Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Collection
2.2.1. Hyperspectral Data Determination
2.2.2. Determination of NBI
2.3. Canopy Hyperspectral Transformation
2.4. Selection of VIs
2.5. Model Development
2.5.1. Univariate Regression
2.5.2. Partial Least Squares Regression
2.5.3. Random Forest Regression
2.5.4. Support Vector Regression
2.6. Evaluation Metrics for Model Accuracy
3. Results
3.1. Descriptive Analysis of Nitrogen Balance Index
3.2. Hyperspectral Features and Nitrogen Balance Index
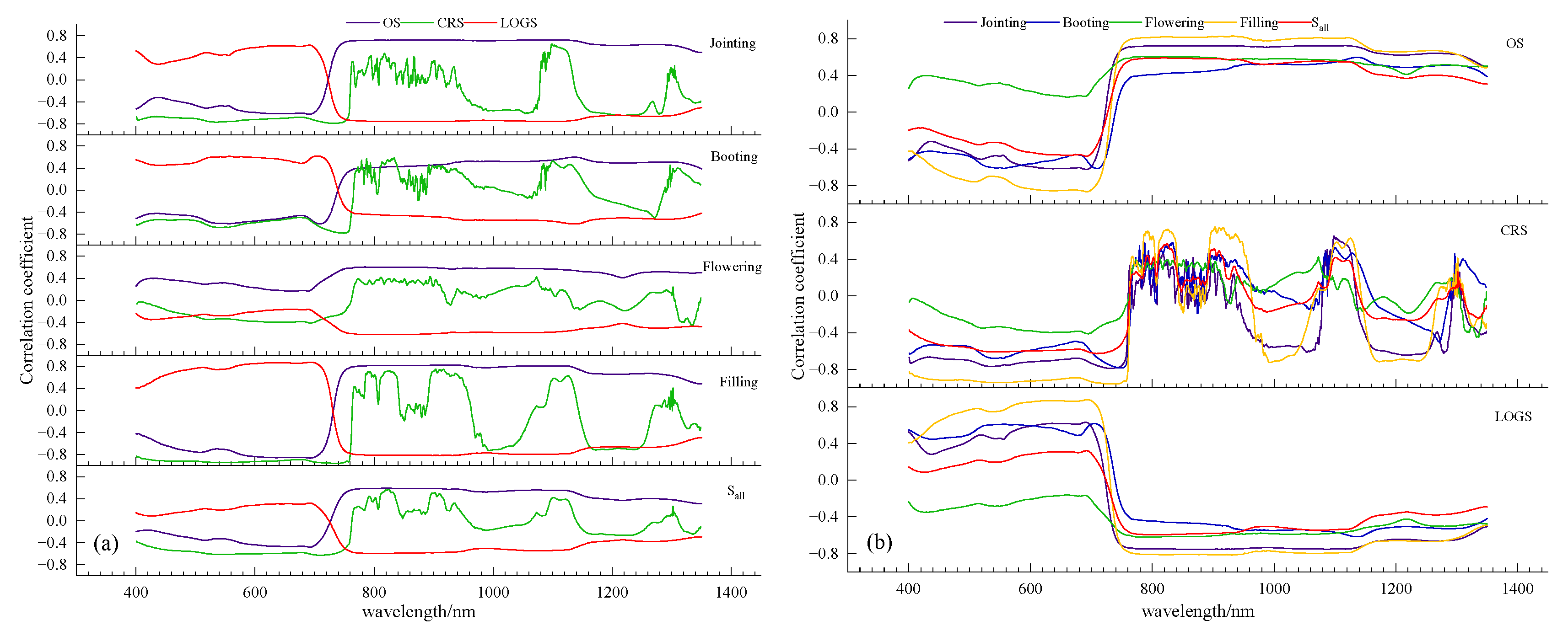
3.2.1. Sensitive Bands and NBI
3.2.2. VIs and NBI
3.3. NBI Estimation Model
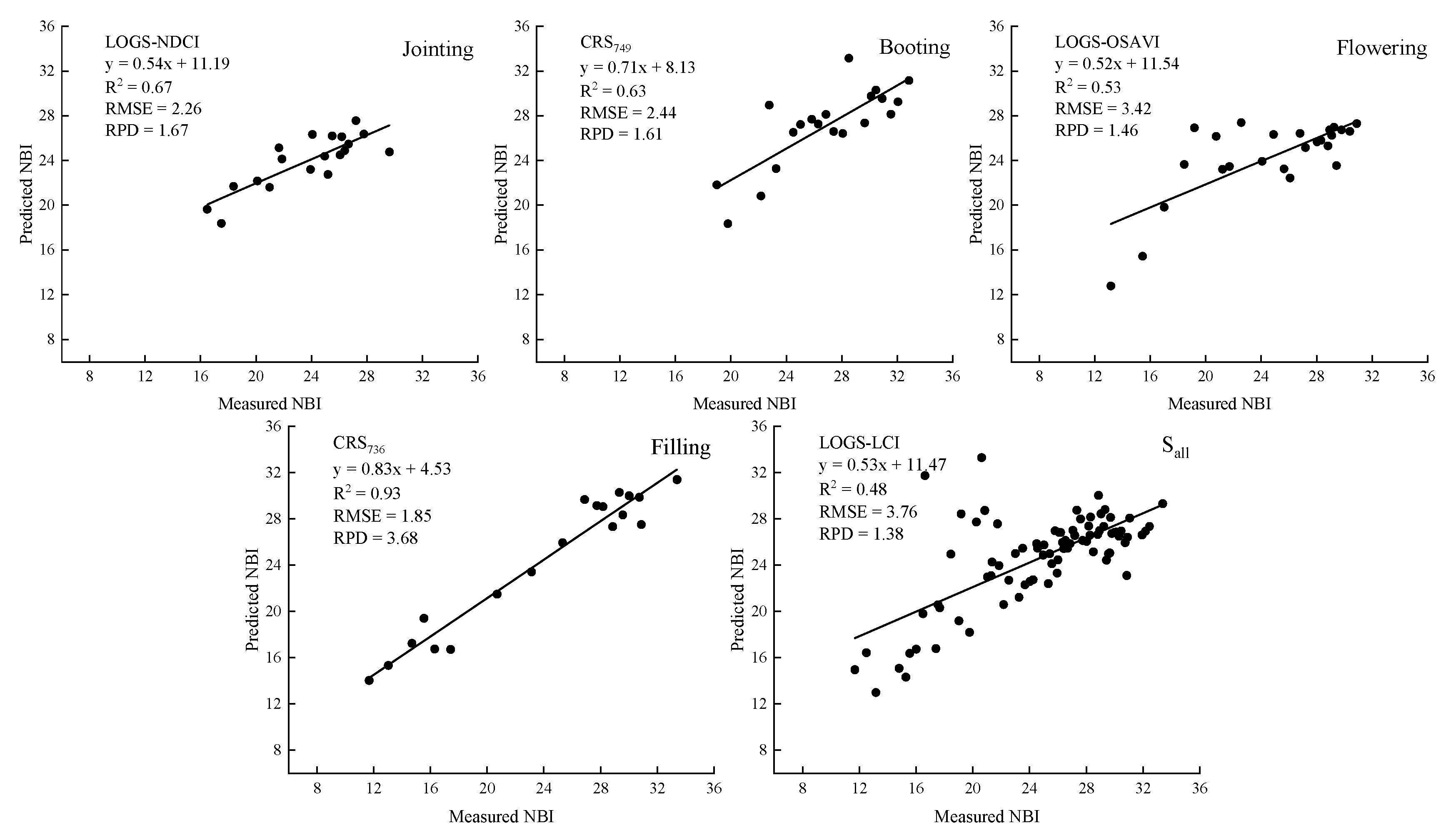
3.3.1. Univariate Regression Model for NBI Estimation (NBI-UR)
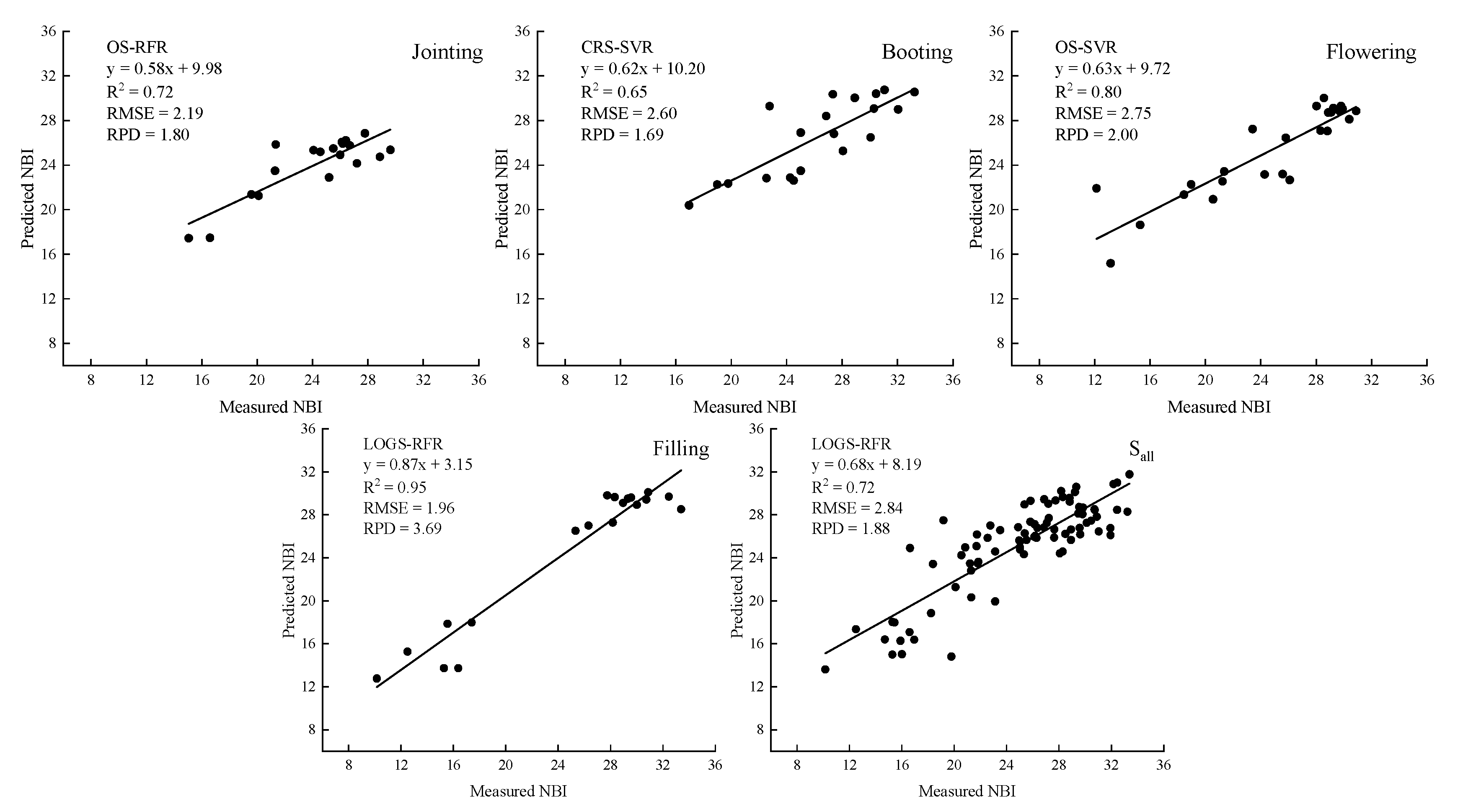
3.3.2. Multivariate Regression Model for NBI Estimation (NBI-MR)
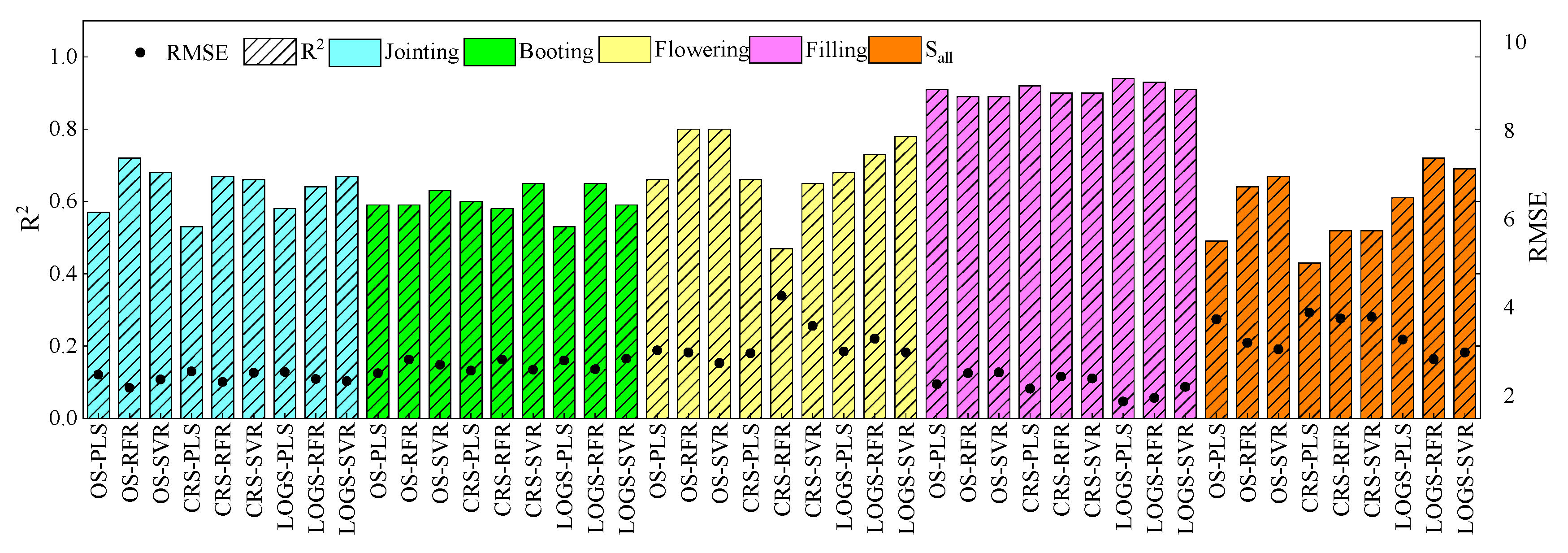
3.4. Model Accuracy Comparison
4. Discussion
4.1. Feasibility of Sensitive Bands and VIs to Estimate NBI
4.2. Potential for Estimating NBI in Each Growth Stage
4.3. Model Selection for NBI
4.4. Challenges and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Bracke, J.; Elsen, A.; Adriaenssens, S.; Vandendriessche, H.; Van Labeke, M.-C. Utility of proximal plant sensors to support nitrogen fertilization in Chrysanthemum. Sci. Hortic. 2019, 256, 108544. [Google Scholar] [CrossRef]
- Molla, T.; Abera, G.; Beyene, S. Effects of nitrogen fertilizer and mulch application on growth performance and pod yields of hot pepper (Capsicum annuum L.) under irrigated condition. Int. J. Plant Soil Sci. 2019, 27, 1–15. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Bayat, H. Influence of different rates of nitrogen fertilizer on growth, yield and fruit quality of sweet pepper (Capsicum annum L. var. California Wander). J. Hortic. Postharvest Res. 2018, 1, 105–114. [Google Scholar]
- Arregui, L.; Quemada, M. Strategies to improve nitrogen use efficiency in winter cereal crops under rainfed conditions. Agron. J. 2008, 100, 277–284. [Google Scholar] [CrossRef]
- Meisinger, J.J.; Schepers, J.; Raun, W. Crop nitrogen requirement and fertilization. Nitrogen Agric. Syst. 2008, 49, 563–612. [Google Scholar]
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef]
