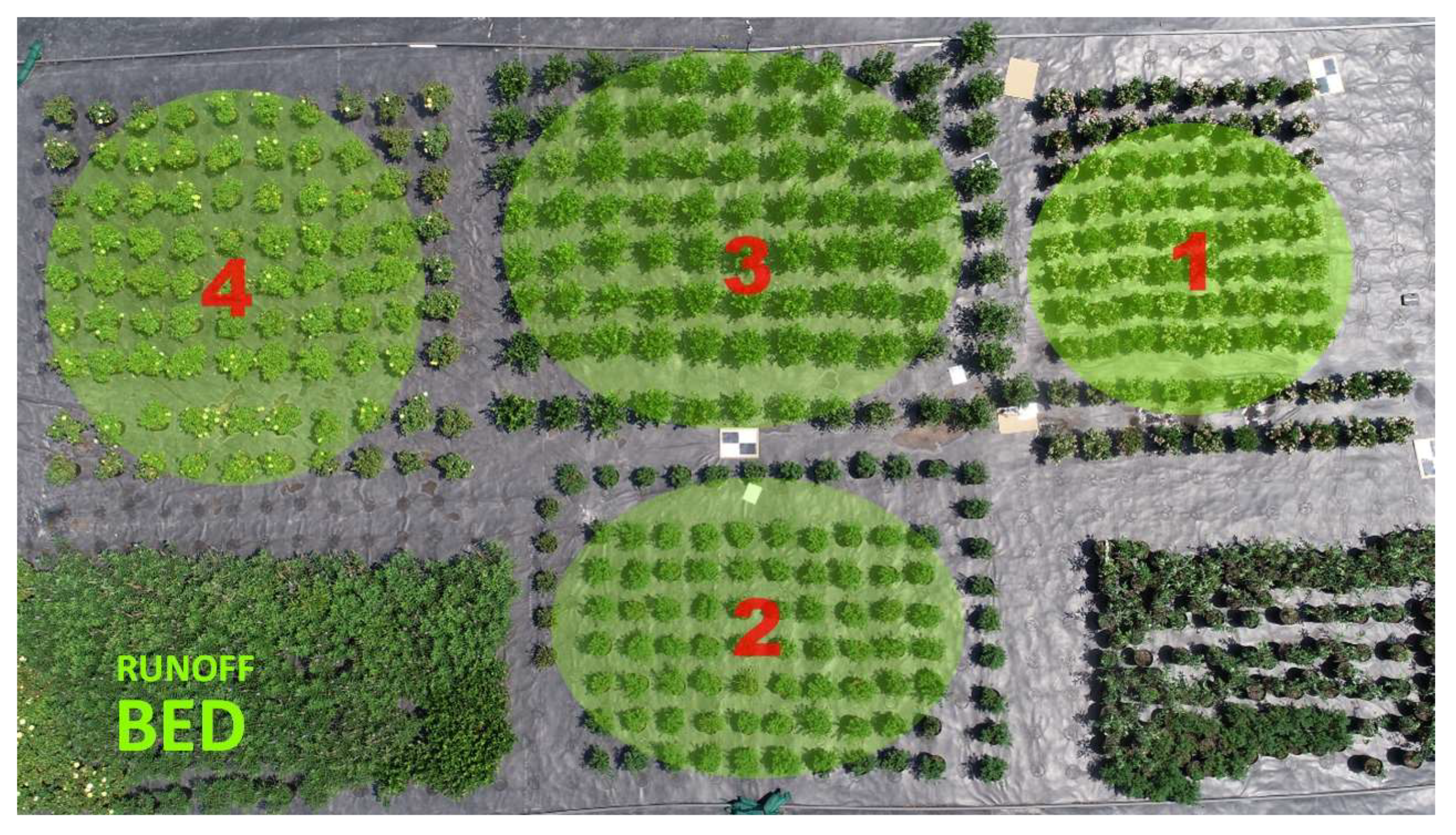
Eleven-liter (#3, C1200, Nursery Supplies, Chambersburg, PA, USA) containers filled with a pine bark substrate amended with dolomite and controlled release fertilizer were used to grow the four taxa under overhead irrigation. Plants were pruned to maintain a healthy and uniform canopy until experiment initiation. All taxa were subjected to four distinct levels of water stress, with five plants (n = 5) allocated to each stress level. To maintain experimental rigor, treated plants were interspersed among 50 non-water stressed control plants of the same taxon. Plants were arranged in a 7 × 10 grid pattern to ensure even spacing which facilitated uniform conditions for the experiment as shown in
Figure 6. Taxa were grouped into four distinct areas on the nursery pad, with each area corresponding to a specific taxon. Area 1 was designated for weigela, Area 2 for spirea, Area 3 for itea, and Area 4 for rose, as indicated by the numbers in the image.
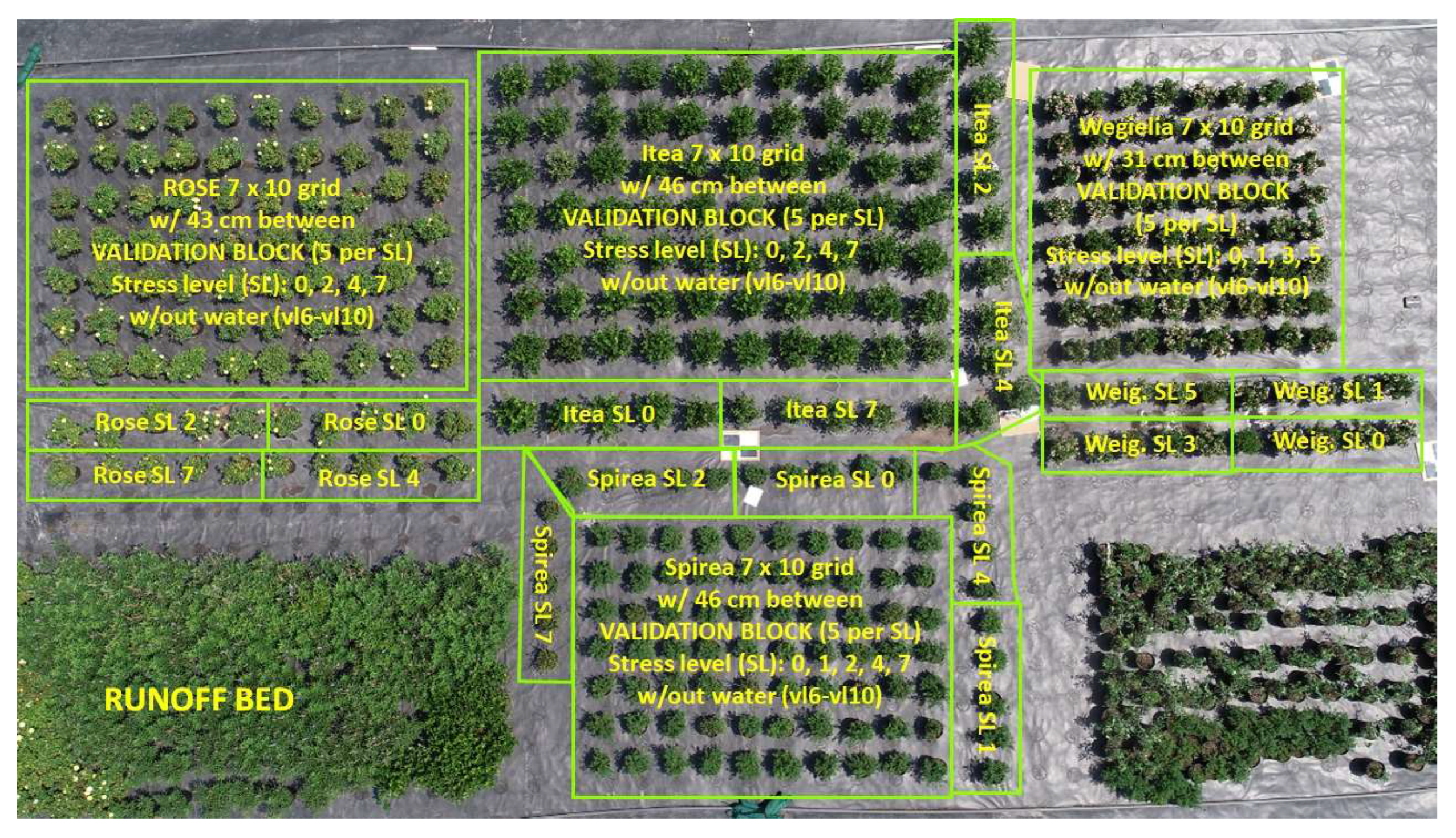
2.3. Training and Validation Areas
The sides of each group, as marked in the image (
Figure 7), were designated as training areas. Here, plants were used to calibrate the hyperspectral imaging sensors and to develop predictive models. These training areas are crucial for establishing baseline spectral data and ensuring the accuracy of the subsequent analysis.
The center of each group served as the validation area. Plants in these sections were used to test the predictive models developed from the training data to assess the models’ accuracy and generalizability under real-world conditions. This approach helped minimize potential external variables, ensuring that the hyperspectral imaging results were both reliable and reproducible.
