A Landscape-Based Habitat Suitability Model (LHS Model) for Oriental Migratory Locust Area Extraction at Large Scales: A Case Study along the Middle and Lower Reaches of the Yellow River
Abstract
1. Introduction
2. Materials and Methods
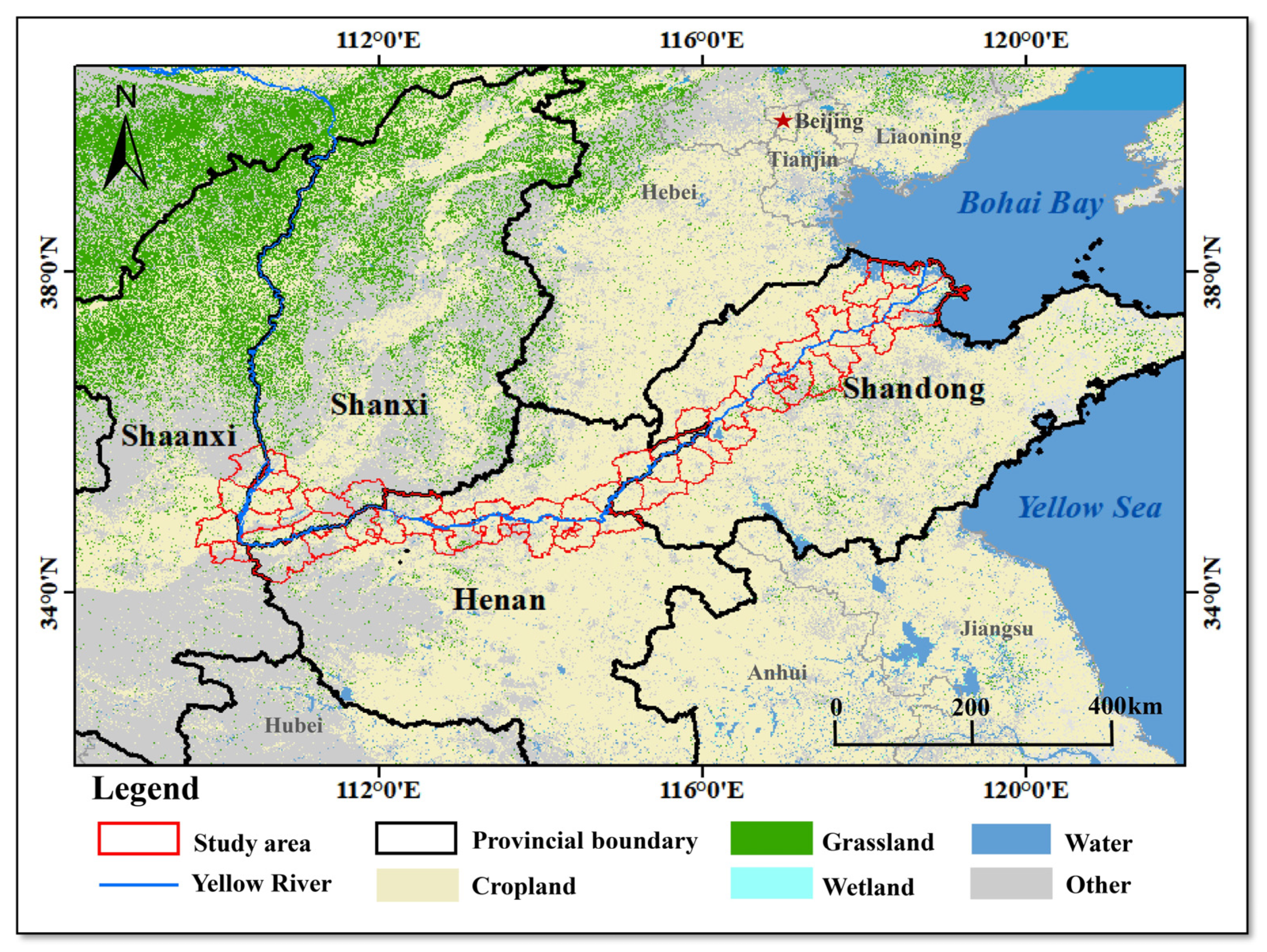
2.1. Study Area
2.2. Data
2.2.1. Remote Sensing Data
2.2.2. Ground Survey Data
2.3. Methods
2.3.1. Locust Development Simulation Using the DD Model
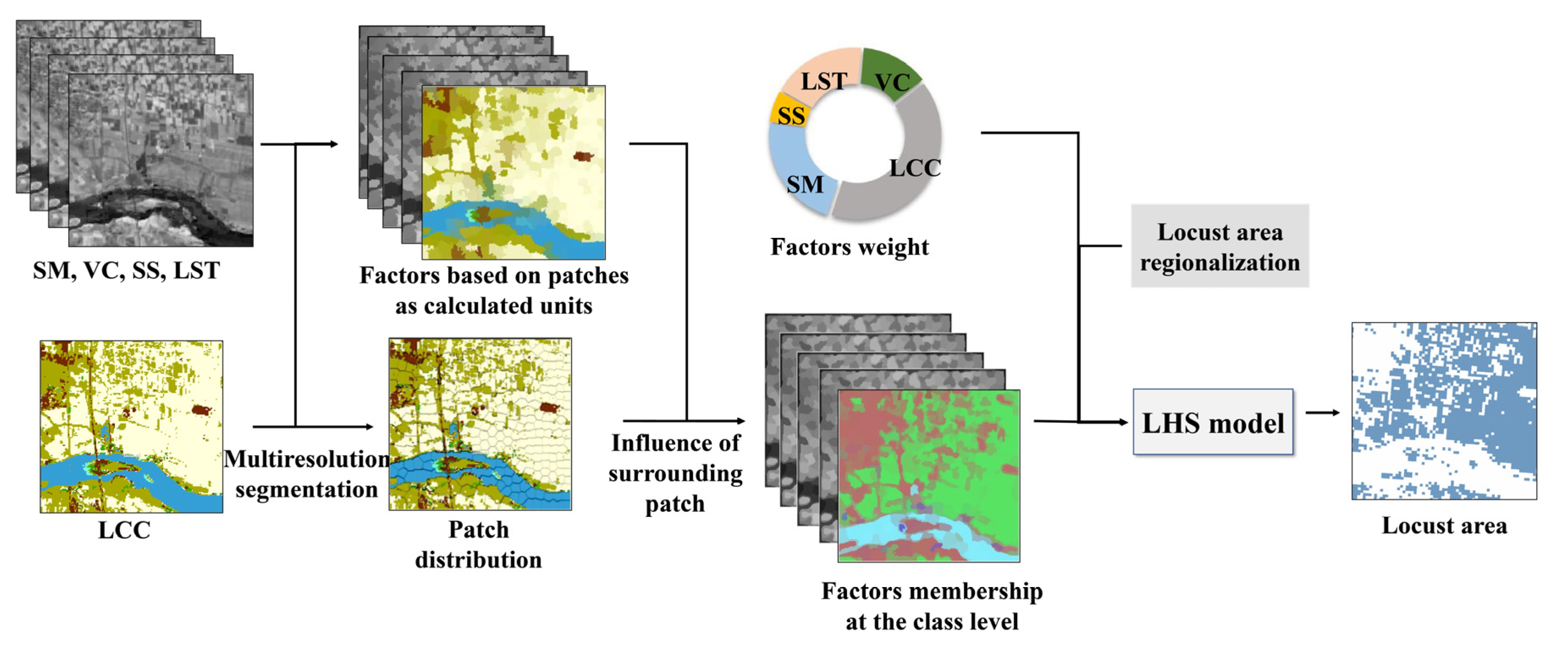
2.3.2. Habitat Factors Obtaining
2.3.3. Locust Area Extraction Using LHS Model
- (1)
- Habitat factors obtained based on patches as calculated units
- (2)
- Habitat factor membership at the class level
- (3)
- Locust area extraction
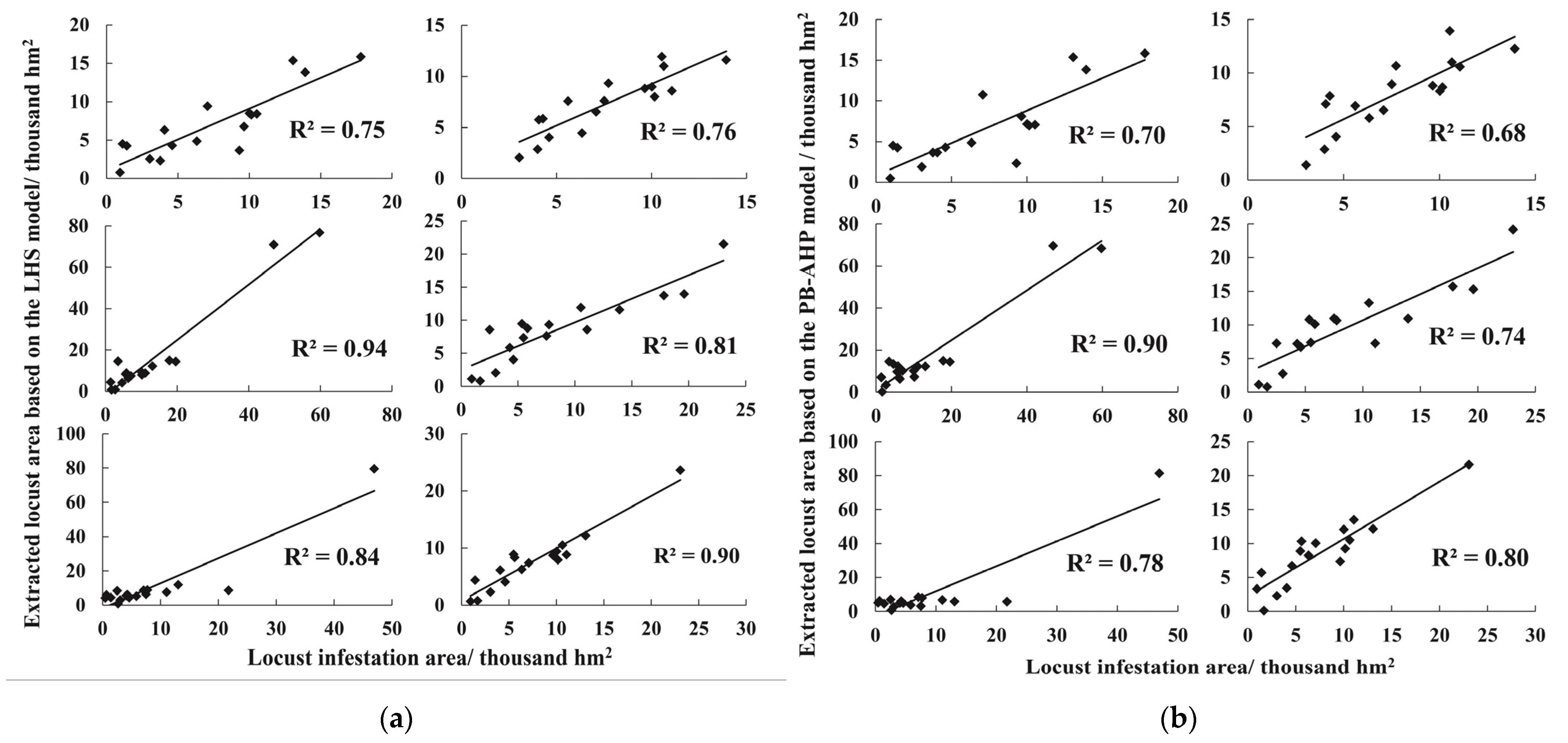
2.3.4. Accuracy Assessment
3. Results
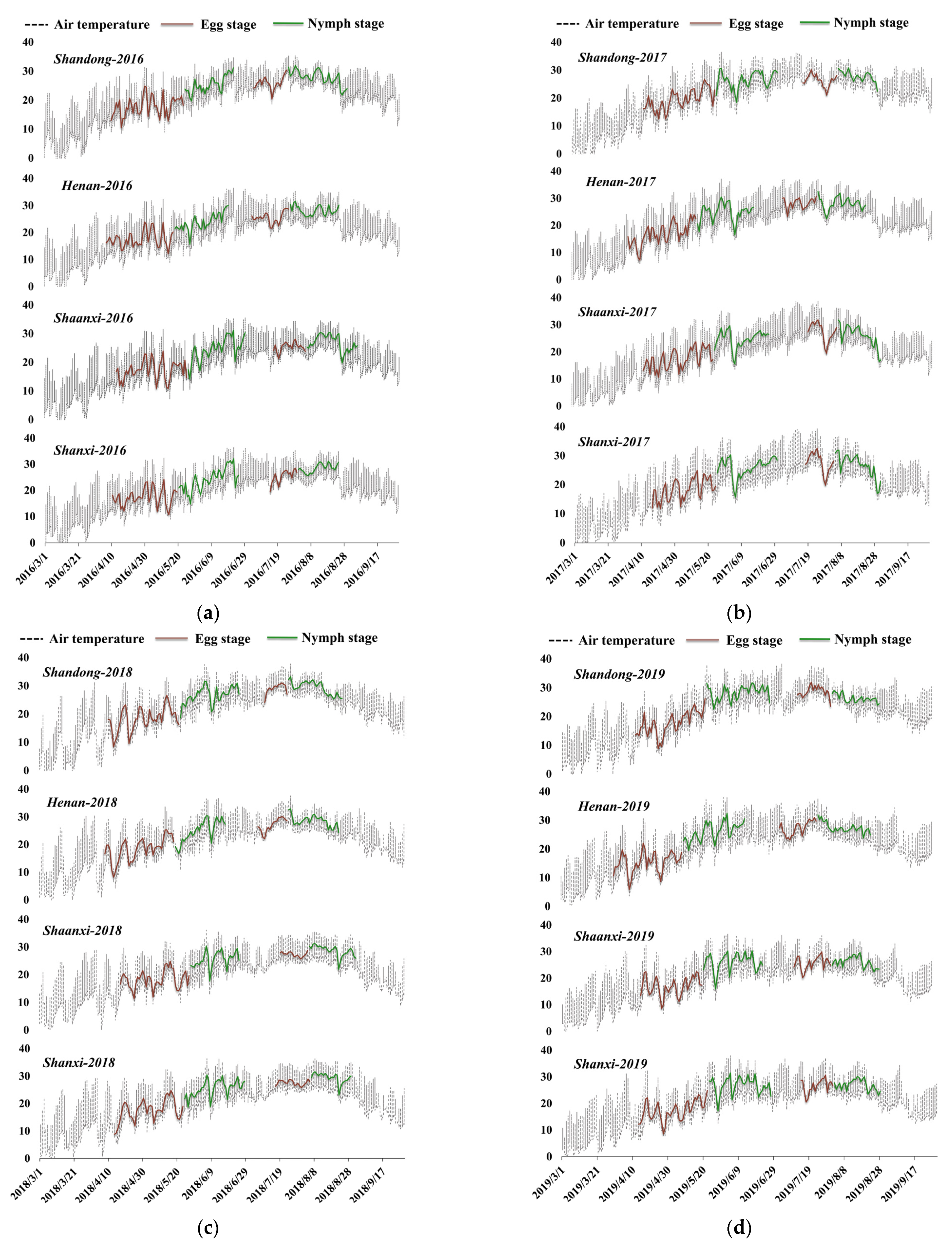
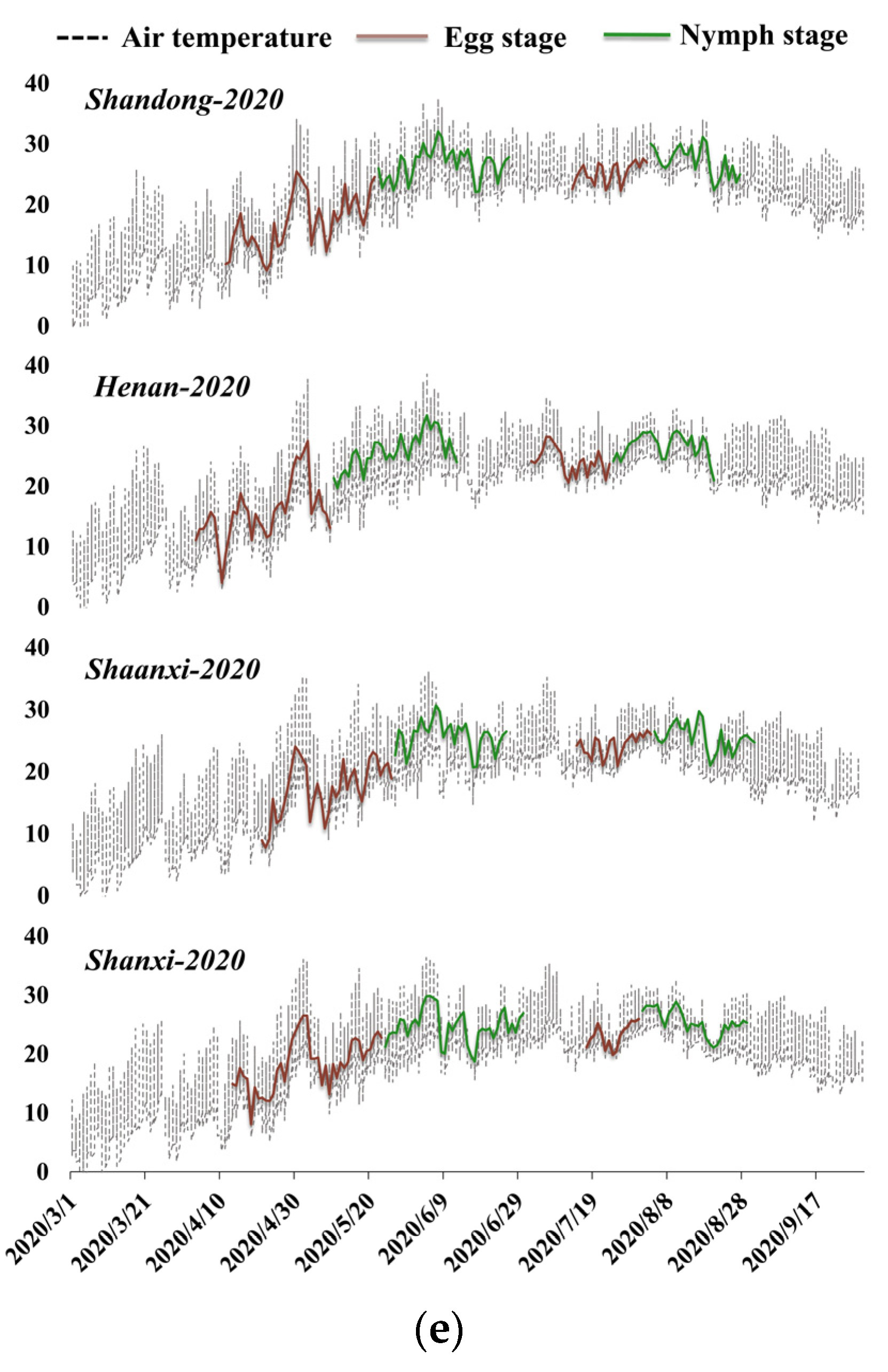
3.1. Locust Development of Each Province
3.2. Habitat Factors
3.3. Locust Area during 2016–2020 Based on the LHS and PB-AHP Models
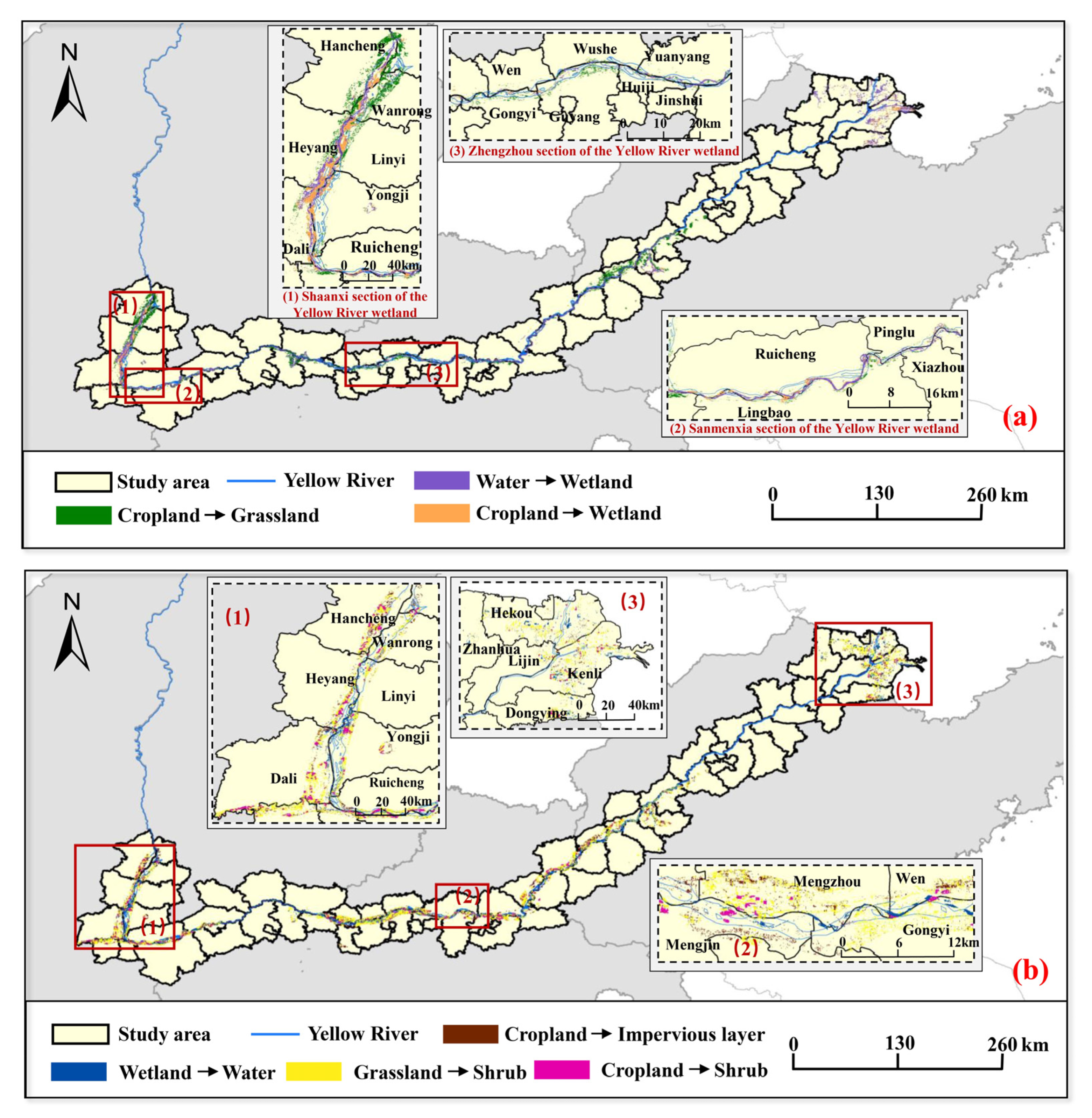
3.4. Locust Area Evolution
4. Discussion
4.1. Assessment and Analysis of Locust Area Extraction
4.2. Locust Area Evolution Trend with LCC Change
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Wang, N.; Li, Z.L. Study on Social Influence, Environmental Significance and Ecological Explanation of the Dynamics of Locust Plagues in China During the Historical Period. Prog. Geogr. 2010, 29, 1375–1384. [Google Scholar] [CrossRef]
- Zheng, X.M. Monitoring Oriental Migratory Locust Damage Based on Multi-Platform Remote Sensing Techniques. Master’s Thesis, Zhejiang University, Hangzhou, China, 2019. (In Chinese). [Google Scholar]
- Feng, X.D. Analysis on occurrence characteristics and causes of Oriental migratory locust in China of recent years. Plant Prot. Cscd. 2007, 10, 34–35. (In Chinese) [Google Scholar]
- Zhu, E.L. Occurrence and Management of Oriental Migratory Locust in China; China Agricultural Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Stige, L.C.; Chan, K.S.; Zhang, Z.; Frank, D.; Stenseth, N.C. Thousand-year-long Chinese time series reveals climatic forcing of decadal locust dynamics. Proc. Natl. Acad. Sci. USA 2007, 104, 16188–16193. [Google Scholar] [CrossRef] [PubMed]
- Calvão, T.; Pessoa, M. Remote sensing in food production—A review. Emir. J. Food Agric. 2015, 27, 138–151. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; Dong, Y.; Peng, D.; Zheng, Q.; Yang, P. The influence of landscape’s dynamics on the Oriental Migratory Locust habitat change based on the time-series satellite data. J. Environ. Manag. 2018, 218, 280–290. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, W.; Chen, J.; Dong, Y.; Ren, B.; Geng, Y. Land use/cover changes in the Oriental migratory locust area of China: Implications for ecological control and monitoring of locust area. Agric. Ecosyst. Environ. 2020, 303, 107110. [Google Scholar] [CrossRef]