- Thompson, R.; Gallardo, M.; Voogt, W. Optimizing nitrogen and water inputs for greenhouse vegetable production. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, Australia, 17–22 August 2014; pp. 15–30. [Google Scholar]
- Fox, R.H.; Walthall, C.L. Crop monitoring technologies to assess nitrogen status. Nitrogen Agric. Syst. 2008, 49, 647–674. [Google Scholar]
- Li, J.; Zhang, F.; Qian, X.; Zhu, Y.; Shen, G. Quantification of rice canopy nitrogen balance index with digital imagery from unmanned aerial vehicle. Remote Sens. Lett. 2015, 6, 183–189. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Ma, Y.; Zhang, R.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. A comparative assessment of measures of leaf nitrogen in rice using two leaf-clip meters. Sensors 2019, 20, 175. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Qian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y. Assessment of portable chlorophyll meters for measuring crop leaf chlorophyll concentration. Remote Sens. 2019, 11, 2706. [Google Scholar] [CrossRef] [Green Version]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Moigne, M.L. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Nondestructive optical sensing of flavonols and chlorophyll in white head cabbage (Brassica oleracea L. var. capitata subvar. alba) grown under different nitrogen regimens. J. Agric. Food Chem. 2016, 64, 85–94. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Yao, X.; Shi, X.; Zeng, Y. Diversity Analysis of Chlorophyll, Flavonoid, Anthocyanin, and Nitrogen Balance Index of Tea Based on Dualex. Phyton 2021, 90, 1549. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Bélec, C. Evaluation of the Dualex for the assessment of corn nitrogen status. J. Plant Nutr. 2007, 30, 1355–1369. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Belec, C. Performance of Dualex in spring wheat for crop nitrogen status assessment, yield prediction and estimation of soil nitrate content. J. Plant Nutr. 2009, 33, 57–70. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Chen, G.; Ji, R.; Shi, W. Effects of Nitrogen Fertilizer Levels on Nitrogen Balance Index and Yield of Hybrid Super Rice. Soils 2021, 53, 700–706. [Google Scholar]
