Estimating Leaf Area Index with a New Vegetation Index Considering the Influence of Rice Panicles
Abstract
1. Introduction
2. Materials and Methods
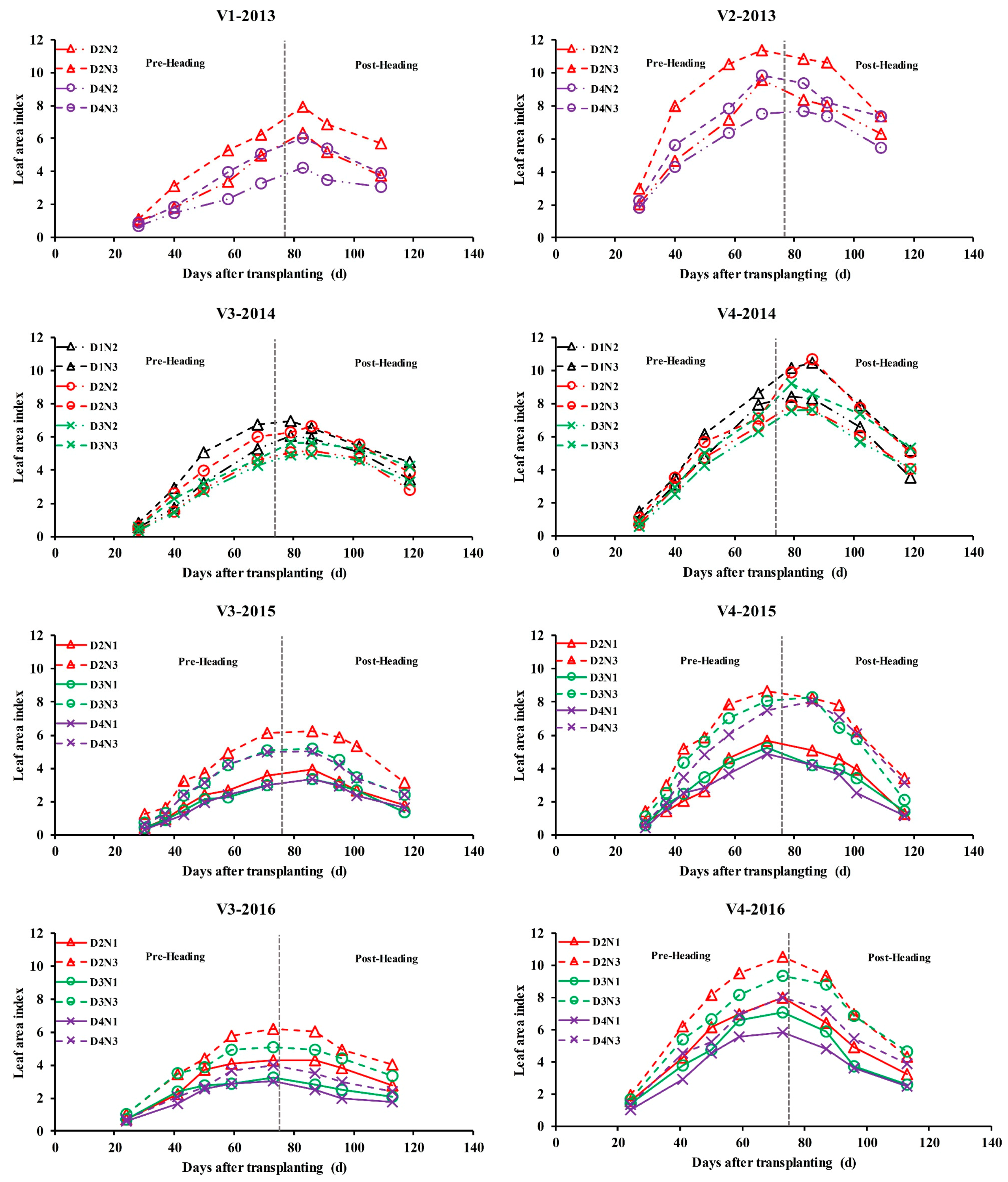
2.1. Study Area and Experimental Details
2.2. Spectral Measurements
2.3. UAV, Sensor, Image Acquisition and Processing
2.4. Determination of LAI
2.5. Development of the Panicle-Adjusted Renormalized Difference Vegetation Index
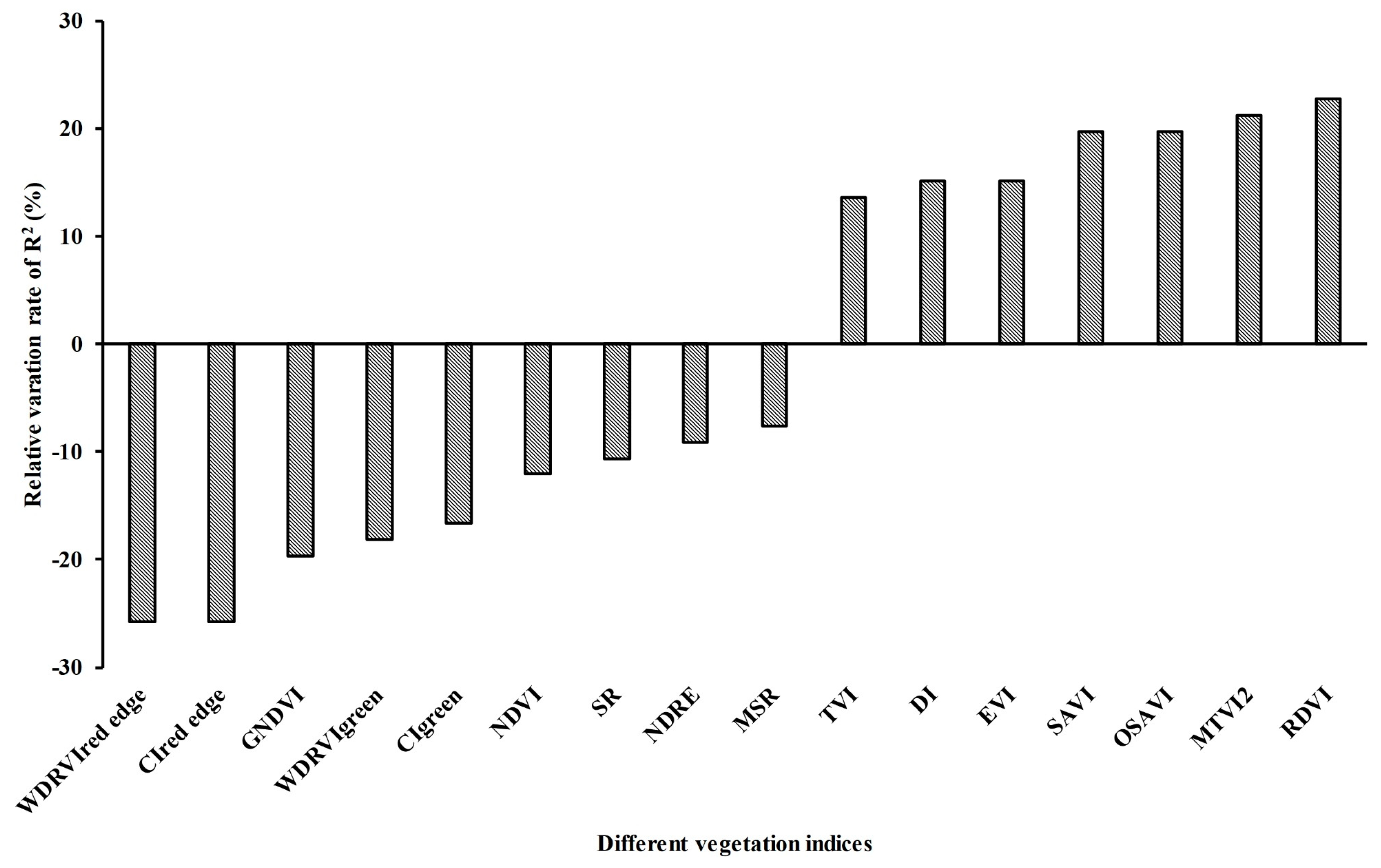
2.5.1. Selection of Vegetation Index Form
2.5.2. Development of the Panicle-Adjusted Renormalized Difference Vegetation Index (PRDVI)
2.6. Data Analysis and Application
3. Results
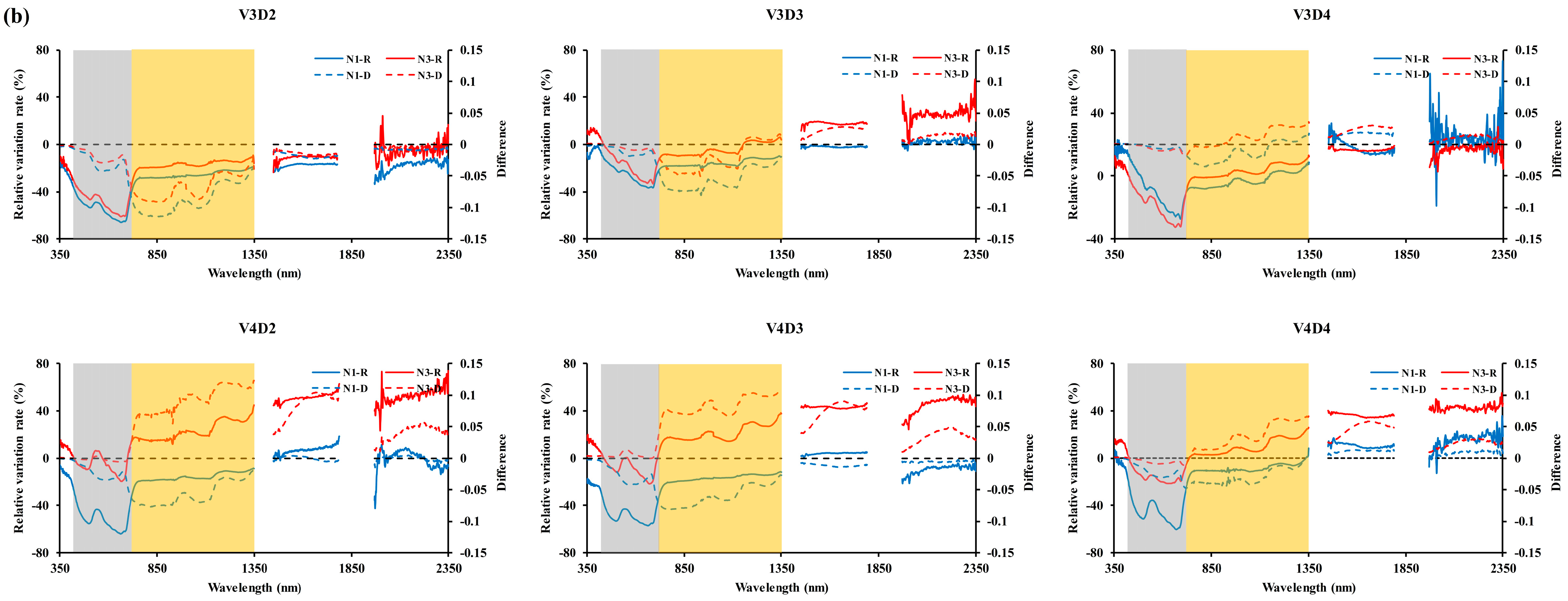
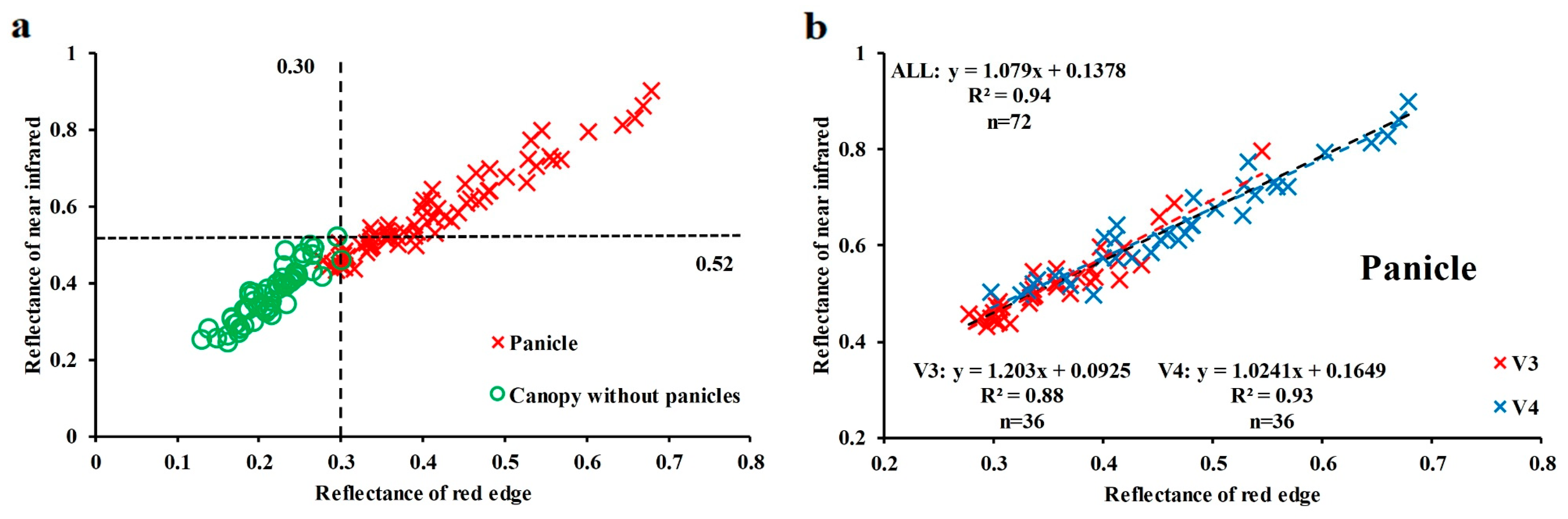
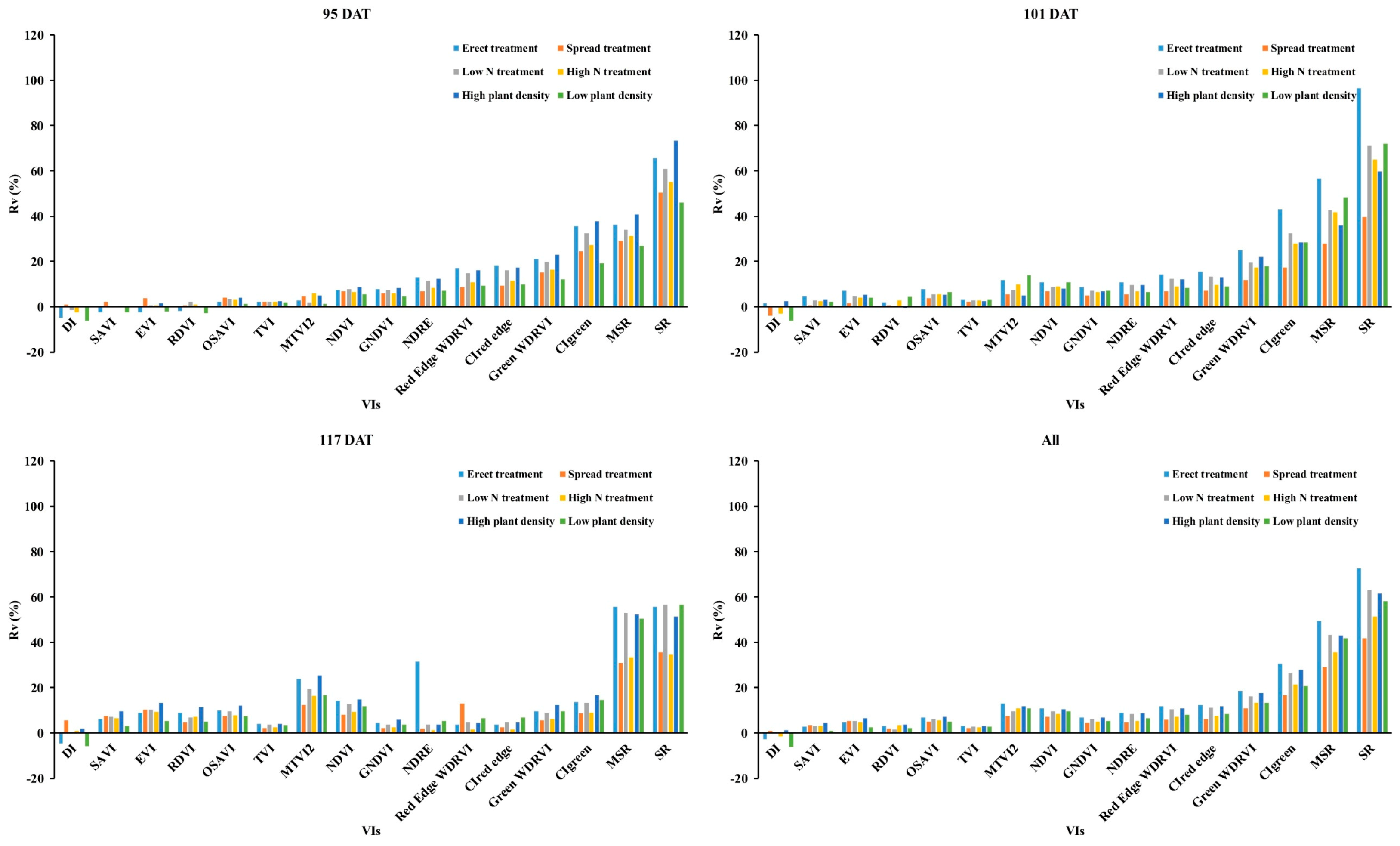
3.1. Influence of Panicles on the Reflectance of Rice Canopy
3.2. Influence of Panicles on LAI Estimation Accuracy
3.2.1. Relationship between LAI and the Reflectance of Rice Canopy before and after the Removal of Panicles
3.2.2. Relationship between LAI and VIs during the Pre-Heading Stage, Post-Heading Stage, and Whole Growth Stage
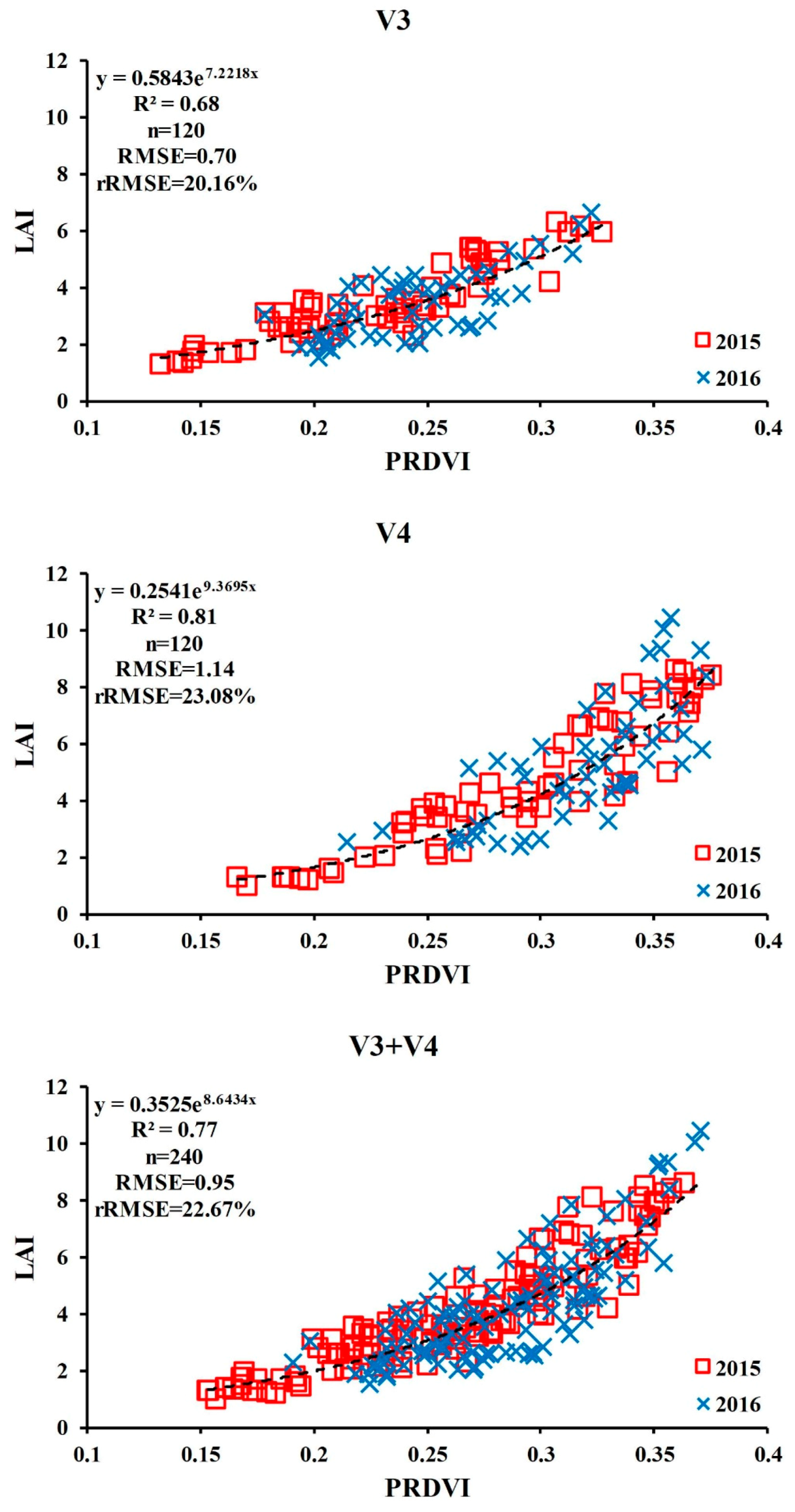
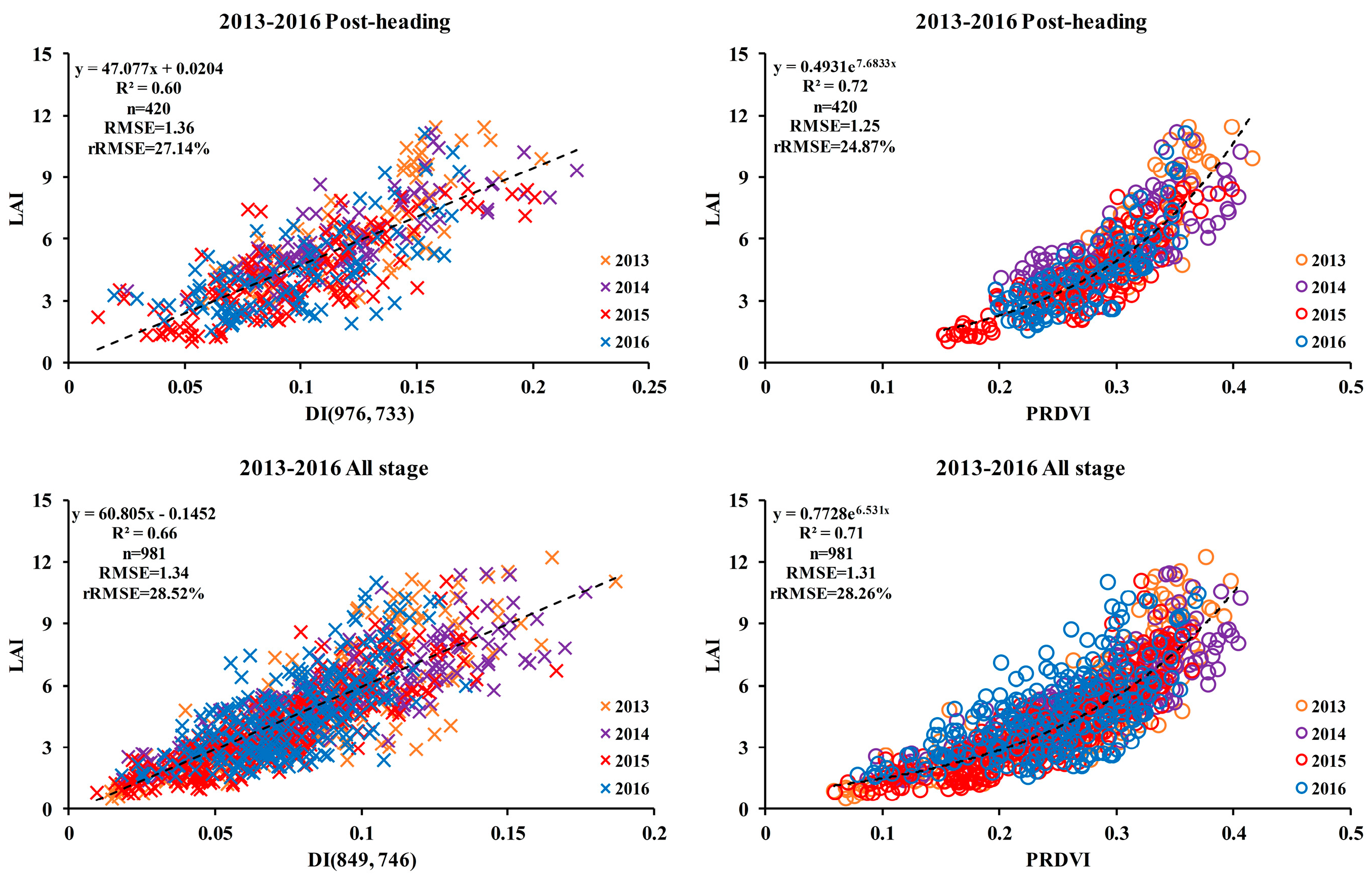
3.3. Relationship between the PRDVI and LAI
4. Discussion
4.1. Influence of Panicle in Reflectance of Rice Canopy
4.2. Influence of Panicles on LAI Estimation Accuracy
4.3. Elimination of the Impact of Panicle and Background
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bulcock, H.H.; Jewitt, G.P.W. Improved spatial mapping of leaf area index using hyperspectral remote sensing for hydrological applications with a particular focus on canopy interception. Hydrol. Earth Syst. Sci. Discuss. 2009, 6, 5783–5809. [Google Scholar] [CrossRef]
- Chen, J.M.; Cihlár, J. Retrieving Leaf Area Index of boreal conifer forests using Landsat TM images. Remote Sens. Environ. 1996, 55, 153–162. [Google Scholar] [CrossRef]
- Bréda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Schepers, J. Relationships between gross primary production, green LAI, and canopy chlorophyll content in maize: Implications for remote sensing of primary production. Remote Sens. Environ. 2014, 144, 65–72. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Aubrey, D.P.; Zhuang, Q.; Teskey, R.O. Global patterns and predictors of stem CO2 efflux in forest ecosystems. Glob. Chang. Boil. 2016, 22, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Xiao, J.F.; Ju, W.M.; Zhu, G.L.; Wu, X.C.; Fan, W.L.; Li, D.Q.; Zhou, Y.L. Satellite-derived LAI products exhibit large discrepancies and can lead to substantial uncertainty in simulated carbon and water fluxes. Remote Sens. Environ. 2018, 206, 174–188. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Rivera, J.P.; Ruiz-Verdú, A.; Moreno, J. Brown and green LAI mapping through spectral indices. Int. J. Appl. Earth Obs. Geoinf. 2015, 35, 350–358. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, G.; Zhang, F. Using negative soil adjustment factor in soil-adjusted vegetation index (SAVI) for aboveground living biomass estimation in arid grasslands. Remote Sens. Environ. 2018, 209, 439–445. [Google Scholar] [CrossRef]