- Low, F.; Waldner, F.; Latchininsky, A.; Biradar, C.; Bolkart, M.; Colditz, R.R. Timely monitoring of Asian Migratory locust habitats in the Amudarya delta, Uzbekistan using time series of satellite remote sensing vegetation index. J. Environ. Manag. 2016, 183, 562–575. [Google Scholar] [CrossRef]
- Deveson, E.D. Satellite normalized difference vegetation index data used in managing Australian plague locusts. J. Appl. Remote Sens. 2013, 7, 075096. [Google Scholar] [CrossRef]
- Ma, J.; Han, X.; Hasibagan; Wang, C.; Zhang, Y.; Tang, J.; Xie, Z.; Deveson, T. Monitoring East Asian migratory locust plagues using remote sensing data and field investigations. Int. J. Remote Sens. 2007, 26, 629–634. [Google Scholar] [CrossRef]
- Latchininsky, A.V.; Sivanpillai, R. Locust Habitat Monitoring and Risk Assessment Using Remote Sensing and GIS Technologies; Springer: Dordrecht, The Netherlands, 2010; pp. 163–188. [Google Scholar] [CrossRef]
- Ji, R.; Xie, B.Y.; Li, D.M.; Li, Z.; Zeng, X.C. Relationships between spatial pattern of Locusta migratoria manilensis eggpods and soil property variability in coastal areas. Soil Biol. Biochem. 2007, 39, 1865–1869. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, J.L. Modelling desert locust presences using 32-year soil moisture data on a large-scale. Ecol. Indic. 2020, 117, 106655. [Google Scholar] [CrossRef]
- Tian, H.D.; Ji, R.; Xie, B.Y.; Li, X.H.; Li, D.M. Using multi–temporal Landsat ETM+ data to monitor the plague of oriental migratory locust. Int. J. Remote Sens. 2008, 29, 1685–1692. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Warner, E.; Ge, Y.; Yu, D.; Ni, S.; Wang, H. Relationship between oriental migratory locust plague and soil moisture extracted from MODIS data. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 84–91. [Google Scholar] [CrossRef]
- Song, P.; Zheng, X.; Li, Y.; Zhang, K.; Huang, J.; Li, H.; Zhang, H.; Liu, L.; Wei, C.; Mansaray, L.R.; et al. Estimating reed loss caused by Locusta migratoria manilensis using UAV-based hyperspectral data. Sci Total Environ. 2020, 719, 137519. [Google Scholar] [CrossRef]
- Latchininsky, A.; Sword, G.; Sergeev, M.; Cigliano, M.M.; Lecoq, M. Locusts and Grasshoppers: Behavior, Ecology, and Biogeography. Psyche A J. Entomol. 2011, 2011, 1–4. [Google Scholar] [CrossRef]