- Gabriel, J.L.; Quemada, M.; Alonso-Ayuso, M.; Lizaso, J.I.; Martín-Lammerding, D. Predicting N status in maize with clip sensors: Choosing sensor, leaf sampling point, and timing. Sensors 2019, 19, 3881. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sun, H.; Qiao, X.; Yan, X.; Feng, M.; Xiao, L.; Song, X.; Zhang, M.; Shafiq, F.; Yang, W. Hyperspectral estimation of canopy chlorophyll of winter wheat by using the optimized vegetation indices. Comput. Electron. Agric. 2022, 193, 106654. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Ta, N.; Chang, Q.; Zhang, Y. Estimation of Apple Tree Leaf Chlorophyll Content Based on Machine Learning Methods. Remote Sens. 2021, 13, 3902. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xiong, S.; Song, Z.; Tian, W.; Shi, L.; Ma, X. Comparison of new hyperspectral index and machine learning models for prediction of winter wheat leaf water content. Plant Methods 2021, 17, 34. [Google Scholar] [CrossRef]
- Changchun, L.; Chunyan, M.; Peng, C.; Yingqi, C.; Jinjin, S.; Yilin, W. Machine learning-based estimation of potato chlorophyll content at different growth stages using UAV hyperspectral data. Zemdirb. Agric. 2021, 108, 181–190. [Google Scholar]
- Li, Z.; Jin, X.; Yang, G.; Drummond, J.; Yang, H.; Clark, B.; Li, Z.; Zhao, C. Remote sensing of leaf and canopy nitrogen status in winter wheat (Triticum aestivum L.) based on N-PROSAIL model. Remote Sens. 2018, 10, 1463. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhang, J.; Xiong, S.; Zhang, Z.; Wei, Q.; Zhang, W.; Feng, W.; Ma, X. Hyperspectral assessment of leaf nitrogen accumulation for winter wheat using different regression modeling. Precis. Agric. 2021, 22, 1634–1658. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Yuan, F.; Cao, Q.; Ye, H.; Lenz-Wiedemann, V.I.; Bareth, G. In-season diagnosis of rice nitrogen status using proximal fluorescence canopy sensor at different growth stages. Remote Sens. 2019, 11, 1847. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Manevski, K.; Kørup, K.; Larsen, R.; Andersen, M.N. Random forest regression results in accurate assessment of potato nitrogen status based on multispectral data from different platforms and the critical concentration approach. Field Crops Res. 2021, 268, 108158. [Google Scholar] [CrossRef]