- Feng, W.; Guo, B.B.; Wang, Z.J.; He, L.; Song, X.; Wang, Y.H.; Guo, T.C. Measuring leaf nitrogen concentration in winter wheat using double-peak spectral reflection remote sensing data. Field Crop. Res. 2014, 159, 43–52. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Qin, Y.; Zhu, Y.; Cao, W.; Tian, Y. Exploring the Vertical Distribution of Structural Parameters and Light Radiation in Rice Canopies by the Coupling Model and Remote Sensing. Remote Sens. 2015, 7, 5203–5221. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q. Developing and validating novel hyperspectral indices for leaf area index estimation: Effect of canopy vertical heterogeneity. Ecol. Indic. 2013, 32, 123–130. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Bréon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Huete, A.; Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanré, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Peddle, D.R.; Riddell, K.D.; Hall, F.G. SCAVI: A Sunlit Canopy Adjusted Vegetation Index. Can. J. Remote Sens. 2015, 41, 20–28. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Kang, Y.; Özdoğan, M.; Zipper, S.C.; Román, M.O.; Walker, J.; Hong, S.Y.; Marshall, M.; Magliulo, V.; Moreno, J.; Alonso, L.; et al. How Universal Is the Relationship between Remotely Sensed Vegetation Indices and Crop Leaf Area Index? A Global Assessment. Remote Sens. 2016, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Diao, W.Y.; Xiao, C.H.; Wang, F.Y.; Chen, B.; Wang, K.R.; Li, S.K. Estimation of Wheat Agronomic Parameters using New Spectral Indices. PLoS ONE 2013, 8, e72736. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Li, Z.H.; Feng, H.K.; Xu, X.G.; Yang, G.J. Newly combined spectral indices to improve estimation of total leaf chlorophyll content in cotton. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4589–4600. [Google Scholar] [CrossRef]
- Luo, J.H.; Ma, R.H.; Feng, H.H.; Li, X.C. Estimating the total nitrogen concentration of reed canopy with hyperspectral measurements considering a non-uniform vertical nitrogen distribution. Remote Sens. 2016, 8, 789. [Google Scholar] [CrossRef]
- Gutierrez, M.; Reynolds, M.P.; Klatt, A.R. Effect of leaf and spike morphological traits on the relationship between spectral reflectance indices and yield in wheat. Int. J. Remote Sens. 2015, 36, 701–718. [Google Scholar] [CrossRef]