- Scanlan, J.; Grant, W.; Hunter, D.M.; Milner, R. Habitat and environmental factors influencing the control of migratory locusts Locusta migratoria with a biopesticide Metarhizium anisopliae. Ecol. Model. Ecol. Model 2001, 136, 223–236. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D. A possible relationship between outbreaks of the oriental migratory locust (Locusta migratoria manilensis Meyen) in China and the El Niño episodes. Ecol. Res. 2002, 14, 267–270. [Google Scholar] [CrossRef]
- Word, M.L.; Hall, S.J.; Robinson, B.E.; Manneh, B.; Beye, A.; Cease, A.J. Soil-targeted interventions could alleviate locust and grasshopper pest pressure in West Africa. Sci. Total Environ. 2019, 663, 632–643. [Google Scholar] [CrossRef]
- Ma, J.W.; Han, X.Z. Oriental Migratory Locust Plague Remote Sensing Monitoring Mechanism and Method; The Science Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Geng, Y.; Zhao, L.; Dong, Y.; Huang, W.; Shi, Y.; Ren, Y.; Ren, B. Migratory Locust Habitat Analysis With PB-AHP Model Using Time-Series Satellite Images. IEEE Access 2020, 8, 166813–166823. [Google Scholar] [CrossRef]
- Scott, J.M.; Heglund, P.; Morrison, M.L.; Wall, W.A.; Haufler, J. Predicting Species Occurrences: Issues of Accuracy and Scale; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Girvetz, E.H.; Greco, S.E. Multi-scale predictive habitat suitability modeling based on hierarchically delineated patches: An example for yellow-billed cuckoos nesting in riparian forests, California, USA. Landsc. Ecol. 2009, 24, 1315–1329. [Google Scholar] [CrossRef]
- Girvetz, E.H.; Greco, S.E. How to define a patch: A spatial model for hierarchically delineating organism-specific habitat patches. Landsc. Ecol. 2007, 22, 1131–1142. [Google Scholar] [CrossRef]
- Kebede, Y.; Bianchi, F.; Baudron, F.; Abraham, K.; de Valenca, A.; Tittonell, P. Implications of changes in land cover and landscape structure for the biocontrol potential of stemborers in Ethiopia. Biol. Control 2018, 122. [Google Scholar] [CrossRef]
- Wu, J. Landscape Ecology: Pattern, Process, Scale and Hierarchy; Higher Education Press: Beijing, China, 2007; p. 266. (In Chinese) [Google Scholar]
- Tu, X.; Li, Z.; Wang, J.; Huang, X.; Yang, J.; Fan, C.; Wu, H.; Wang, Q.; Zhang, Z. Improving the Degree-Day Model for Forecasting Locusta migratoria manilensis (Meyen) (Orthoptera: Acridoidea). PLoS ONE 2014, 9, e89523. [Google Scholar] [CrossRef]
- Healy, K.B.; Dugas, E.; Fonseca, D.M. Development of a Degree-Day Model to Predict Egg Hatch of Aedes albopictus. J. Am. Mosq. Control Assoc. 2019, 35, 249–257. [Google Scholar] [CrossRef]