- Dong, R.; Miao, Y.; Wang, X.; Yuan, F.; Kusnierek, K. Combining leaf fluorescence and active canopy reflectance sensing technologies to diagnose maize nitrogen status across growth stages. Precis. Agric. 2022, 23, 939–960. [Google Scholar] [CrossRef]
- Xu, X.; Fan, L.; Li, Z.; Meng, Y.; Feng, H.; Yang, H.; Xu, B. Estimating leaf nitrogen content in corn based on information fusion of multiple-sensor imagery from UAV. Remote Sens. 2021, 13, 340. [Google Scholar] [CrossRef]
- Zhao, B.; Duan, A.; Ata-Ul-Karim, S.T.; Liu, Z.; Chen, Z.; Gong, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Qin, A. Exploring new spectral bands and vegetation indices for estimating nitrogen nutrition index of summer maize. Eur. J. Agron. 2018, 93, 113–125. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Liu, J.; Wang, Y.; Chang, Q. Evaluation of leaf N concentration in winter wheat based on discrete wavelet transform analysis. Remote Sens. 2019, 11, 1331. [Google Scholar] [CrossRef] [Green Version]
- Zha, H.; Miao, Y.; Wang, T.; Li, Y.; Zhang, J.; Sun, W.; Feng, Z.; Kusnierek, K. Improving unmanned aerial vehicle remote sensing-based rice nitrogen nutrition index prediction with machine learning. Remote Sens. 2020, 12, 215. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.M.; Fontes, P.C.R.; Milagres, C.d.C.; Garcia Junior, E. Application of proximal optical sensors to assess nitrogen status and yield of bell pepper grown in slab. J. Soil Sci. Plant Nutr. 2021, 21, 229–237. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L.; Zarco-Tejada, P. Airborne hyperspectral images and ground-level optical sensors as assessment tools for maize nitrogen fertilization. Remote Sens. 2014, 6, 2940–2962. [Google Scholar] [CrossRef] [Green Version]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Wang, Y.; Shi, B.; Hou, Y.; Chang, Q. Estimation of winter wheat leaf area index based on UAV hyperspectral remote sensing. Trans. Chin. Soc. Agric. Eng. 2020, 36, 40–49. [Google Scholar]
- Wang, R.; Song, X.; Li, Z.; Yang, G.; Guo, W.; Tan, C.; Chen, L. Estimation of winter wheat nitrogen nutrition index using hyperspectral remote sensing. Trans. Chin. Soc. Agric. Eng. 2014, 30, 191–198. [Google Scholar]