Water stress was induced by removing the selected plants from their regular irrigation schedule and withholding water for varying durations of up to 7 days. The water stress levels were defined as follows: Level 0 for well-watered plants, Level 1 for 1 day without water, Level 2 for 2 days without water, Level 3 for 3 days without water, Level 4 for 4 days without water, Level 5 for 5 days without water, and Level 7 for 7 days without water. The non-water stressed plants continued to receive regular irrigation throughout the experiment.
2.4. Preparation and Handling of Plants
Plants were carefully removed from their respective growing areas and saturated to effective container capacity as outlined in Fields et al. [
25]. This saturation process was achieved through hand watering using a wand and breaker. Each plant received three rounds of watering, with each round lasting a minimum of 3 s. To ensure thorough saturation, a 1 min interval was maintained between each watering event, allowing the substrate to fully absorb water between rounds.
Following this saturation procedure, plants were allowed to drain for a standardized period of 30 min to remove excess water while retaining sufficient moisture within the container capacity. After draining, each plant was weighed individually using a Defender 3000 scale (model #D30BR, Ohaus Corp, Parsippany, NJ, USA) to obtain accurate weight measurements corresponding to their fully saturated state.
On August 21, all plants were initially positioned on a bench in a glass covered greenhouse. To protect them from any precipitation and ensure consistent drying conditions, plants were then transferred to gravel ground cover adjacent to the greenhouse on August 22, where they remained until August 27. This gravel-covered area minimized unintended water input while allowing for a controlled drying environment. Plants designated for drying treatments (i.e., those not receiving any water) stayed on the gravel ground cover to ensure consistency in water stress application. On August 28, plants were weighed again before being assigned as training plants or randomly allocated as validation plants within the experimental setup. This preparation ensured a consistent and controlled baseline for both the training and validation phases of the study.
2.5. Hyperspectral Imaging
Five #3 flowering shrubs at each of the four water stress levels (n = 5) and five non-water-stressed plants were grouped by treatment. These plants were placed adjacent to the experimental block and used to train and calibrate the hyperspectral imaging sensors.
The hyperspectral camera (Rikola, Senop, Helsinki, FI, USA) (