- Li, H.L.; Zhao, C.J.; Yang, G.J.; Feng, H.K. Variations in crop variables within wheat canopies and responses of canopy spectral characteristics and derived vegetation indices to different vertical leaf layers and spikes. Remote Sens. Environ. 2015, 169, 358–374. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, X.; Zhang, M.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Development and testing of an ear-leaf model for rice canopy reflectance. J. Appl. Remote Sens. 2018, 12, 016016. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikeura, H.; Phongchanmaixay, S.; Khanthavong, P. Canopy Hyperspectral Sensing of Paddy Fields at the Booting Stage and PLS Regression can Assess Grain Yield. Remote Sens. 2018, 10, 1249. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Wang, C. Potential of X-Band Images from High-Resolution Satellite SAR Sensors to Assess Growth and Yield in Paddy Rice. Remote Sens. 2014, 6, 5995–6019. [Google Scholar] [CrossRef]
- Asilo, S.; Nelson, A.; De Bie, K.; Skidmore, A.; Laborte, A.; Maunahan, A.; Quilang, E.J.P. Relating X-band SAR Backscattering to Leaf Area Index of Rice in Different Phenological Phases. Remote Sens. 2019, 11, 1462. [Google Scholar] [CrossRef]
- Broge, N.; Mortensen, J. Deriving green crop area index and canopy chlorophyll density of winter wheat from spectral reflectance data. Remote Sens. Environ. 2002, 81, 45–57. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.; Xu, X.; He, J.; Ge, X.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef]
- Ali, M.; Montzka, C.; Stadler, A.; Menz, G.; Thonfeld, F.; Vereecken, H. Estimation and Validation of RapidEye-Based Time-Series of Leaf Area Index for Winter Wheat in the Rur Catchment (Germany). Remote Sens. 2015, 7, 2808–2831. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.; Schlerf, M. Mapping grassland leaf area index with airborne hyperspectral imagery: A comparison study of statistical approaches and inversion of radiative transfer models. ISPRS J. Photogramm. Remote Sens. 2011, 66, 894–906. [Google Scholar] [CrossRef]
- Pu, R.; Cheng, J. Mapping forest leaf area index using reflectance and textural information derived from WorldView-2 imagery in a mixed natural forest area in Florida, US. Int. J. Appl. Earth Obs. Geoinf. 2015, 42, 11–23. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation. NASA/GSFC Type III Final Report; NASA/GSFC: Greenbelt, MD, USA, 1974.
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A. Application of chlorophyll-related vegetation indices for remote estimation of maize productivity. Agric. For. Meteorol. 2011, 151, 1267–1276. [Google Scholar] [CrossRef]
- Broge, N.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Huete, A.R.; Didan, K.; Miura, H.; Rodriguez, E.P.; Gao, X.; Ferreira, L.F. Overview of the radiometric and biopyhsical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Rundquist, D.C.; Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30, 52-1–52-4. [Google Scholar]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Xu, N.; Tian, J.; Tian, Q.; Xu, K.; Tang, S. Analysis of Vegetation Red Edge with Different Illuminated/Shaded Canopy Proportions and to Construct Normalized Difference Canopy Shadow Index. Remote Sens. 2019, 11, 1192. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawamura, K.; Maki, M.; Muramoto, Y.; Yoshida, K.; Akiyama, T. Spectral index of quantifying leaf area index of winter wheat by field hyperspectral measurements: A case study in Gifu Prefectrue, Central Japan. Remote Sens. 2015, 7, 5329–5346. [Google Scholar] [CrossRef]