- Li, D.; Li, F.; Hu, Y. Study on the Estimation of Nitrogen Content in Wheat and Maize Canopy Based on Band Optimization of Spectral Parameters. Spectrosc. Spectr. Anal. 2016, 36, 1150–1157. [Google Scholar] [CrossRef]
- Ranjan, R.; Chopra, U.K.; Sahoo, R.N.; Singh, A.K.; Pradhan, S. Assessment of plant nitrogen stress in wheat (Triticum aestivum L.) through hyperspectral indices. Int. J. Remote Sens. 2012, 33, 6342–6360. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Wang, W.; Chen, X.; Chang, Q. Monitoring of winter wheat nitrogen nutrition based on UAV hyperspectral images. Trans. Chin. Soc. Agric. Eng. 2020, 36, 31–39. [Google Scholar]
- Yue, J.; Feng, H.; Jin, X.; Yuan, H.; Li, Z.; Zhou, C.; Yang, G.; Tian, Q. A comparison of crop parameters estimation using images from UAV-mounted snapshot hyperspectral sensor and high-definition digital camera. Remote Sens. 2018, 10, 1138. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Feng, H.; Yang, G.; Li, Z. A comparison of regression techniques for estimation of above-ground winter wheat biomass using near-surface spectroscopy. Remote Sens. 2018, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Cui, L.; Wang, J.; Fei, T.; Chen, Y.; Wu, G. Comparison of multivariate methods for estimating soil total nitrogen with visible/near-infrared spectroscopy. Plant Soil 2013, 366, 363–375. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chang, Q.; Liu, X.; Yan, L.; Luo, D.; Wang, S. Estimation of maize leaf SPAD value based on hyperspectrum and BP neural network. Trans. Chin. Soc. Agric. Eng. 2016, 32, 135–142. [Google Scholar]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Daughtry, C.S.; Walthall, C.; Kim, M.; De Colstoun, E.B.; McMurtrey Iii, J. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, H.-Y.; Zhang, Y.-S.; Qi, S.-L.; Heng, Y.-R.; Guo, B.-B.; Ma, D.-Y.; Guo, T.-C. Remote detection of canopy leaf nitrogen concentration in winter wheat by using water resistance vegetation indices from in-situ hyperspectral data. Field Crops Res. 2016, 198, 238–246. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gamon, J.A.; Solovchenko, A. Multiple drivers of seasonal change in PRI: Implications for photosynthesis 2. Stand level. Remote Sens. Environ. 2017, 190, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Ravier, C.; Quemada, M.; Jeuffroy, M.-H. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Field Crops Res. 2017, 214, 73–82. [Google Scholar] [CrossRef]