Figure 8a) was used to collect hyperspectral data. The camera is a snapshot with spectral region from 503~898 nm. The selected configuration measured 55 bands equally distributed in 7 nm steps with 8~12 nm Full Width at Half Maximum (FWHM). It has a 1010 × 1010 pixel spatial resolution and 1.5 s measurement time. Calibration was performed using a 50% grey reference panel (SphereOptics GmbH, Herrsching, Germany) on each flight. The reference panel images were captured immediately before and after the imaging of the plants to ensure consistent normalization under varying lighting conditions. The hyperspectral camera was mounted to an unmanned aerial system (Matrice 600 Pro, DJI, Shenzhen, China) (
Figure 8b) with a 3-axis gimbal (Ronin, DJI, Shenzhen, China).
For each plant, hyperspectral data were collected from three distinct points in the canopy. The average of these three points was calculated to represent the spectral signature of each individual plant. This approach helped to account for any intra-plant variability and ensured a more reliable representation of the plant’s water stress level. The raw hyperspectral data were corrected for sensor-specific distortions and noise using the manufacturer’s software (Senop HSI-2 v.2020.01.03.1), which included radiometric and geometric corrections. Radiometric correction accounted for the camera’s sensitivity across different wavelengths, and geometric correction ensured proper alignment of spectral bands. Reflectance data were then normalized using the grey reference to generate consistent spectral signatures. Outlier pixels or anomalous data were identified and excluded during preprocessing to ensure the quality and reliability of the spectral data.
2.6. Data Analysis
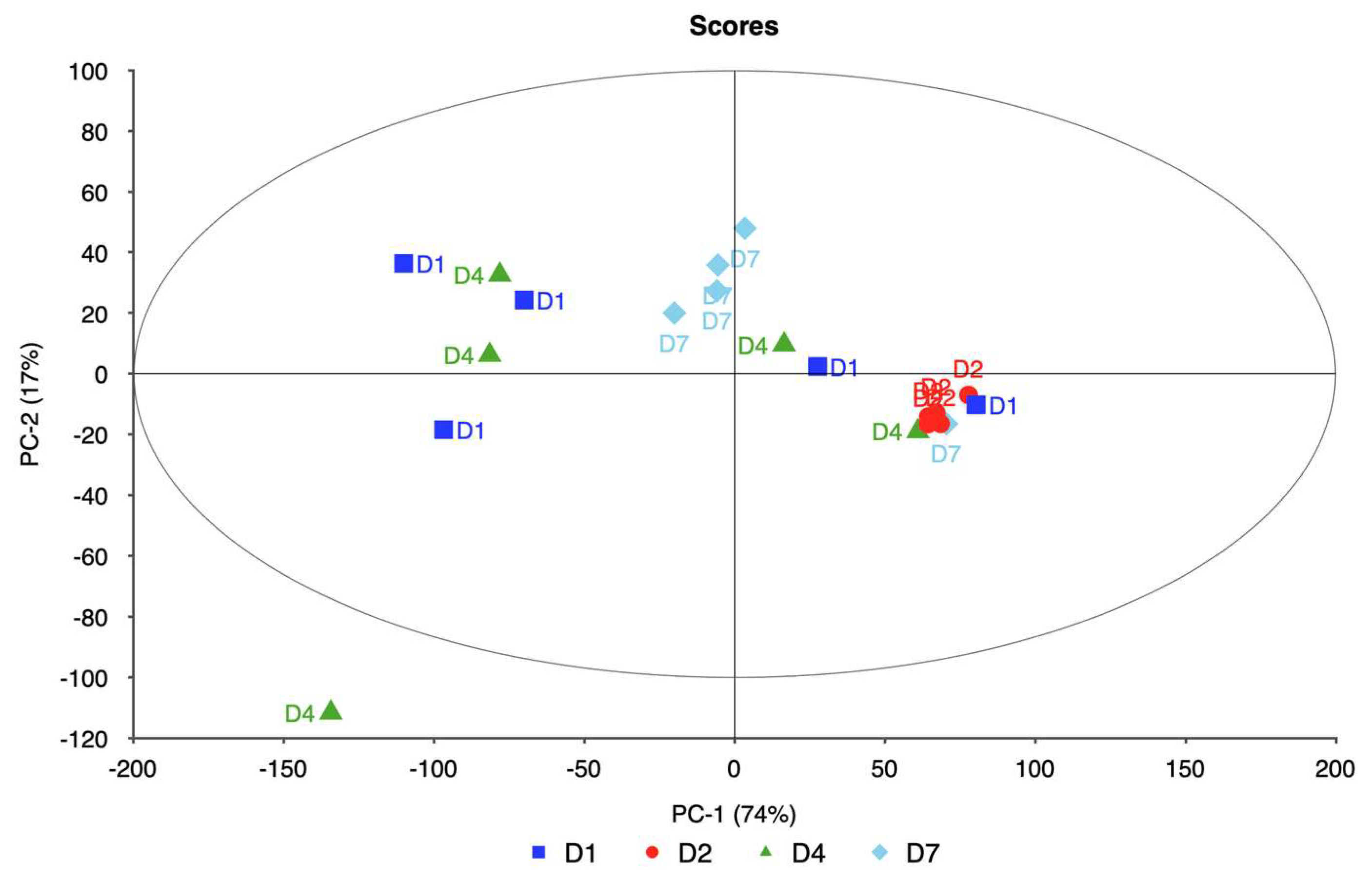
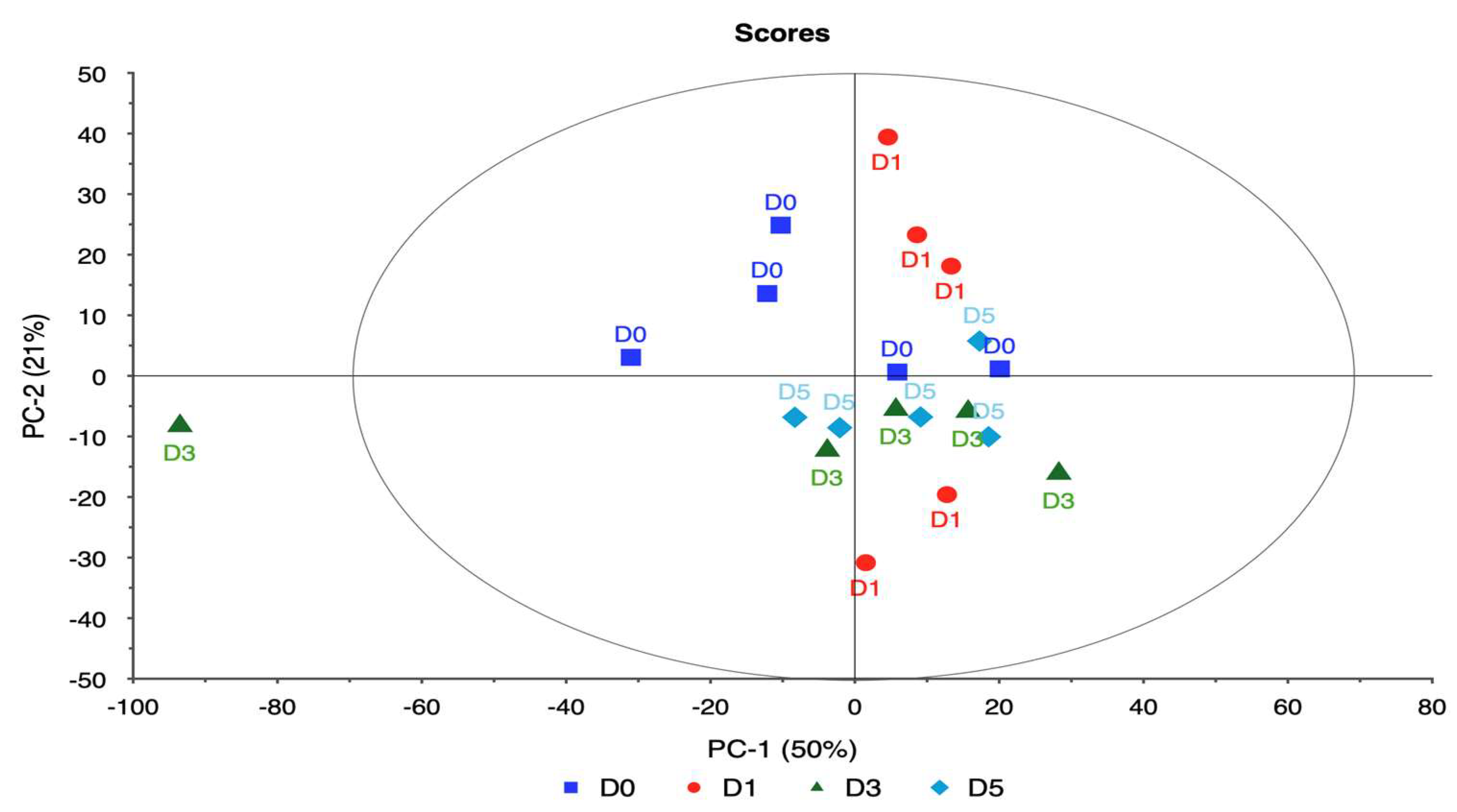
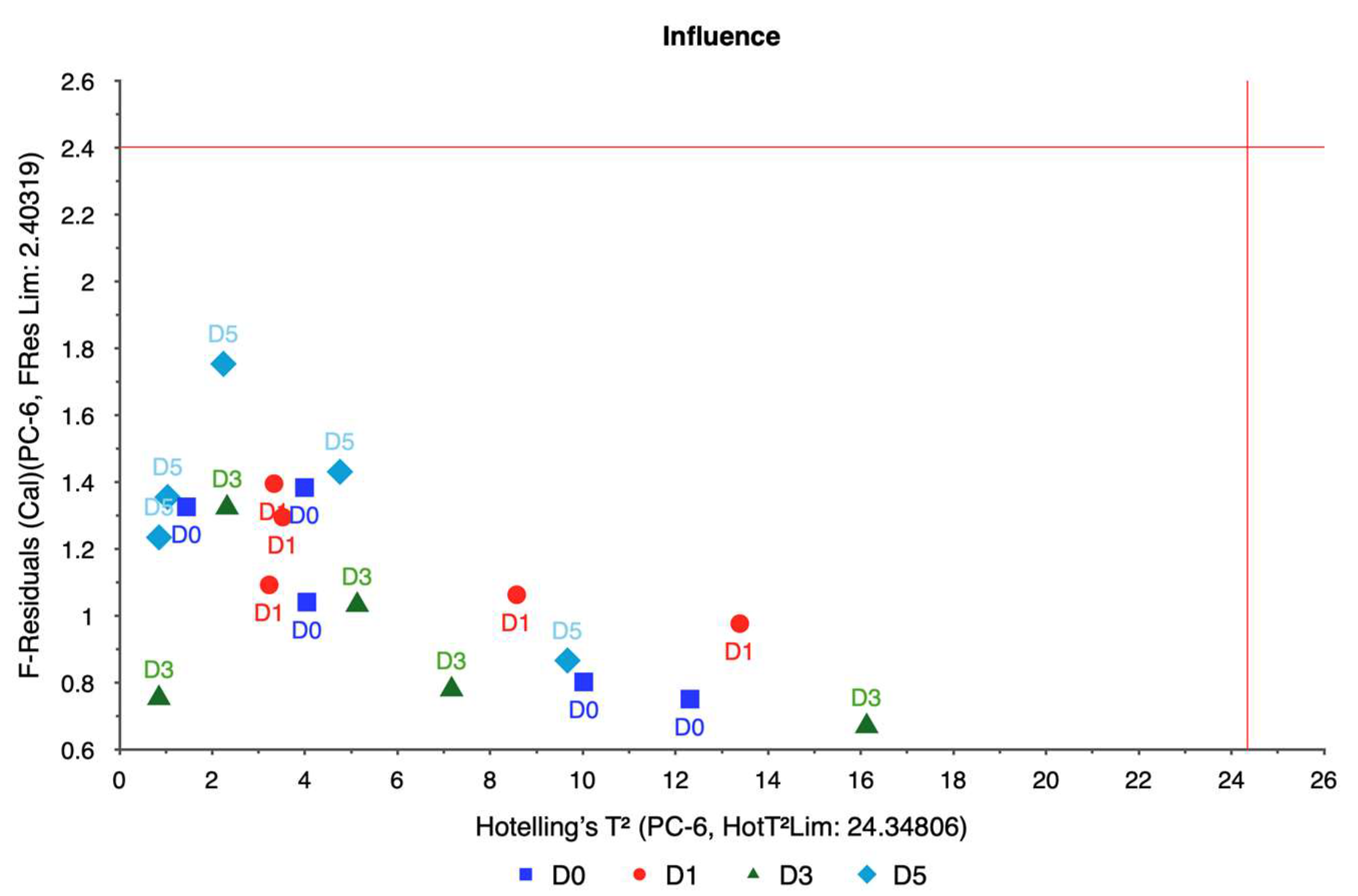
Data analysis in this study involved hyperspectral imaging data collected from four ornamental plant taxa—rose, itea, spirea, and weigela—subjected to varying levels of water stress. For each taxon, spectral data were collected across multiple wavelengths, capturing the reflectance characteristics of the plants under different stress levels. Data points for this study were selected through a systematic approach aimed at capturing representative measurements across varying water stress levels and ensuring consistency within each taxon. For each plant, spectral data were collected from three distinct points to account for intra-plant variability and provide a comprehensive representation of each plant’s physiological state. These three data points were averaged to produce a single, consolidated spectral signature per plant, minimizing potential variability and noise due to localized differences within the canopy.
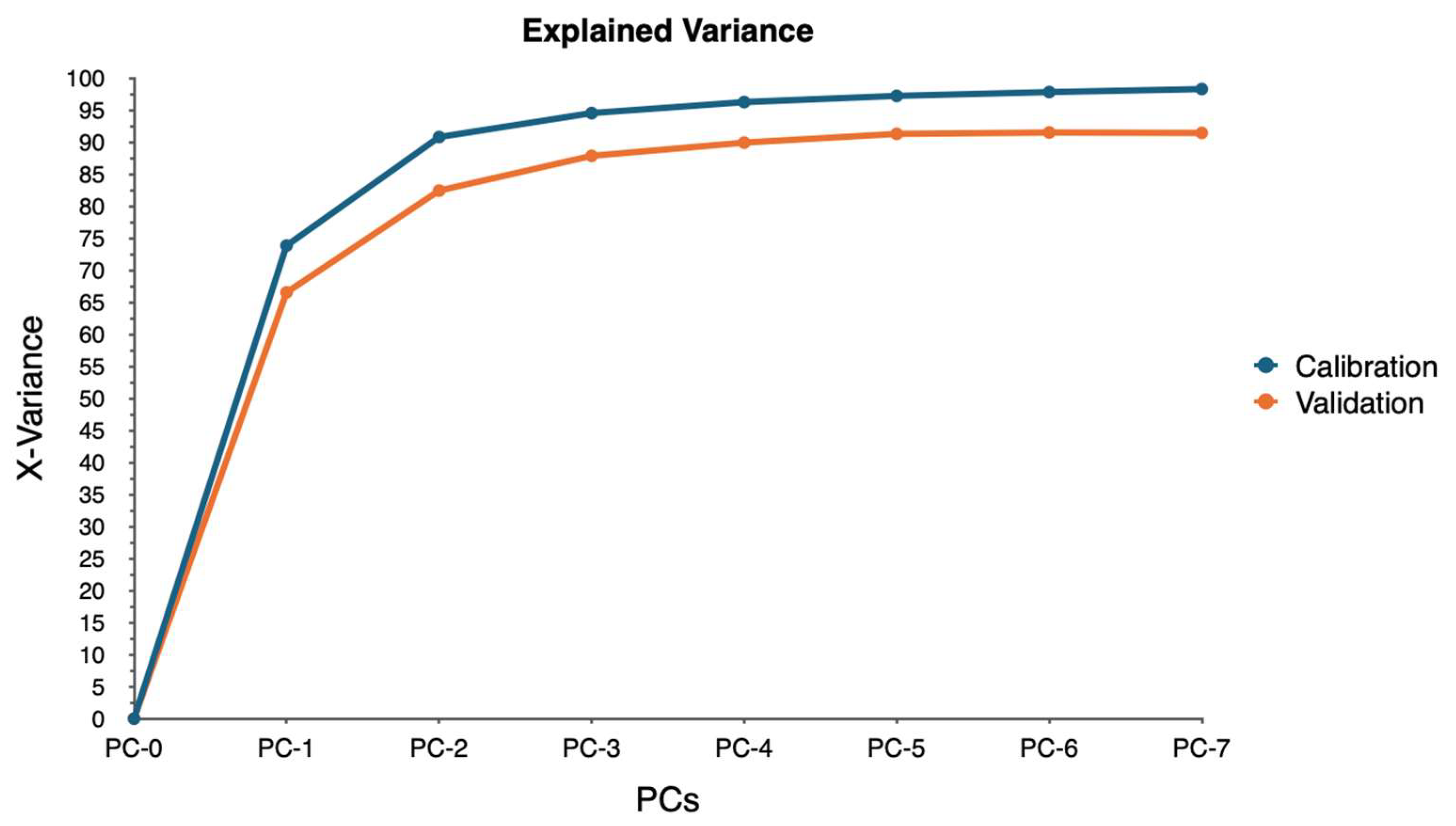
PCA was employed to analyze this high-dimensional data utilizing the singular value decomposition (SVD) algorithm. SVD was chosen for its robustness and ability to handle large datasets efficiently. The analysis aimed to reduce the dimensionality of the data while retaining the most significant variance, enabling the identification of key spectral bands associated with water stress.