- Hallik, L.; Kuusk, A.; Lang, M.; Kuusk, J. Reflectance Properties of Hemiboreal Mixed Forest Canopies with Focus on Red Edge and Near Infrared Spectral Regions. Remote Sens. 2019, 11, 1717. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.; Noland, T.; Mohammed, G.; Sampson, P. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Sui, B.; Feng, X.; Tian, G.; Hu, X.; Shen, Q.; Guo, S. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crop. Res. 2013, 150, 99–107. [Google Scholar] [CrossRef]
- Tian, Y.C.; Gu, K.J.; Chu, X.; Yao, X.; Cao, W.X.; Zhu, Y. Comparison of different hyperspectral vegetation indices for canopy leaf nitrogen concentration estimation in rice. Plant Soil 2014, 376, 193–209. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Zhao, W.J.; Zhou, D.M.; Gong, H.L. Sensitivity analysis of vegetation reflectance to biochemical and biophysical variables at leaf, canopy, and regional scales. IEEE Trans. Geosci. Remote Sens. 2014, 52, 4014–4024. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.; Zhang, Y. The applicability of empirical vegetation indices for determining leaf chlorophyll content over different leaf and canopy structures. Ecol. Complex. 2014, 17, 119–130. [Google Scholar] [CrossRef]
- Boegh, E.; Houborg, R.; Bienkowski, J.; Braban, C.F.; Dalgaard, T.; Van Dijk, N.; Dragosits, U.; Holmes, E.; Magliulo, V.; Schelde, K.; et al. Remote sensing of LAI, chlorophyll and leaf nitrogen pools of crop- and grasslands in five European landscapes. Biogeosciences 2013, 10, 6279–6307. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192, 140–148. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Qian, B.; Zhao, T.; Jing, Q.; Geng, X.; Wang, J.; Huffman, T.; Shang, J. Estimating winter wheat biomass by assimilating leaf area index derived from fusion of Landsat-8 and MODIS data. Int. J. Appl. Earth Obs. Geoinf. 2016, 49, 63–74. [Google Scholar] [CrossRef]
- Huang, M.; Yang, C.; Ji, Q.; Jiang, L.; Tan, J.; Li, Y. Tillering responses of rice to plant density and nitrogen rate in a subtropical environment of southern China. Field Crop. Res. 2013, 149, 187–192. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating Hyperspectral Vegetation Indices for Leaf Area Index Estimation of Oryza sativa L. at Diverse Phenological Stages. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Richter, K.; Hank, T.B.; Vuolo, F.; Mauser, W.; D’Urso, G. Optimal Exploitation of the Sentinel-2 Spectral Capabilities for Crop Leaf Area Index Mapping. Remote Sens. 2012, 4, 561–582. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Bao, Y.; Luo, J.; Jin, X.; Xu, X.; Song, X.; Yang, G. Exploring the Best Hyperspectral Features for LAI Estimation Using Partial Least Squares Regression. Remote Sens. 2014, 6, 6221–6241. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]