- Pancorbo, J.; Camino, C.; Alonso-Ayuso, M.; Raya-Sereno, M.; Gonzalez-Fernandez, I.; Gabriel, J.L.; Zarco-Tejada, P.J.; Quemada, M. Simultaneous assessment of nitrogen and water status in winter wheat using hyperspectral and thermal sensors. Eur. J. Agron. 2021, 127, 126287. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Ma, C.; Ding, F.; Li, Y.; Chen, W.; Li, J.; Xiao, Z. Hyperspectral Estimation of Winter Wheat Leaf Area Index Based on Continuous Wavelet Transform and Fractional Order Differentiation. Sensors 2021, 21, 8497. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Zhang, X.; Cheng, X.; Feng, G.; Wang, Y.; Gao, Q. Estimating the maize biomass by crop height and narrowband vegetation indices derived from UAV-based hyperspectral images. Ecol. Indic. 2021, 129, 107985. [Google Scholar] [CrossRef]
- Mistele, B.; Schmidhalter, U. Estimating the nitrogen nutrition index using spectral canopy reflectance measurements. Eur. J. Agron. 2008, 29, 184–190. [Google Scholar] [CrossRef]
- Ryu, C.; Suguri, M.; Iida, M.; Umeda, M.; Lee, C. Integrating remote sensing and GIS for prediction of rice protein contents. Precis. Agric. 2011, 12, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Wang, J.; Zhu, X.; Wang, Y.; Wang, J.; Tong, L.; Guo, W. Monitoring Main Growth Status Parameters at Jointing Stage in Winter Wheat Based on Landsat TM Images. Sci. Agric. Sin. 2011, 44, 1358–1366. [Google Scholar]
- Wang, Y.J.; Li, T.H.; Jin, G.; Wei, Y.M.; Li, L.Q.; Kalkhajeh, Y.K.; Ning, J.M.; Zhang, Z.Z. Qualitative and quantitative diagnosis of nitrogen nutrition of tea plants under field condition using hyperspectral imaging coupled with chemometrics. J. Sci. Food Agric. 2020, 100, 161–167. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Lin, Z. Identification of ginseng according to geographical origin by near-infrared spectroscopy and pattern recognition. Vib. Spectrosc. 2020, 110, 8. [Google Scholar] [CrossRef]
- Sheng, X.H.; Li, Z.P.; Li, Z.W.; Dong, J.H.; Wang, J.; Yin, J.J. Nondestructive determination of lignin content in Korla fragrant pear based on near-infrared spectroscopy. Spectr. Lett. 2020, 53, 306–314. [Google Scholar] [CrossRef]