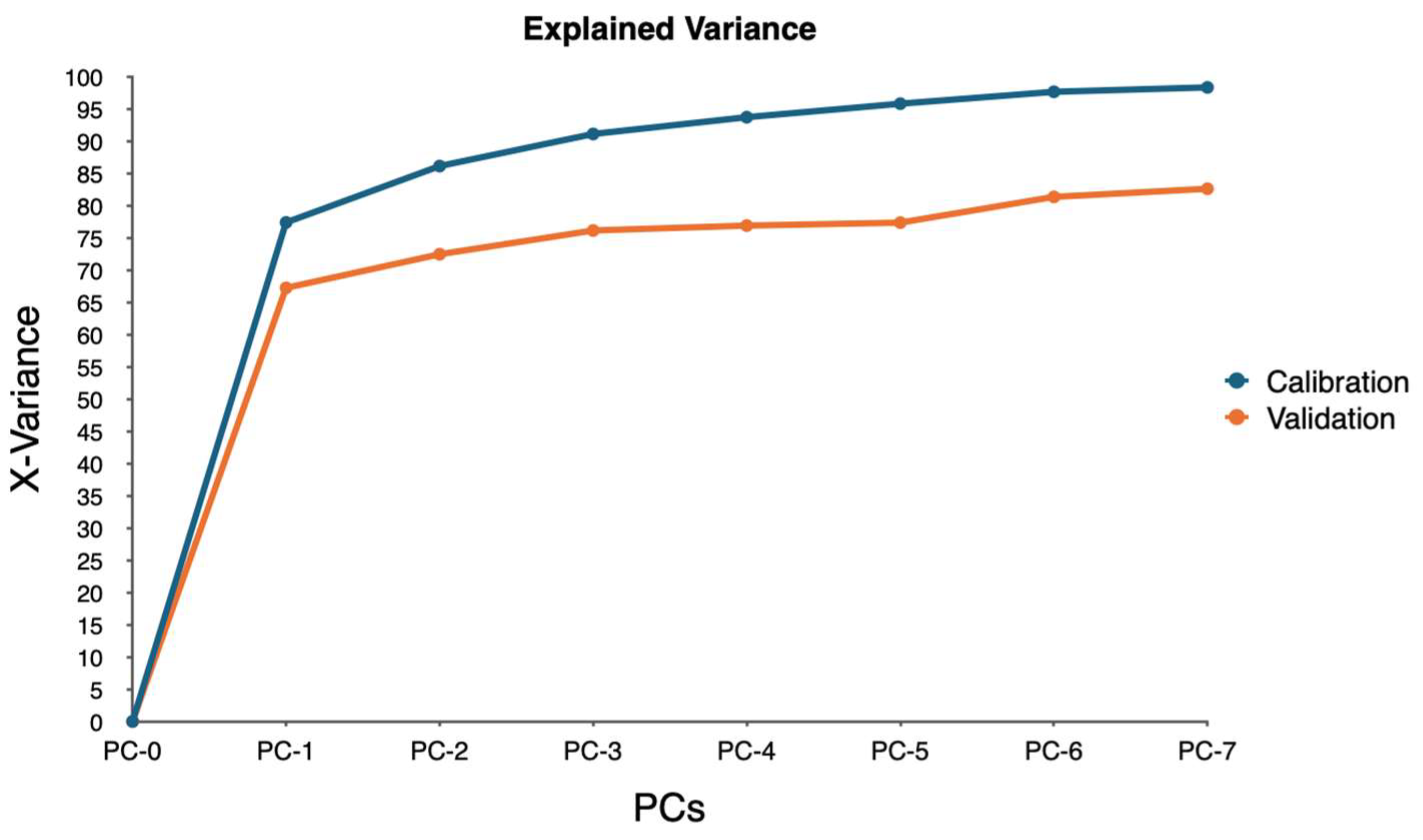
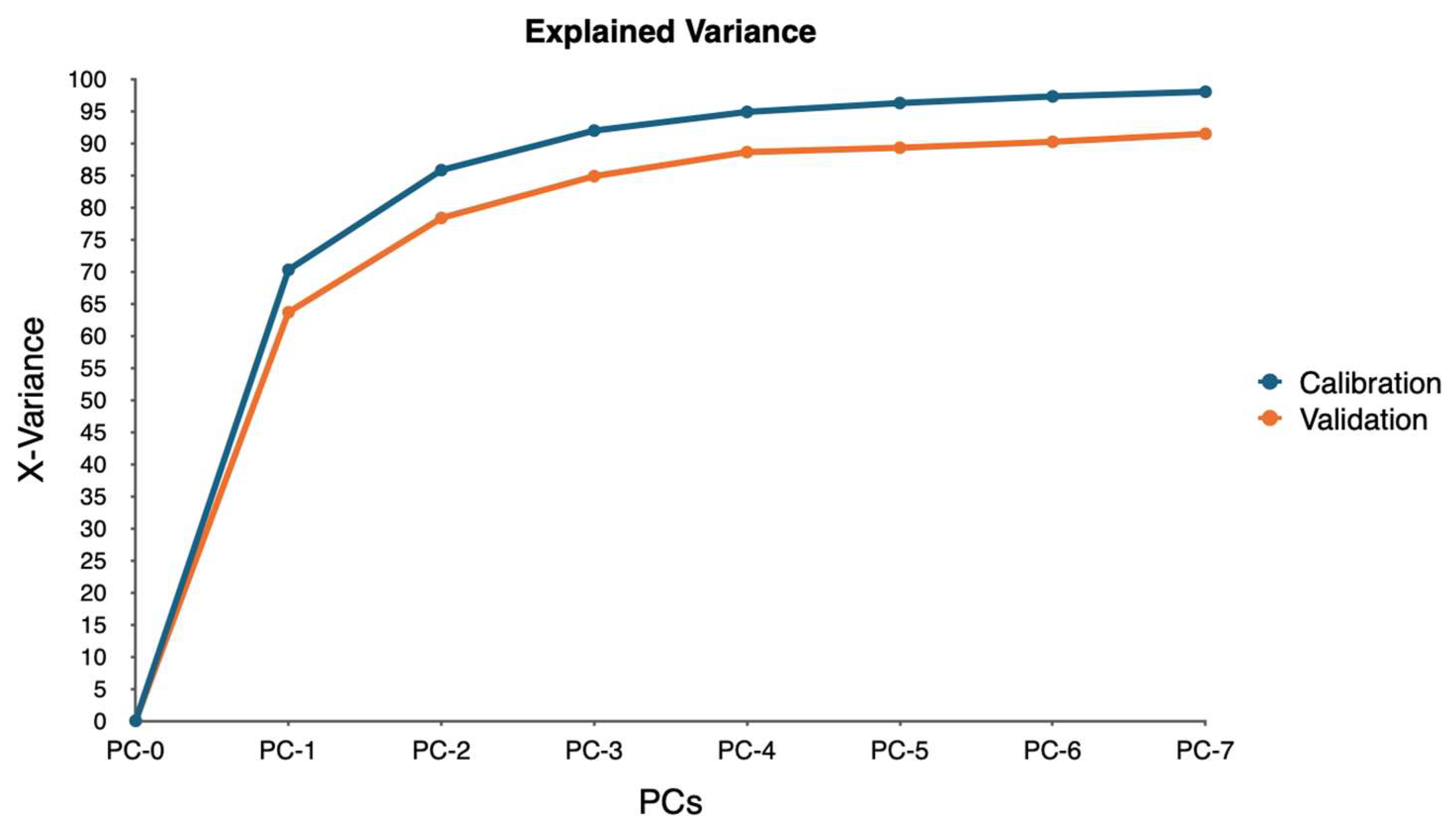
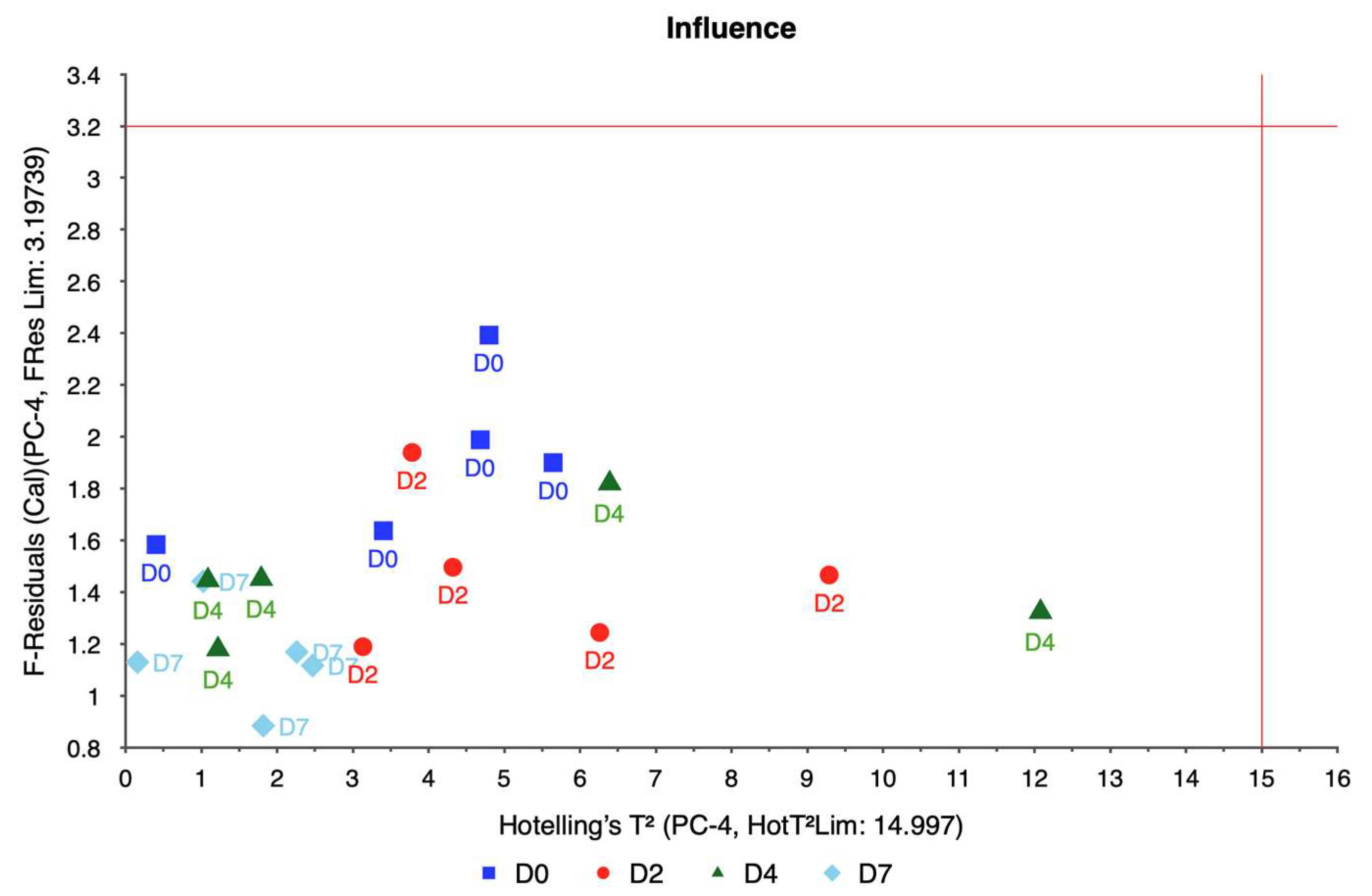
The PCA model was validated using cross-validation, specifically a full cross-validation method with 20 segments. This approach allowed for a thorough assessment of the model’s performance and ensured that the results were reliable and generalizable. The analysis considered seven components, with the model suggesting four as the optimal number of components. This optimal number was determined to be four based on the explained variance and the ability to distinguish between different levels of water stress.
4. Discussion
This study focused on evaluating the spectral responses of four ornamental plant taxa—rose, itea, spirea, and weigela—under varying levels of water stress using hyperspectral imaging combined with PCA. The goal was to identify key spectral bands associated with water stress and to assess the potential of these bands for early detection and monitoring of plant health as shown in