| Cultivar | Nitrogen Fertilization Rate (kg·ha−1) | Plant Density (Plants·m−2) | Sampling and Spectral Measurement Data (DATa) | |
|---|---|---|---|---|
| Exp.1 (2013) | Wuxiangjing 14 (WXJ14, V1) Shanyou 63 (SY63, V2) | 150 (N2) 300 (N3) | 22.22 (D2) 13.33 (D4) | 28, 40, 58, 69, 83, 91, 109 |
| Exp.2 (2014) | Wuyunjing 24 (WYJ24, V3) Eryou 728 (EY728, V4) | 150 (N2) 300 (N3) | 33.33 (D1) 22.22 (D2) 16.66 (D3) | 28, 40, 50, 68, 79, 86, 102, 119 |
| Exp.3 (2015) | Wuyunjing 24 (WYJ24, V3) Eryou 728 (EY728, V4) | 100 (N1) 300 (N3) | 22.22 (D2) 16.66 (D3) 13.33 (D4) | 37, 43, 50, 58, 71, 86, 95, 96b, 101, 102b, 117, 118b |
| Exp.4 (2016) | Wuyunjing 24 (WYJ24, V3) Eryou 728 (EY728, V4) | 100 (N1) 300 (N3) | 22.22 (D2) 16.66 (D3) 13.33 (D4) | 24, 41, 50, 59, 73, 87, 89b, 96, 98b, 113, 114b |
| Exp.5c (2015) | Wuyunjing 24 (WYJ24, V3) Y Liangyou 1 (YL1, V5) | 0 (N0) 100 (N1) 300 (N3) | 22.22 (D2) 13.33 (D4) | 82, 99 |
| Dataset | Year | Description | Number of Samples | ||
|---|---|---|---|---|---|
| Japonica | Indica | Total | |||
| 1 | 2015–2016 | Pre-heading stages | 159 | 162 | 321 |
| 2 | Post-heading stages | 120 | 120 | 240 | |
| 3 | All stages | 279 | 282 | 561 | |
| 4 | Panicles | 36 | 36 | 72 | |
| 5 | Original canopy | 36 | 36 | 72 | |
| 6 | Canopy without panicles | 36 | 36 | 72 | |
| 7 | Pre-heading + Canopy without panicles | 195 | 198 | 393 | |
| 8 | Post-heading + Original canopy | 156 | 156 | 312 | |
| 9 | 2013–2016 | Pre-heading stages | 279 | 282 | 561 |
| 10 | Post-heading stages | 210 | 210 | 420 | |
| 11 | All stages | 489 | 492 | 981 | |
| 12 | 2015 | UAV data | 36 | 36 | 72 |
| Vegetation Index | Formula a | Coefficient of Determination | Reference | |
|---|---|---|---|---|
| Entire Canopy | Canopy without Panicles | |||
| DI | b | 0.66 | 0.76 | [10] |
| SR | 0.38 | 0.31 | [10] | |
| NDVI | 0.37 | 0.29 | [38] | |
| GNDVI | 0.49 | 0.36 | [39] | |
| RDVI | 0.43 | 0.58 | [14] | |
| MSR | 0.33 | 0.28 | [15] | |
| Green WDRVI | c | 0.51 | 0.39 | [40,41] |
| Red Edge WDRVI | c | 0.60 | 0.43 | [40,41] |
| SAVI | d | 0.44 | 0.57 | [16] |
| OSAVI | e | 0.40 | 0.53 | [17] |
| TVI | 0.28 | 0.37 | [42] | |
| MTVI2 | 0.38 | 0.52 | [20] | |
| EVI | 0.51 | 0.61 | [43] | |
| CIred edge | 0.60 | 0.43 | [44] | |
| CIgreen | 0.50 | 0.39 | [44] | |
| NDRE | 0.41 | 0.35 | [45] | |
| Treatments | 96 DAT | 102 DAT | 117 DAT | |||||
|---|---|---|---|---|---|---|---|---|
| Original Canopy | Canopy without Panicles | Original Canopy | Canopy without Panicles | Original Canopy | Canopy without Panicles | |||