- Fei, J.; Zhou, J. The drought and locust plague of 942–944 AD in the Yellow River Basin, China. Quat. Int. 2016, 394, 115–122. [Google Scholar] [CrossRef]
- Salih, A.A.M.; Baraibar, M.; Mwangi, K.K.; Artan, G. Climate change and locust outbreak in East Africa. Nat. Clim. Chang. 2020, 10, 584–585. [Google Scholar] [CrossRef]
- Tratalos, J.A.; Cheke, R.A. Corrigendum to “Can NDVI GAC imagery be used to monitor desert locust breeding areas?”. J. Arid Environ. 2006, 65, 512. [Google Scholar] [CrossRef]
- Gong, P.; Liu, H.; Zhang, M.; Li, C.; Wang, J.; Huang, H.; Clinton, N.; Ji, L.; Li, W.; Bai, Y. Stable classification with limited sample: Transferring a 30-m resolution sample set collected in 2015 to mapping 10-m resolution global land cover in 2017. Sci. Bull. 2019, 64, 370–373. [Google Scholar] [CrossRef]
- Cushman, S.A.; Macdonald, E.A.; Landguth, E.L.; Malhi, Y.; Macdonald, D.W. Multiple-scale prediction of forest loss risk across Borneo. Landsc. Ecol. 2017, 32, 1581–1598. [Google Scholar] [CrossRef]
- Sivanpillai, R.; Latchininsky, A.V. Can late summer Landsat data be used for locating Asian migratory locust, Locustamigratoriamigratoria, oviposition sites in the Amudarya River delta, Uzbekistan? Entomol. Exp. Et Appl. 2008, 128, 346–353. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Y.L.; Lu, B.L. The Biology of the Migratory Locusts in China; Shandong Science and Technology Press: Jinan, China, 1991. (In Chinese) [Google Scholar]
- Wu, D.; Wu, H.; Zhao, X.; Zhou, T.; Tang, B.; Zhao, W.; Jia, K. Evaluation of Spatiotemporal Variations of Global Fractional Vegetation Cover Based on GIMMS NDVI Data from 1982 to 2011. Remote Sens. 2014, 6, 4217–4239. [Google Scholar] [CrossRef]
- Ermida, S.L.; Soares, P.; Mantas, V.; Göttsche, F.-M.; Trigo, I.F. Google Earth Engine Open-Source Code for Land Surface Temperature Estimation from the Landsat Series. Remote Sens. 2020, 12, 1471. [Google Scholar] [CrossRef]
- Douaoui, A.E.K.; Nicolas, H.; Walter, C. Detecting salinity hazards within a semiarid context by means of combining soil and remote-sensing data. Geoderma 2006, 134, 217–230. [Google Scholar] [CrossRef]
- Nguyen, K.-A.; Liou, Y.-A.; Tran, H.-P.; Hoang, P.-P.; Nguyen, T.-H. Soil salinity assessment by using near-infrared channel and Vegetation Soil Salinity Index derived from Landsat 8 OLI data: A case study in the Tra Vinh Province, Mekong Delta, Vietnam. Prog. Earth Planet. Sci. 2020, 7, 1. [Google Scholar] [CrossRef]