- Chen, X.; Huan, K.W.; Zhao, H.; Fan, H.Y.; Han, X.Y. Variable Selection of Near Infrared Spectroscopy Based on Variable Frequency Weighted Bootstrap Sampling. Chin. J. Anal. Chem. 2021, 49, 1743–1749. [Google Scholar]





| Spectral Indices | Definitions |
|---|---|
| OSAVI (Optimized soil-adjusted vegetation index) [38] | (1 + 0.16) (R800 − R670)/(R800 + R670 + 0.16) |
| mSR705 (Modified red edge simple ratio index) [42] | (R750 − R445)/(R705 − R445) |
| MTCI (MERIS terrestrial chlorophyll index) [35] | (R754 − R709)/(R709 − R681) |
| SIPI (Structure intensive pigment index) [25] | (R800 − R445)/(R800 − R680) |
| NPCI680 (Normalized pigment chlorophyll index) [25] | (R680 − R430)/(R680 + R430) |
| NRI (Nitrogen reflectance index) [35] | (R570 − R670)/(R570 + R670) |
| NDRE (Normalized difference red-edge) [35] | (R790 − R720)/(R790 + R720) |
| DCNI (Double-peak canopy nitrogen index) [42] | (R720 − R700)/(R700 − R670)/(R720 − R670 + 0.03) |
| GNDVI (Green normalized difference vegetation index) [35] | (R750 − R550)/(R750 + R550) |
| MCARI2 (Modified triangular vegetation index 2) [35] | 1.5(1.2(R800 − R550) − 2.5(R670 − R550))/sqrt((2R800 + 1)2 − (6R800 − 5sqrt(R670)) − 0.5) |
| CI red (Red-edge chlorophyll index) [43] | R790/R720 − 1 |
| CI green (Green chlorophyll index) [43] | R790/R550 − 1 |
| RVI800 (Ratio vegetation index) [43] | R800/R680 |
| NDCI (Normalized difference chlorophyll index) [44] | (R762 − R527)/(R762 + R527) |
| GRVI (Green ratio vegetation index) [25] | (R620 − R506)/(R620 + R506) |
| TCARI (Transformed chlorophyll absorption in reflectance index) [38] | 3 [(R700 − R670) − 0.2(R700 − R550)/(R700/R670)] |
| NPCI642 (Normalized pigment chlorophyll index) [25] | (R642 − R432)/(R642 + R432) |
| PPR (Plant pigment ratio) [25] | (R503 − R436)/(R503 + R436) |
| NDSI (Normalized difference spectral index) [25] | (R813 − R763)/(R813 + R763) |
| LCI (Leaf chlorophyll index) [25] | (R850 − R710)/(R850 − R680) |
| PRI (Photochemical reflectance index) [42] | (R570 − R539)/(R570 + R539) |
| VOG (Vogelman red edge index) [42] | R740/R720 |
| REP LI780 (Red edge position: linear interpolation method) [42] | 700 + 40 [(R670 + R780)/2 − R700]/(R740 − R700) |