Table 2.
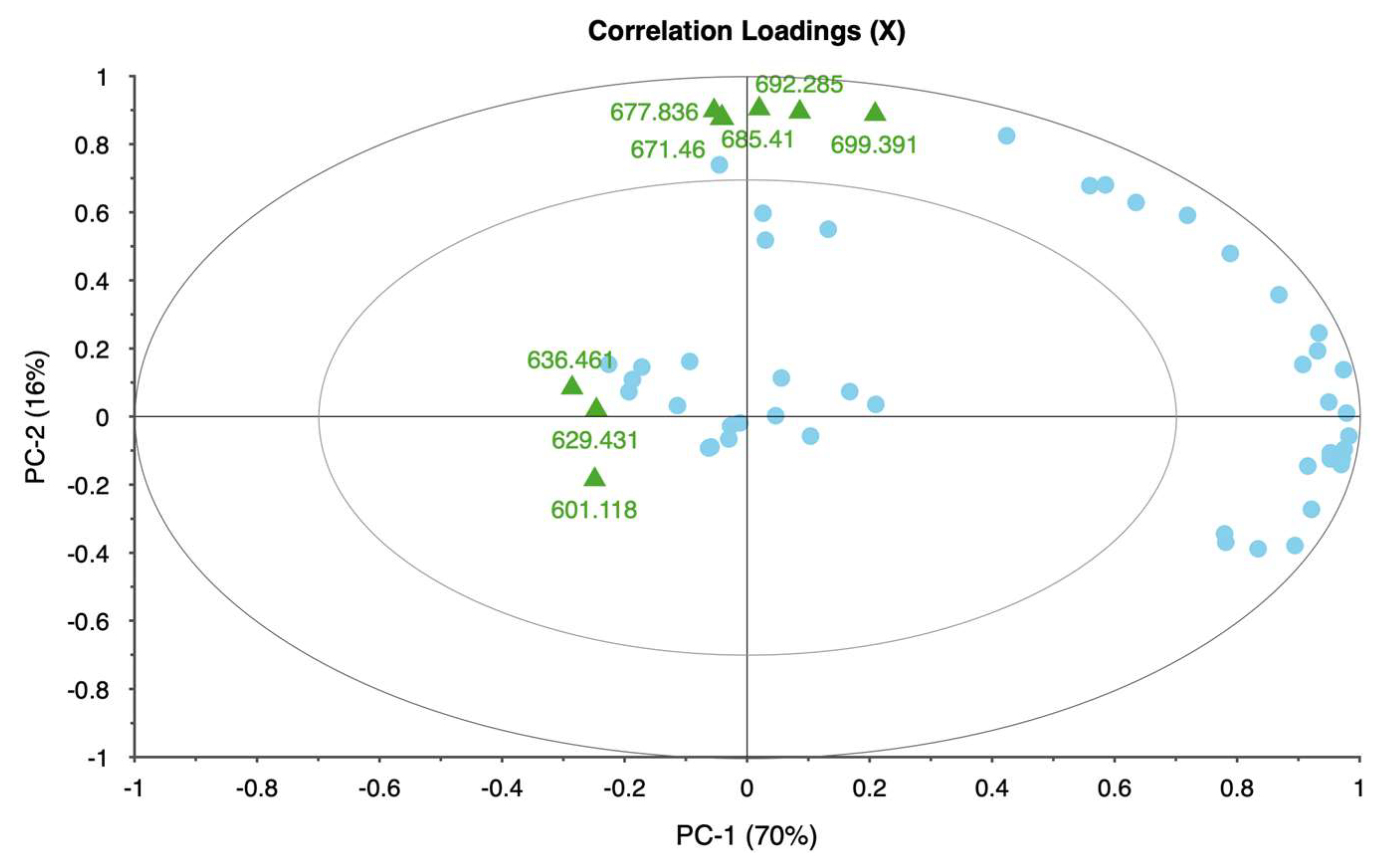
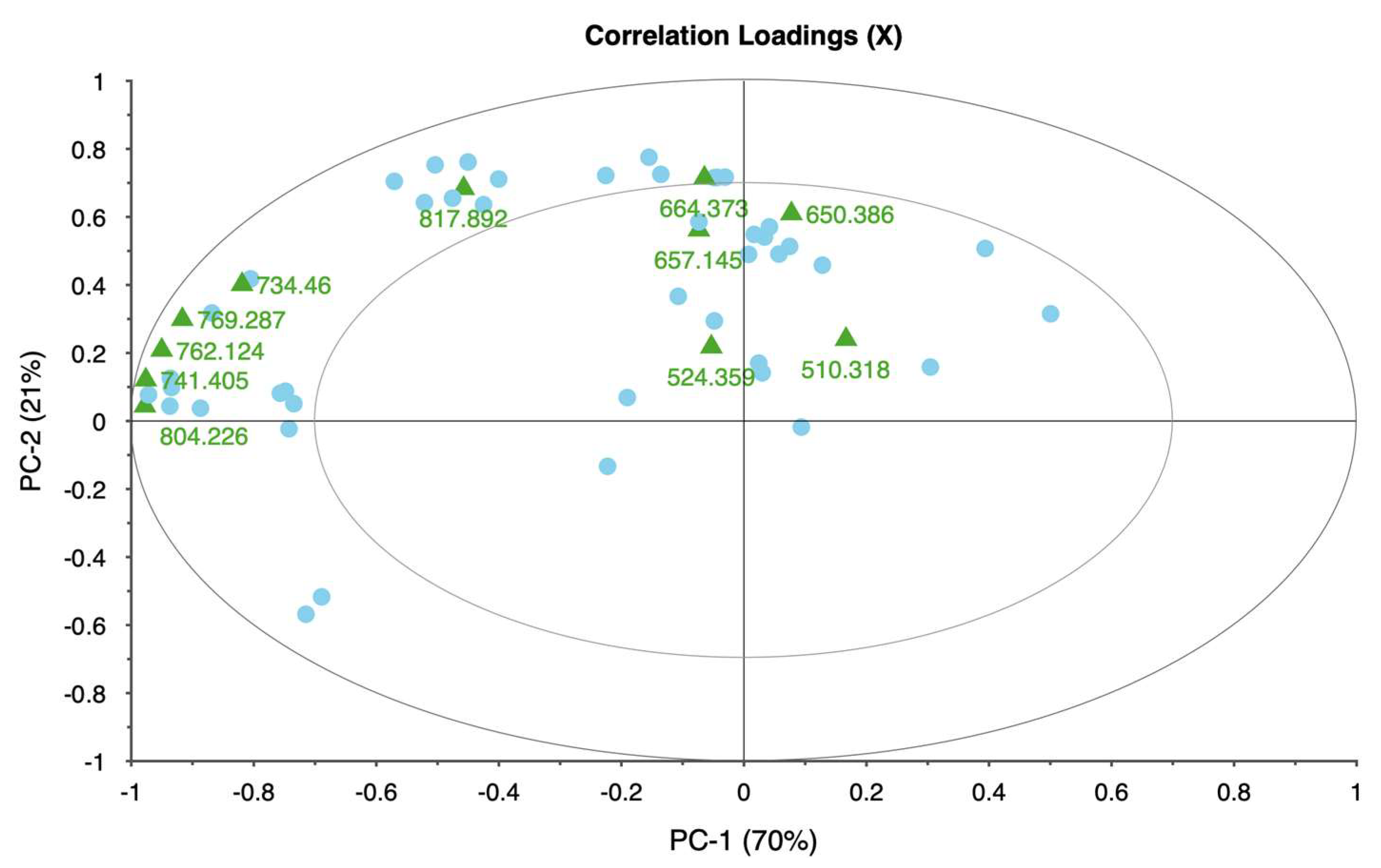
Each taxon exhibited distinct patterns in reflectance across spectral bands, demonstrating unique physiological adaptations and responses to water deficits. Rose displayed significant changes in the red and near-infrared regions, specifically at 650.386, 692.285, 734.460, and 762.124 nm. These shifts in reflectance were strongly associated with water stress, allowing for clear differentiation between stressed and unstressed samples. The high explained variance achieved in rose’s principal components analysis suggests that these spectral bands reliably captured the effects of water stress, making rose a responsive model for studying drought sensitivity in ornamental plants.
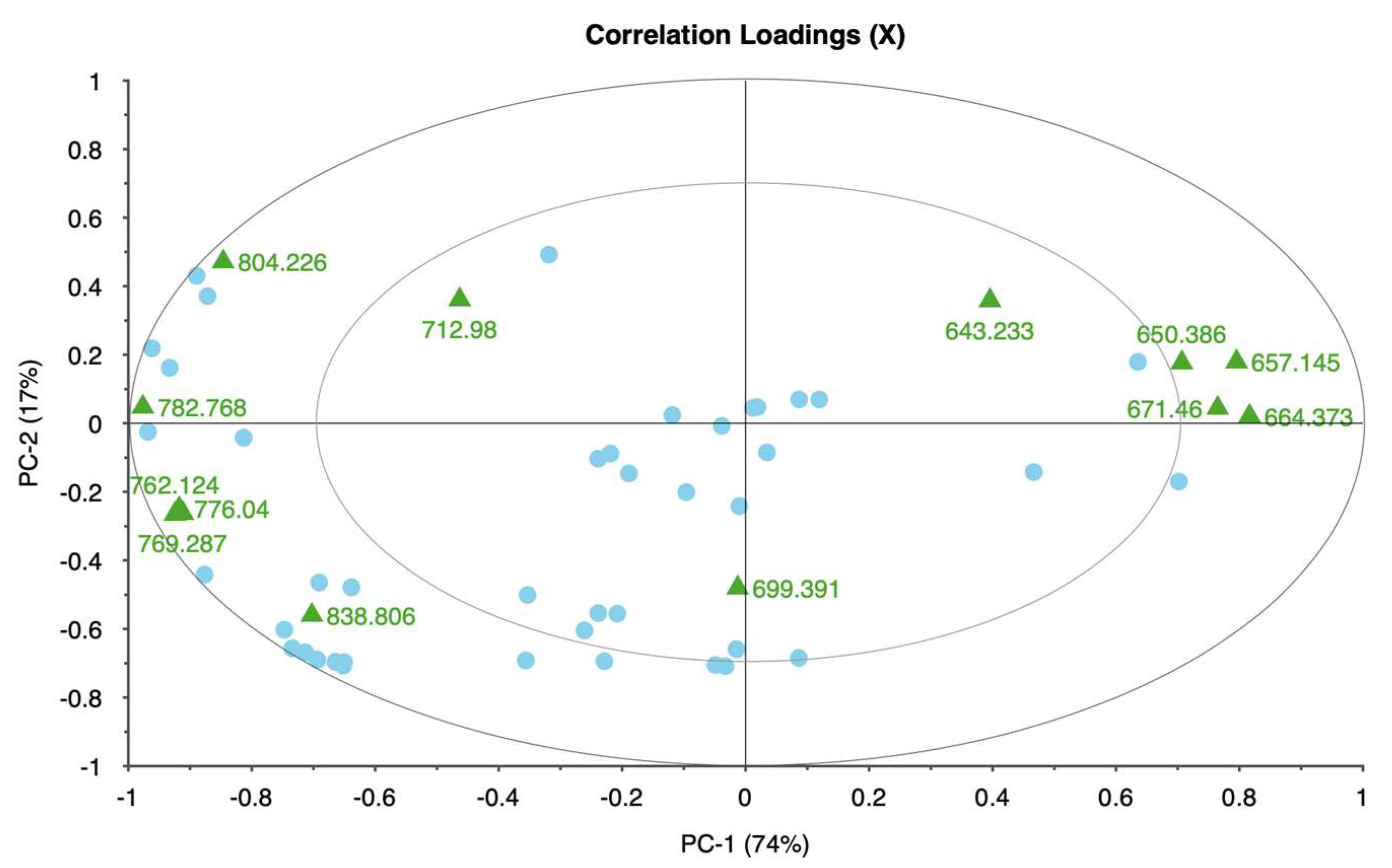
In itea, the spectral bands at 664.373, 685.410, 706.114, and 727.202 nm showed noticeable reflectance changes, especially in the early stages of water stress. While itea’s response was moderate compared to rose, the high explained variance in its PCA indicates that the selected spectral bands were effective in capturing the initial impact of water stress. This finding is significant for early detection of stress in nursery settings, where timely intervention could mitigate potential growth or quality impacts on itea plants.
The spirea’s spectral response was pronounced in the near-infrared bands, with key shifts observed at 643.233, 677.836, 711.460, and 734.460 nm. These bands allowed for robust differentiation of stress levels, as evidenced by the model’s high reliability. The distinct spectral changes observed in spirea suggest that it may be well-suited for hyperspectral stress monitoring, particularly for identifying moderate to severe stress levels. The explained variance for spirea further supports the accuracy of the spectral data in capturing water stress indicators.