- Ismayilov, A.I.; Mamedov, A.I.; Fujimaki, H.; Tsunekawa, A.; Levy, G.J. Soil Salinity Type Effects on the Relationship between the Electrical Conductivity and Salt Content for 1:5 Soil-to-Water Extract. Sustainability 2021, 13, 3395. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, P.; Qi, S.; Liu, C.; Xiong, T. Mapping bamboo with regional phenological characteristics derived from dense Landsat time series using Google Earth Engine. Int. J. Remote Sens. 2019, 40, 9541–9555. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Qin, Y.; Dong, J.; Geissler, G.; Zhang, G.; Cejda, N.; Alikhani, B.; Doughty, R.B. Mapping the dynamics of eastern redcedar encroachment into grasslands during 1984–2010 through PALSAR and time series Landsat images. Remote Sens. Environ. 2017, 190, 233–246. [Google Scholar] [CrossRef]
- Chen, B.; Xiao, X.; Li, X.; Pan, L.; Doughty, R.; Ma, J.; Dong, J.; Qin, Y.; Zhao, B.; Wu, Z.; et al. A mangrove forest map of China in 2015: Analysis of time series Landsat 7/8 and Sentinel-1A imagery in Google Earth Engine cloud computing platform. ISPRS J. Photogramm. Remote Sens. 2017, 131, 104–120. [Google Scholar] [CrossRef]
- Zhu, Z.; Woodcock, C. Object-based cloud and cloud shadow detection in Landsat imagery. Remote Sens. Environ. 2012, 118, 83–94. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguz, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Xu, H. A study on information extraction of water body with the modified normalized difference water index (MNDWI). J. Remote Sens. 2005, 9, 589–595. [Google Scholar]
- Du, Z.; Linghu, B.; Ling, F.; Li, W.; Tian, W.; Wang, H.; Gui, Y.; Sun, B.; Zhang, X. Estimating Surface Water Area Changes Using Time-Series Landsat Data in the Qingjiang River Basin, China. J. Appl. Remote Sens. 2012, 6, 3609. [Google Scholar] [CrossRef]
- Baatz, M.; Schäpe, A. An Optimization Approach for High Quality Multi-Scale Image Segmentation. In Angewandte Geographische Informationsverarbeitung XII; Strobl, J., Blaschke, T., Griesebner, G., Eds.; Wichmann-Verlag: Heidelberg, Germany, 2000; pp. 12–23. [Google Scholar]
- Rezaee, M.R.; Van der Zwet, P.M.; Lelieveldt, B.P.E.; Van der Geest, R.J.; Reiber, J.H. A multiresolution image segmentation technique based on pyramidal segmentation and fuzzy clustering. IEEE Trans. Image Processing 2000, 9, 1238–1248. [Google Scholar] [CrossRef]
- Wang, J.Z.; Jia, L.; Gray, R.M.; Wiederhold, G. Unsupervised multiresolution segmentation for images with low depth of field. IEEE Trans. Pattern Anal. Mach. Intell. 2001, 23, 85–90. [Google Scholar] [CrossRef]
- Wu, J. Effects of changing scale on landscape pattern analysis: Scaling relations. Landsc. Ecol. 2004, 19, 125–138. [Google Scholar] [CrossRef]