| V3 | D2 | N1 | 725 nm | 725 nm | 715 nm | 715 nm | 697 nm | 725 nm |
| N3 | 727 nm | 727 nm | 727 nm | 727 nm | 723 nm | 727 nm | ||
| D3 | N1 | 718 nm | 718 nm | 716 nm | 716 nm | 697 nm | 718 nm | |
| N3 | 727 nm | 727 nm | 723 nm | 723 nm | 722 nm | 727 nm | ||
| D4 | N1 | 723 nm | 723 nm | 723 nm | 723 nm | 697 nm | 723 nm | |
| N3 | 727 nm | 727 nm | 723 nm | 723 nm | 698 nm | 727 nm | ||
| V4 | D2 | N1 | 725 nm | 725 nm | 716 nm | 716 nm | 694 nm | 725 nm |
| N3 | 726 nm | 726 nm | 723 nm | 723 nm | 697 nm | 727 nm | ||
| D3 | N1 | 718 nm | 718 nm | 723 nm | 723 nm | 692 nm | 725 nm | |
| N3 | 729 nm | 729 nm | 723 nm | 723 nm | 697 nm | 725 nm | ||
| D4 | N1 | 725 nm | 725 nm | 716 nm | 716 nm | 695 nm | 725 nm | |
| N3 | 729 nm | 729 nm | 728 nm | 728 nm | 700 nm | 729 nm | ||
| Cultivar | Data | DI | SAVI | RDVI | CI | WDRVI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | OBC | RMSE | rRMSE (%) | R2 | OBC | RMSE | rRMSE (%) | R2 | OBC | RMSE | rRMSE (%) | R2 | OBC | RMSE | rRMSE (%) | R2 | OBC | RMSE | rRMSE (%) | ||
| All (V1+V2+V4+V4) | 1 | 0.73 | 851, 756 | 1.15 | 27.88 | 0.76 | 854, 752 | 1.09 | 26.42 | 0.75 | 851, 753 | 1.09 | 26.43 | 0.72 | 854, 751 | 1.17 | 28.24 | 0.72 | 854, 751 | 1.17 | 28.27 |
| 2 | 0.59 | 774, 744 | 1.24 | 29.68 | 0.60 | 934, 723 | 1.23 | 29.39 | 0.60 | 934, 724 | 1.23 | 29.27 | 0.49 | 955, 724 | 1.39 | 33.21 | 0.50 | 955, 724 | 1.38 | 33.06 | |
| 3 | 0.63 | 785, 753 | 1.28 | 30.76 | 0.65 | 897, 738 | 1.25 | 30.03 | 0.65 | 851, 742 | 1.25 | 29.94 | 0.58 | 853, 742 | 1.36 | 32.66 | 0.59 | 897, 739 | 1.35 | 32.52 | |
| 5 | 0.66 | 958, 732 | 0.88 | 22.30 | 0.68 | 958, 732 | 0.87 | 22.14 | 0.69 | 951, 733 | 0.85 | 21.74 | 0.55 | 958, 734 | 1.06 | 27.06 | 0.55 | 958, 734 | 1.06 | 27.06 | |
| 6 | 0.70 | 934, 731 | 0.93 | 23.62 | 0.70 | 974, 733 | 0.90 | 23.01 | 0.71 | 958, 732 | 0.89 | 22.63 | 0.53 | 974, 737 | 1.08 | 27.58 | 0.54 | 974, 737 | 1.08 | 27.56 | |
| 7 | 0.69 | 851, 750 | 1.17 | 28.57 | 0.72 | 867, 748 | 1.11 | 27.07 | 0.73 | 855, 749 | 1.11 | 26.98 | 0.67 | 854, 748 | 1.20 | 29.36 | 0.67 | 854, 748 | 1.21 | 29.39 | |
| 8 | 0.59 | 774, 744 | 1.19 | 28.94 | 0.60 | 934, 724 | 1.92 | 46.61 | 0.61 | 934, 725 | 1.17 | 28.28 | 0.50 | 960, 727 | 1.33 | 32.25 | 0.50 | 960, 727 | 1.33 | 32.17 | |
| 9 | 0.76 | 851, 753 | 1.24 | 27.58 | 0.74 | 779, 749 | 1.27 | 28.45 | 0.76 | 855, 749 | 1.23 | 27.39 | 0.70 | 779, 749 | 1.37 | 30.65 | 0.70 | 779, 749 | 1.37 | 30.69 | |
| 10 | 0.60 | 976, 733 | 1.41 | 28.24 | 0.53 | 1490, 708 | 1.53 | 30.46 | 0.60 | 961, 729 | 1.42 | 28.39 | 0.56 | 1490, 708 | 1.49 | 29.67 | 0.57 | 1490, 708 | 1.48 | 29.61 | |
| 11 | 0.66 | 849, 746 | 1.40 | 29.76 | 0.64 | 897, 741 | 1.44 | 30.59 | 0.65 | 896, 742 | 1.42 | 30.15 | 0.57 | 930, 740 | 1.59 | 33.79 | 0.57 | 930, 740 | 1.59 | 33.78 | |