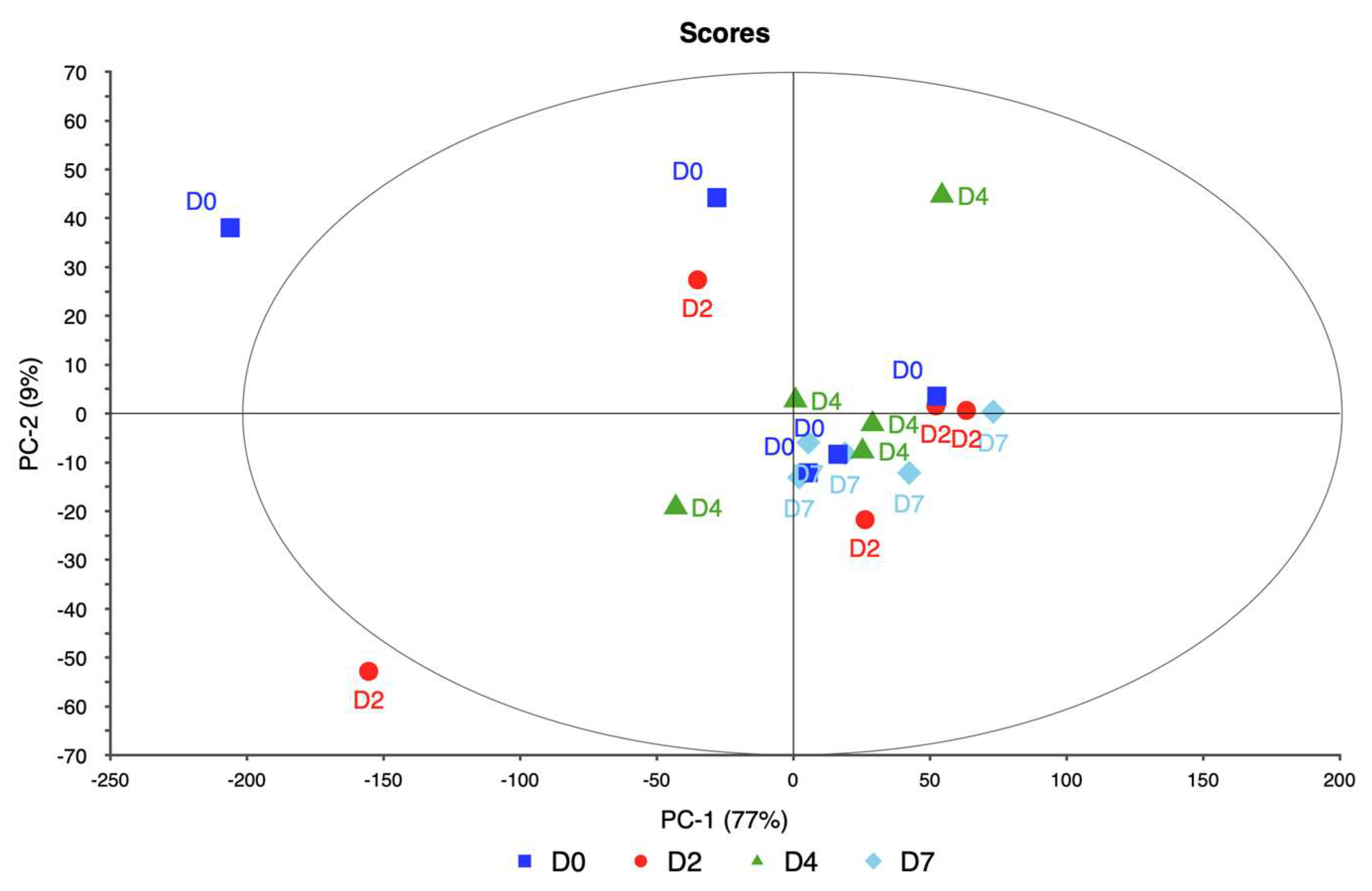
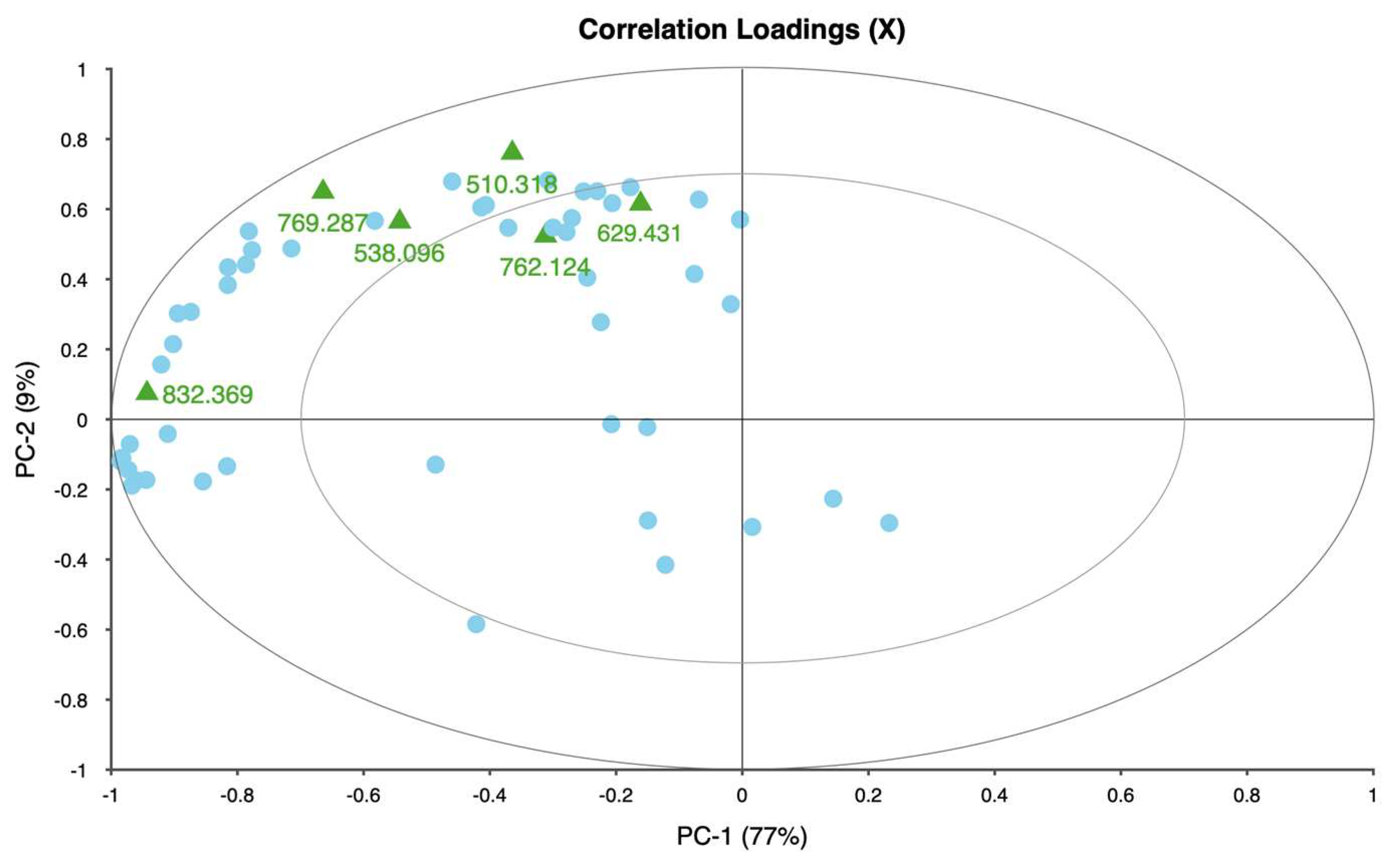
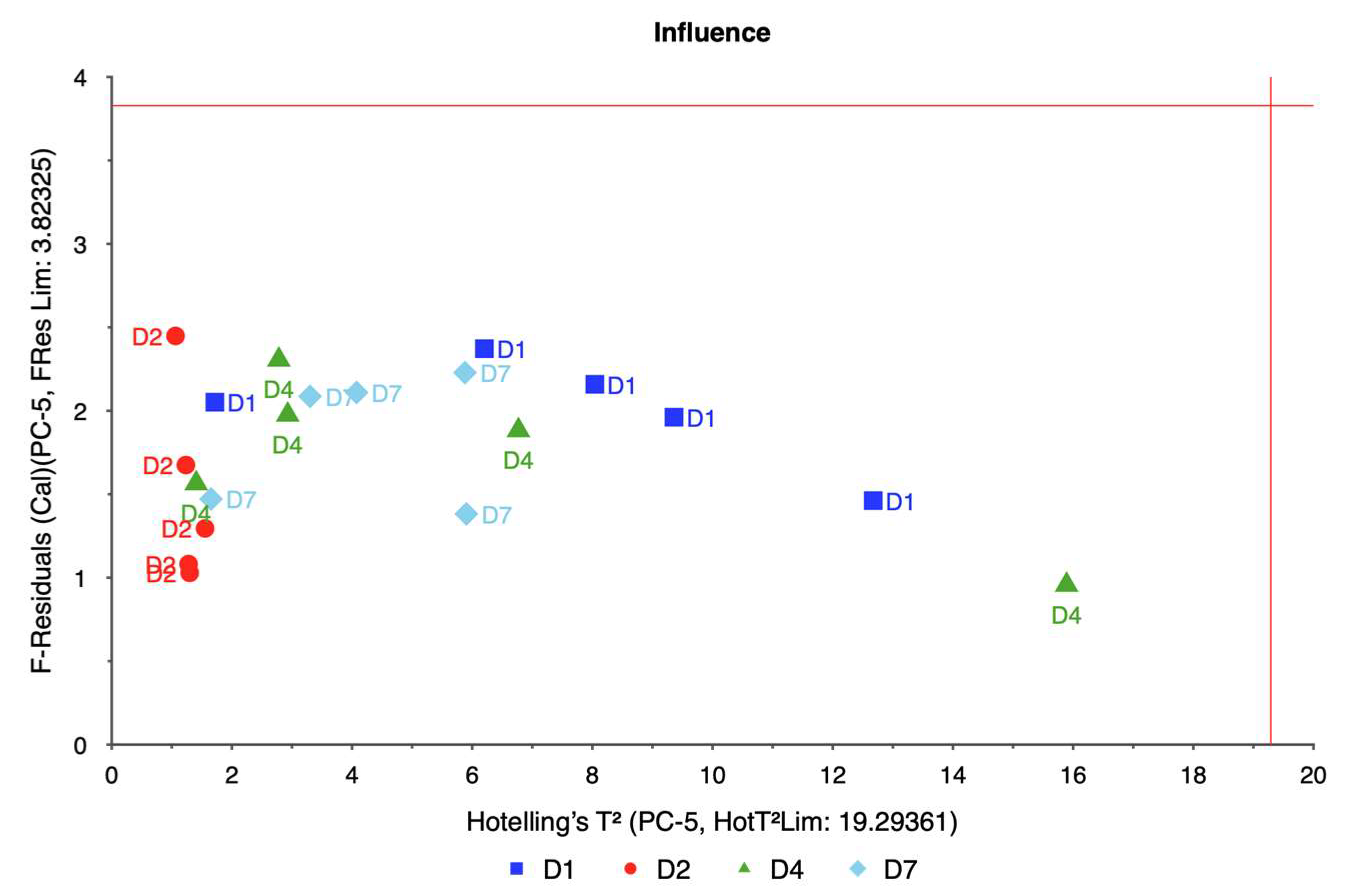
The weigela showed the most varied response to water stress among the four taxa, with key spectral bands located at 636.461, 664.373, 692.285, and 762.124 nm. This variation suggests a heightened sensitivity to prolonged water deficits, as reflected in the influence plot where a wider distribution of data points was observed. The high variability in weigela’s spectral response highlights its diverse physiological responses to drought stress. Weigela’s results underscore the importance of using a multi-band approach to fully capture its stress dynamics, making it a potentially complex model for monitoring plant health under drought conditions.
The differences in spectral responses across taxa can be attributed to their unique physiological adaptations to drought. For instance, rose exhibited higher sensitivity due to its limited water-holding capacity and greater reliance on stomatal regulation. In contrast, weigela demonstrated a broader range of stress responses, likely due to its robust root system and efficient water use strategies. These physiological differences explain the taxa-specific patterns observed in the spectral bands and highlight the importance of considering plant morphology and drought tolerance in hyperspectral studies.
While PCA proved effective in reducing the dimensionality of hyperspectral data and identifying key spectral bands associated with water stress, the method has inherent limitations and assumptions that must be considered. First, PCA assumes linear relationships between variables, which may not fully capture the complex, non-linear interactions present in biological systems such as plants. This assumption could limit the interpretability of certain principal components, especially when non-linearities dominate the spectral data. Second, PCA is sensitive to noise, which can disproportionately affect the derived components and potentially obscure meaningful patterns. In this study, rigorous preprocessing steps, including radiometric and geometric corrections and the removal of outliers, were employed to minimize noise and ensure the reliability of the analysis. Finally, the interpretability of principal components can be challenging, as they represent linear combinations of original variables that may not directly correspond to specific biological traits. This underscores the importance of coupling PCA with domain knowledge to relate spectral data to physiological plant responses accurately.
The identified spectral bands (e.g., 762 nm and 810 nm) have significant implications for practical water management in horticultural settings. For irrigation scheduling, these bands can serve as early indicators of water stress, enabling nursery operators to implement precision irrigation strategies. Monitoring reflectance at these wavelengths allows for stress detection before visible symptoms appear, optimizing the timing and amount of water applied. This can result in significant water savings and improved plant health.
Furthermore, the taxa-specific spectral responses observed in this study provide a foundation for adaptive water management strategies. For instance,
Rosa hybrid, which exhibited significant stress signals at key bands, may require more frequent or targeted irrigation. In contrast,
Weigela florida, with its broader stress tolerance, can be managed with interventions like mulching or shading to reduce water loss. These approaches enhance water use efficiency while maintaining plant quality and marketability in ornamental nurseries. Comparisons with recent studies, such as Virnodkar et al. and Wong et al., highlight the potential of hyperspectral imaging in distinguishing water stress responses across different taxa [
19,
20]. While these studies focus on agricultural crops, their findings support the broader application of hyperspectral imaging in precision agriculture. This alignment underscores the value of extending these methodologies to ornamental species, as demonstrated in our research.