| Dataset | Growth Stage | Sample Numbers | Range | Mean | Standard Deviation | Coefficient of Variation/% |
|---|---|---|---|---|---|---|
| Modeling set | Jointing | 37 | 15.06–28.87 | 23.56 | 3.61 | 15.32 |
| Booting | 40 | 16.95–33.23 | 26.83 | 4.02 | 14.98 | |
| Flowering | 51 | 12.13–32.44 | 24.85 | 4.99 | 20.08 | |
| Filling | 37 | 10.16–33.45 | 23.49 | 6.81 | 28.99 | |
| Sall | 165 | 10.16–33.23 | 24.74 | 5.14 | 20.78 | |
| Validation set | Jointing | 19 | 16.48–29.62 | 23.72 | 3.60 | 15.18 |
| Booting | 20 | 18.99–32.84 | 26.85 | 3.94 | 14.67 | |
| Flowering | 25 | 13.15–30.89 | 24.68 | 4.98 | 20.18 | |
| Filling | 19 | 11.69–33.39 | 23.87 | 6.82 | 28.57 | |
| Sall | 83 | 11.69–33.39 | 24.79 | 5.17 | 20.86 |
| Growth Stage | OS | CRS | LOGS | |||
|---|---|---|---|---|---|---|
| Wavelength/nm | Correlation Coefficients | Wavelength/nm | Correlation Coefficients | Wavelength/nm | Correlation Coefficients | |
| Jointing | 929 | 0.72 | 733 | −0.79 | 867 | −0.75 |
| Booting | 709 | −0.61 | 749 | −0.78 | 1135 | −0.62 |
| Flowering | 748 | 0.60 | 1336 | −0.45 | 784 | −0.62 |
| Filling | 692 | −0.87 | 736 | −0.95 | 694 | 0.87 |
| Sall | 817 | 0.59 | 708 | −0.63 | 817 | −0.60 |
| Growth Stage | Variables |
|---|---|
| Jointing | SB, NDRE, GNDVI, CI red, CI green, NDCI, VOG |
| Booting | SB, MTCI, NDRE, CI red, LCI, REP, LI780 |
| Flowering | SB, OSAVI, NPCI680, GRVI, NPCI642, PPR |
| Filling | SB, MTCI, NDRE, CI red, LCI, VOG |
| Sall | SB, OSAVI, NDRE, NDCI, LCI, REP, LI780 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.; Li, F.; Chen, X.; Li, Z.; Mulla, D.J. Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning. Remote Sens. 2022, 14, 3504. https://doi.org/10.3390/rs14143504
Fan K, Li F, Chen X, Li Z, Mulla DJ. Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning. Remote Sensing. 2022; 14(14):3504. https://doi.org/10.3390/rs14143504
Chicago/Turabian StyleFan, Kai, Fenling Li, Xiaokai Chen, Zhenfa Li, and David J. Mulla. 2022. "Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning" Remote Sensing 14, no. 14: 3504. https://doi.org/10.3390/rs14143504
APA StyleFan, K., Li, F., Chen, X., Li, Z., & Mulla, D. J. (2022). Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning. Remote Sensing, 14(14), 3504. https://doi.org/10.3390/rs14143504