| Japonica rice (V1+V3) | 1 | 0.70 | 786, 758 | 0.77 | 25.41 | 0.77 | 801, 758 | 0.94 | 31.11 | 0.77 | 801, 758 | 0.67 | 22.26 | 0.80 | 802, 757 | 0.62 | 20.64 | 0.80 | 802, 757 | 0.62 | 20.65 |
| 2 | 0.49 | 507, 476 | 0.90 | 26.15 | 0.58 | 776, 746 | 0.82 | 23.72 | 0.57 | 775, 745 | 0.83 | 24.00 | 0.63 | 777, 747 | 0.77 | 22.23 | 0.63 | 777, 747 | 0.77 | 22.23 | |
| 3 | 0.58 | 786, 753 | 0.88 | 27.47 | 0.67 | 788, 754 | 0.78 | 24.34 | 0.66 | 787, 754 | 0.79 | 24.61 | 0.70 | 789, 755 | 0.74 | 22.99 | 0.70 | 789, 755 | 0.74 | 22.99 | |
| 5 | 0.57 | 929, 741 | 0.69 | 20.33 | 0.63 | 865, 748 | 0.67 | 19.70 | 0.63 | 897, 745 | 0.66 | 19.55 | 0.54 | 822, 752 | 0.77 | 22.75 | 0.54 | 822, 752 | 0.77 | 22.74 | |
| 6 | 0.63 | 933, 737 | 0.74 | 21.89 | 0.65 | 931, 739 | 0.69 | 20.31 | 0.66 | 931, 739 | 0.69 | 20.23 | 0.51 | 930, 740 | 0.80 | 23.49 | 0.51 | 930, 740 | 0.79 | 23.45 | |
| 7 | 0.66 | 796, 758 | 0.78 | 25.39 | 0.73 | 802, 757 | 0.70 | 22.68 | 0.73 | 802, 757 | 0.71 | 22.83 | 0.75 | 810, 757 | 0.68 | 22.13 | 0.75 | 810, 757 | 0.68 | 22.14 | |
| 8 | 0.46 | 776, 746 | 0.91 | 26.44 | 0.55 | 777, 747 | 0.83 | 24.19 | 0.54 | 777, 747 | 0.84 | 24.30 | 0.56 | 788, 747 | 0.82 | 23.87 | 0.56 | 788, 748 | 0.82 | 23.88 | |
| 9 | 0.74 | 786, 755 | 0.82 | 25.17 | 0.78 | 789, 755 | 0.75 | 22.83 | 0.78 | 789, 754 | 0.75 | 23.03 | 0.79 | 789, 755 | 0.74 | 22.53 | 0.79 | 789, 755 | 0.74 | 22.54 | |
| 10 | 0.49 | 510, 480 | 1.04 | 25.50 | 0.54 | 934, 733 | 0.99 | 24.24 | 0.54 | 934, 732 | 0.99 | 24.30 | 0.60 | 934, 731 | 0.92 | 22.52 | 0.60 | 934, 731 | 0.92 | 22.50 | |
| 11 | 0.61 | 787, 756 | 1.00 | 27.50 | 0.69 | 788, 756 | 0.89 | 24.45 | 0.69 | 788, 756 | 0.90 | 24.75 | 0.72 | 823, 749 | 0.85 | 23.39 | 0.72 | 823, 749 | 0.85 | 23.38 | |
| Indica rice (V2+V4) | 1 | 0.74 | 813, 763 | 1.19 | 22.65 | 0.79 | 851, 755 | 1.07 | 20.34 | 0.78 | 851, 756 | 1.09 | 20.85 | 0.80 | 849, 753 | 1.03 | 19.64 | 0.80 | 849, 753 | 1.03 | 19.64 |
| 2 | 0.62 | 1575, 719 | 1.37 | 27.79 | 0.67 | 955, 727 | 1.28 | 25.96 | 0.66 | 955, 727 | 1.28 | 26.10 | 0.66 | 965, 728 | 1.29 | 26.11 | 0.67 | 965, 728 | 1.28 | 26.06 | |
| 3 | 0.60 | 786, 756 | 1.44 | 28.25 | 0.67 | 785, 755 | 1.31 | 25.64 | 0.66 | 785, 755 | 1.32 | 25.90 | 0.67 | 786, 753 | 1.32 | 25.83 | 0.67 | 784, 754 | 1.32 | 25.81 | |
| 5 | 0.71 | 1207, 725 | 0.86 | 19.25 | 0.73 | 1157, 724 | 0.84 | 18.83 | 0.74 | 1157, 724 | 0.83 | 18.62 | 0.73 | 1342, 720 | 0.93 | 20.90 | 0.72 | 1341, 720 | 0.94 | 20.98 | |
| 6 | 0.77 | 773, 743 | 1.07 | 23.90 | 0.78 | 996, 733 | 0.93 | 20.83 | 0.78 | 996, 732 | 0.91 | 20.37 | 0.66 | 1211, 732 | 1.05 | 23.48 | 0.65 | 1211, 732 | 1.04 | 23.45 | |
| 7 | 0.67 | 795, 758 | 1.30 | 25.48 | 0.74 | 851, 749 | 1.14 | 22.43 | 0.73 | 794, 758 | 1.16 | 22.81 | 0.75 | 851, 748 | 1.13 | 22.12 | 0.75 | 851, 748 | 1.13 | 22.13 | |
| 8 | 0.63 | 776, 746 | 1.31 | 27.12 | 0.68 | 955, 728 | 1.20 | 24.95 | 0.68 | 955, 728 | 1.20 | 25.02 | 0.66 | 963, 729 | 1.24 | 25.70 | 0.67 | 966, 729 | 1.23 | 25.63 | |