These findings highlight the potential of hyperspectral imaging as a non-invasive tool to integrate into real-world horticultural practices, contributing to sustainable water management and improved drought resilience. The taxa-specific spectral responses observed in this study, particularly in the red and near-infrared regions, provide unique insights into the physiological changes associated with water stress. These findings not only contribute to the limited body of knowledge on hyperspectral imaging in ornamental species but also highlight the potential for broader applications in precision nursery management.
The findings of this study align with recent research demonstrating the capability of hyperspectral imaging for stress detection in agriculture. For example, Osco et al. highlighted the potential of hyperspectral indices derived from artificial neural networks for identifying water stress in lettuce under controlled conditions [
15]. Similarly, Nagasubramanian et al. showcased the efficacy of hyperspectral imaging integrated with 3D deep learning in detecting plant diseases [
17]. Unlike these studies, which focus on food crops, our work extends this technology to ornamental plants, representing a novel application with economic and aesthetic significance.
The study’s findings emphasize the utility of hyperspectral imaging as a tool for detecting water stress in ornamental plants. The species-specific responses across rose, itea, spirea, and weigela suggest that while each taxon responds differently to water stress, key spectral bands in the red and near-infrared regions serve as reliable indicators across all taxa. The high explained variance in each taxa’s PCA results supports the robustness of the spectral data in capturing stress indicators. Overall, hyperspectral imaging enables precise monitoring of water stress, providing insights for nursery management and drought resilience in ornamental plant production.
5. Conclusions
This study demonstrates that hyperspectral imaging, combined with PCA, provides a powerful approach for detecting and monitoring water stress in ornamental plants. The spectral responses of four ornamental woody shrub taxa produced in 11 L containers revealed distinct, species-specific patterns across key bands in the red and near-infrared regions. These bands—such as 650.386, 664.373, 692.285, and 734.460 nm—proved effective in distinguishing between stress levels, highlighting the potential of hyperspectral data for early and accurate stress detection in containerized nursery crop production. The high explained variance across taxa further supports the robustness and reliability of these spectral indicators, emphasizing the value of hyperspectral imaging in precision agriculture for ornamental species.
While the findings underscore the ability of hyperspectral imaging to identify water stress in these four taxa, future research should focus on expanding the scope of this approach to other ornamental plant species and environmental stress factors. Investigating how different plant varieties within the same taxa respond to stress could provide more nuanced insights into species resilience. Additionally, integrating hyperspectral data with other physiological measurements, such as leaf water potential and stomatal conductance, may enhance the understanding of the underlying mechanisms driving spectral changes. Exploring machine learning techniques to complement PCA could improve the ability to analyze non-linear relationships in hyperspectral datasets, potentially increasing the accuracy and applicability of stress detection.
This study was conducted in a controlled environment, which, while advantageous for isolating water stress variables, may not fully replicate the variability present in real-world nursery settings. Future research should validate these findings under field conditions to ensure robustness across diverse environments. Furthermore, the sensitivity of PCA to noise and its linearity assumption may limit the interpretation of spectral data. Additional studies incorporating advanced computational approaches or combining hyperspectral imaging with other technologies could address these limitations.
Research on real-time, field-based hyperspectral imaging systems would also be beneficial, as it would allow for continuous monitoring of plant health in dynamic nursery environments. Ultimately, integrating hyperspectral imaging into operational nursery practices has the potential to revolutionize water management strategies, enabling more sustainable and efficient production of ornamental plants.