- Wu, J.; Li, H. Perspectives and methods of scaling. In Scaling and Uncertainty Analysis in Ecology; Wu, J., Jones, K.B., Li, H., Loucks, O.L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 17–44. [Google Scholar]
- Ren, C. Outbreak of Oriental migratory locust Locusta migratoria manilensis (Meyen) in Baiyandian Lake. Kun Chong Zhi Shi 2001, 38, 128–130. [Google Scholar]
- Qiu, Z.Y. Study on Oriental Migratory Locust Habitat Assessing Information System. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2006. (In Chinese). [Google Scholar]
- Zhang, C.L. Retrieval of Land Surface Temperature Using Remotely Sensed Data and Its Application in Monitoring Oriental Migratory Locust. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2006. (In Chinese). [Google Scholar]
- Basu, T.; Pal, S. A GIS-based factor clustering and landslide susceptibility analysis using AHP for Gish River Basin, India. Environ. Dev. Sustain. 2019, 22, 4787–4819. [Google Scholar] [CrossRef]
- Bajec, P.; Tuljak-Suban, D. An Integrated Analytic Hierarchy Process—Slack Based Measure-Data Envelopment Analysis Model for Evaluating the Efficiency of Logistics Service Providers Considering Undesirable Performance Criteria. Sustainability 2019, 11, 2330. [Google Scholar] [CrossRef]
- Yu, G.; Shen, H.; Liu, J. Impacts of climate change on historical locust outbreaks in China. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Shi, R.; Liu, C.; Li, D.; Xie, B. Distribution of Locusta migatoria manilensis and soil in the locust plague are at Baiyangdian. Kun Chong Zhi Shi 2004, 41, 29–33. [Google Scholar]
- Propastin, P. Satellite-based monitoring system for assessment of vegetation vulnerability to locust hazard in the River Ili delta (Lake Balkhash, Kazakhstan). J. Appl. Remote Sens. 2013, 7, 075094. [Google Scholar] [CrossRef][Green Version]
- Trebitz, A.S.; Brazner, J.C.; Tanner, D.K.; Meyer, R. Interacting watershed size and landcover influences on habitat and biota of Lake Superior coastal wetlands. Aquat. Ecosyst. Health Manag. 2011, 14, 443–455. [Google Scholar] [CrossRef]
- Matinfar, H.R.; Alavi Panah, S.K.; Zand, F.; Khodaei, K. Detection of soil salinity changes and mapping land cover types based upon remotely sensed data. Arab. J. Geosci. 2013, 6, 913–919. [Google Scholar] [CrossRef]
- Wang, J.R.; Lv, G.Q. The occurrence and control history of Oriental migratory locust and sustainable control measures in Henan Province. Mod. Agric. Sci. Technol. 2019, 2, 2. (In Chinese) [Google Scholar]