| 9 | 0.78 | 813, 763 | 1.19 | 21.16 | 0.79 | 796, 766 | 1.23 | 21.62 | 0.79 | 796, 766 | 1.23 | 21.61 | 0.79 | 795, 758 | 1.22 | 21.54 | 0.79 | 795, 758 | 1.22 | 21.55 | |
| 10 | 0.63 | 991, 733 | 1.53 | 25.75 | 0.65 | 992, 731 | 1.48 | 24.94 | 0.66 | 992, 731 | 1.47 | 24.83 | 0.58 | 1193, 727 | 1.64 | 27.59 | 0.63 | 1193, 727 | 1.61 | 27.23 | |
| 11 | 0.64 | 795, 758 | 1.55 | 26.84 | 0.67 | 848, 749 | 1.50 | 25.91 | 0.67 | 847, 749 | 1.50 | 25.89 | 0.64 | 848, 749 | 1.55 | 26.80 | 0.64 | 848, 749 | 1.55 | 26.78 | |
| Vegetation Index | Bands (nm) | R2 | RMSE | rRMSE (%) | Equation |
|---|---|---|---|---|---|
| PRDVI | 800, 720 | 0.77 | 1.01 | 19.91 | y = 0.7247e5.0572x |
| DI | 800, 720 | 0.70 | 1.32 | 25.87 | y = 1.2232e6.9031x |
| SR | 800, 680 | 0.21 | 1.97 | 43.52 | y = 2.8269e0.0087x |
| NDVI | 800, 680 | 0.32 | 1.99 | 39.12 | y = 0.0577e4.6542x |
| GNDVI | 800, 550 | 0.44 | 1.81 | 35.50 | y = 0.1675e4.1117x |
| RDVI | 800, 680 | 0.53 | 1.65 | 32.42 | y = 0.4082e4.7314x |
| MSR | 800, 680 | 0.38 | 1.84 | 37.30 | y = 0.3966x + 2.249 |
| Green WDRVI | 800, 550 | 0.47 | 1.80 | 35.29 | y = 1.1515e1.6901x |
| Red-Edge WDRVI | 800, 720 | 0.73 | 1.38 | 27.07 | y = 1.1867e4.8563x |
| SAVI | 800, 680 | 0.54 | 1.65 | 32.40 | y = 0.5715e3.9851x |
| OSAVI | 800, 680 | 0.51 | 1.66 | 32.54 | y = 0.2662e4.0489x |
| TVI | 720, 680, 550 | 0.00 | 2.38 | 46.74 | y = 0.1088x + 5.7682 |
| MTVI2 | 800, 680, 550 | 0.51 | 1.62 | 32.97 | y = 8.7745x − 0.8143 |
| EVI | 800, 680, 490 | 0.57 | 1.59 | 31.32 | y = 0.6818e3.4347x |
| CIred edge | 800, 720 | 0.72 | 1.26 | 24.77 | y = 2.6279x − 0.1794 |
| CIgreen | 800, 550 | 0.43 | 1.80 | 35.30 | y = 0.3486x + 1.7115 |
| NDRE | 800, 720 | 0.73 | 1.27 | 24.92 | y = 0.5498e4.3685x |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhang, N.; Su, X.; Lu, J.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Estimating Leaf Area Index with a New Vegetation Index Considering the Influence of Rice Panicles. Remote Sens. 2019, 11, 1809. https://doi.org/10.3390/rs11151809
He J, Zhang N, Su X, Lu J, Yao X, Cheng T, Zhu Y, Cao W, Tian Y. Estimating Leaf Area Index with a New Vegetation Index Considering the Influence of Rice Panicles. Remote Sensing. 2019; 11(15):1809. https://doi.org/10.3390/rs11151809
Chicago/Turabian StyleHe, Jiaoyang, Ni Zhang, Xi Su, Jingshan Lu, Xia Yao, Tao Cheng, Yan Zhu, Weixing Cao, and Yongchao Tian. 2019. "Estimating Leaf Area Index with a New Vegetation Index Considering the Influence of Rice Panicles" Remote Sensing 11, no. 15: 1809. https://doi.org/10.3390/rs11151809
APA StyleHe, J., Zhang, N., Su, X., Lu, J., Yao, X., Cheng, T., Zhu, Y., Cao, W., & Tian, Y. (2019). Estimating Leaf Area Index with a New Vegetation Index Considering the Influence of Rice Panicles. Remote Sensing, 11(15), 1809. https://doi.org/10.3390/rs11151809