- Guo, H.P.; Feng, X.J.; Wei, J.F.; Liang, C.L.; Wei, H.X.; Shi, J.N. The occurrence and distribution status of locusts and ecological management countermeasures in Shaanxi Province. Shaanxi J. Agric. Sci. 2014, 60, 3. (In Chinese) [Google Scholar]
- Xia, H.; Liu, L.; Bai, J.; Kong, W.; Guo, F. Wetland Ecosystem Service Dynamics in the Yellow River Estuary under Natural and Anthropogenic Stress in the Past 35 Years. Wetlands 2020, 40, 2741–2754. [Google Scholar] [CrossRef]

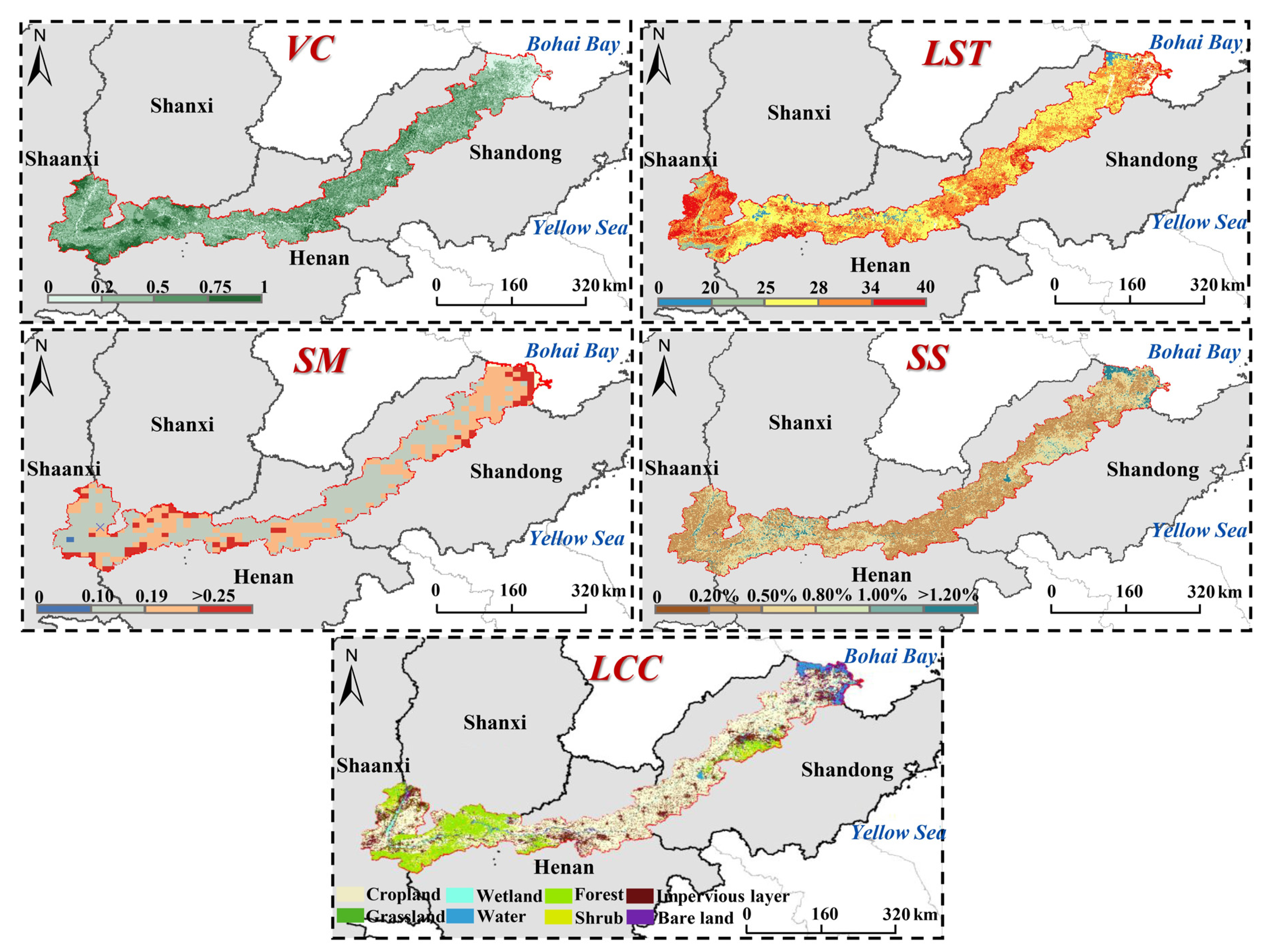


| Factors | Obtaining Method | Data Source | Reference |
|---|---|---|---|
| Vegetation coverage (VC) | Landsat-OLI | [38] | |
| Land surface temperature (LST) | Statistical mono window (SMW) algorithm | [39] | |
| Soil salinity (SS) | [40,41,42] | ||
| Land cover class (LCC) | Phenology-based random forest model | [43] | |
| Soil moisture (SM) | - | SMAP | - |
| Suitability (M) | 1 (Poor) | 2 (General) | 3 (Good) | 4 (Optimal) | |
|---|---|---|---|---|---|
| Factor (V) | |||||
| VC | <20% | >75% | >50% and ≤75% | ≥20% and ≤50% | |
| LCC | other | cropland | grassland | water, wetland | |
| SM | - | <10% or >25% | >19% and ≤25% | ≥10% and ≤19% | |
| SS | >0.80% | >0.50% and ≤0.80% | >0.20% and ≤0.50% | ≤0.20% | |
| LST | <20°C | ≥20 °C and <25 °C or >40 °C and ≤42 °C | >34 °C and ≤40 °C or ≥25°C and <28 °C | ≥28 °C and ≤34 °C | |
| Cropland | Forest | Grassland | Shrub | Wetland | Water | Impervious Layer | Bare Land | UA | OA | Kappa | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cropland | 101 | 2 | 10 | 11 | 2 | 0 | 9 | 1 | 0.74 | 0.73 | 0.73 |
| Forest | 3 | 146 | 0 | 15 | 0 | 0 | 0 | 0 | 0.89 | ||
| Grassland | 7 | 0 | 98 | 23 | 1 | 3 | 12 | 5 | 0.66 | ||
| Shrub | 14 | 5 | 20 | 112 | 0 | 0 | 1 | 0 | 0.74 | ||
| Wetland | 1 | 0 | 3 | 0 | 80 | 35 | 18 | 6 | 0.56 | ||
| Water | 0 | 0 | 0 | 0 | 11 | 146 | 3 | 1 | 0.91 | ||
| Impervious layer | 17 | 0 | 15 | 1 | 0 | 1 | 110 | 6 | 0.73 | ||
| Bare land | 2 | 0 | 8 | 0 | 16 | 18 | 11 | 85 | 0.61 | ||
| PA | 0.70 | 0.95 | 0.64 | 0.69 | 0.73 | 0.72 | 0.67 | 0.82 |
| Factors | Initial Importance | Final Weights |
|---|---|---|
| VC | 0.28 | 0.13 |
| LCC | 0.87 | 0.41 |
| SM | 0.47 | 0.22 |
| SS | 0.13 | 0.06 |
| LST | 0.39 | 0.18 |
| Locust Area Evolution | 2016 | 2020 | Area/Thousand hm2 | Total Area/Thousand hm2 |
|---|---|---|---|---|
| Increase | Cropland | Wetland | 37.59 | 74.39 |
| Water | Wetland | 24.59 | ||
| Cropland | Grassland | 12.21 | ||
| Decrease | Grassland | Shrub | 39.92 | 92.29 |
| Cropland | Shrub | 26.01 | ||
| Cropland | Impervious layer | 13.47 | ||
| Wetland | Water | 12.89 |
| Locust Area Evolution | Factors | LCC Change | Specific Reasons for Evolution |
|---|---|---|---|
| Increase | Natural factors | Water–wetland | The decrease in rainfall in the Yellow River basin from 2016 to 2020 and the flow of the Yellow River. |
| Human factors | Water–wetland | The continuous cultivation of forests and swamps caused the weakening of the Yellow River’s capacity to store water and the decrease in the flow of the Yellow River. | |
| Cropland–wetland | Policies and measures for ecological restoration of the Yellow River wetlands implemented in recent years. | ||
| Cropland–grassland | The relocation of residents along the MLYR resulted in the abandonment of cropland and the formation of new wasteland. | ||
| Decrease | Natural factors | Wetland–water | The flow of the Yellow River. |
| Human factors | Grassland–shrub | The changes of gramineous plants that locusts prefer to eat into planting peanuts, asparagus, and other plants that locusts do not like. | |
| Cropland–shrub | |||
| Cropland–impervious layer | The continuous increase in urbanization. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Y.; Zhao, L.; Huang, W.; Dong, Y.; Ma, H.; Guo, A.; Ren, Y.; Xing, N.; Huang, Y.; Sun, R.; et al. A Landscape-Based Habitat Suitability Model (LHS Model) for Oriental Migratory Locust Area Extraction at Large Scales: A Case Study along the Middle and Lower Reaches of the Yellow River. Remote Sens. 2022, 14, 1058. https://doi.org/10.3390/rs14051058
Geng Y, Zhao L, Huang W, Dong Y, Ma H, Guo A, Ren Y, Xing N, Huang Y, Sun R, et al. A Landscape-Based Habitat Suitability Model (LHS Model) for Oriental Migratory Locust Area Extraction at Large Scales: A Case Study along the Middle and Lower Reaches of the Yellow River. Remote Sensing. 2022; 14(5):1058. https://doi.org/10.3390/rs14051058
Chicago/Turabian StyleGeng, Yun, Longlong Zhao, Wenjiang Huang, Yingying Dong, Huiqin Ma, Anting Guo, Yu Ren, Naichen Xing, Yanru Huang, Ruiqi Sun, and et al. 2022. "A Landscape-Based Habitat Suitability Model (LHS Model) for Oriental Migratory Locust Area Extraction at Large Scales: A Case Study along the Middle and Lower Reaches of the Yellow River" Remote Sensing 14, no. 5: 1058. https://doi.org/10.3390/rs14051058
APA StyleGeng, Y., Zhao, L., Huang, W., Dong, Y., Ma, H., Guo, A., Ren, Y., Xing, N., Huang, Y., Sun, R., & Wang, J. (2022). A Landscape-Based Habitat Suitability Model (LHS Model) for Oriental Migratory Locust Area Extraction at Large Scales: A Case Study along the Middle and Lower Reaches of the Yellow River. Remote Sensing, 14(5), 1058. https://doi.org/10.3390/rs14051058










