Assessment of Relationships between Earthworms and Soil Abiotic and Biotic Factors as a Tool in Sustainable Agricultural
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
3. Results and Discussion
3.1. Earthworm Biomass, Density and Diversity in Differently Managed Agroecosystems
3.2. Earthworms in Relation to Soil Type and Soil Chemical Properties
3.3. Earthworms in Relation to Climate Related and Physical Soil Parameters
3.4. Earthworms in Relation to Arthropods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Jouquet, P.; Dauber, J.; Lagerlof, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Suthar, S. Earthworm communities as bio-indicator of arable land management practices: A case study in semiarid region of India. Ecol. Indic. 2009, 9, 588–594. [Google Scholar] [CrossRef]
- Bertrand, M.; Blouin, M.; Barot, S.; Charlier, A.; Marhcnad, D.; Roger-Estrade, J. Biocontrol of eyespot disease on two winter wheat cultivars by an anecic earthworm (Lumbricus terrestris). Appl. Soil Ecol. 2015, 96, 33–41. [Google Scholar] [CrossRef]
- Burke, J.L.; Maerz, J.C.; Milanovich, J.R.; Melany, C.F.; Gandhi, K.J.K. Invasion by exotic earthworms alters biodiversity and communities of litter- and soil-dwelling oribatid mites. Diversity 2011, 3, 155–175. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Curry, J.P. Factors affecting the abundance of earthworms in soil. In Earthworm Ecology; Edwards, C.A., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; pp. 91–114. [Google Scholar]
- Christensen, O.; Mather, J.G. Dynamics of lumbricid earthworm cocoons in relation to habitat conditions at three different arable sites. Pedobiologia 1990, 34, 227–238. [Google Scholar]
- Kernecker, M.; Whalen, J.K.; Bradley, R.L. Agricultural management and flooding shape habits for non-native earthworms in southern Quebec, Canada. Appl. Soil Ecol. 2015, 96, 240–250. [Google Scholar] [CrossRef]
- Sheu, S. Effects of earthworms on plant growth patterns and perspecitves. Pedobiologia 2003, 47, 846–856. [Google Scholar] [CrossRef]
- Cortez, J. Field decomposition of leaf litters: Relationships between decomposition rates and soil moisture, soil temperature and earthworm activity. Soil Biol. Biochem. 1998, 30, 783–793. [Google Scholar] [CrossRef]
- Goswami, R. Determination of ecological diversity indices to assess the interrelationship between earthworm diversity and different habitats of Indian Botanic garden, Howrah, India. Biol. Forum Int. J. 2015, 7, 128–136. [Google Scholar]
- Jones, H.D.; Santoro, G.; Boag, B.; Neilson, R. The diversity of earthworms in 200 Scottish fields and the possible effect of New Zeland land flatworms (Arthurdendyus triangulates) on earthworm populations. Ann. Appl. Biol. 2001, 139, 75–92. [Google Scholar] [CrossRef]
- Ranson, T.S.; Billa, B.J. Differences in soil characteristics between field and forest may influence the distribution of an invasive earthworm. Invertebr. Biol. 2015, 134, 78–87. [Google Scholar] [CrossRef]
- Ravenek, J. C and N Mineralization and Earthworm Populations in a Norway Spruce Forest at Hasslov (SW Sweden), 25 Years after Liming; SLU, Department of Ecology: Uppsala, Sweden, 2009. [Google Scholar]
- Wever, L.A.; Timothy, J.L.; Clapperton, M.J. The influence of soil moisture and temperature on the survival, aestivation, growth and development of juvenile Aporrectodea tuberculate (eisen) (Lumbiricidae). Pedobiologia 2001, 45, 121–133. [Google Scholar] [CrossRef]
- Lavelle, P.; Barois, I.; Martin, A.; Zaidi, Z.; Schaefer, P. Management of earthworm populations in agro-ecosystems. A possible way to maintain soil quality? In Ecology of Arable Land—Perspectives and Challenges; Clarholm, M., Bergström, L., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 109–122. [Google Scholar]
- Chan, K.Y. An overview of some tillage impacts on earthworm population abundance and diversity—Implications for functioning in soils. Soil Tillage Res. 2001, 57, 179–191. [Google Scholar] [CrossRef]
- Paoletti, M.G. The role of earthworms for assessment of sustainability and as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 137–155. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. Lond. B 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Mele, P.M.; Carter, M.R. Species abundance of earthworms in arable and pasture soils in south-eastern Australia. Appl. Soil. Ecol. 1999, 12, 129–137. [Google Scholar] [CrossRef]
- Manono, B. Agro-ecological role of earthworms (Oligochaetes) in sustainable agriculture and nutrient use efficiency: A review. J. Agric. Ecol. Res. Int. 2016, 8, 1–18. [Google Scholar] [CrossRef]
- MESR. National Biodiversity Strategy of Slovakia; MESR: Bratislava, Slovakia, 1998. [Google Scholar]
- Nikitin, V.; Fishman, V. On the improvement of methods for determination of soil carbon. Chem. Agric. 1969, 3, 76–77. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Zajonc, I. Earthworms (Oligochaeta; Lumbricidae) of Slovakia. In Biological Studies; VEDA: Bratislava, Slovakia, 1981. [Google Scholar]
- Bhadauria, T.; Saxena, K.G. Role of earthworms in Soil fertility Maintenance through the production of Biogenic Structures. Appl. Environ. Soil Sci. 2010. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Wurst, S.; De Deyn, G.B.; Orwin, K. Soil biodiversity and functions. In Soil Ecology and Ecosystem Services; Wall, D.H., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 28–44. [Google Scholar]
- Kanianska, R.; Jaďuďuová, J.; Kizeková, M.; Makovníková, J. Earthworms as bioindicator of land use in agroecosystems. Ecol. Saf. 2015, 9, 34–41. [Google Scholar]
- Fromm, H.; Winter, K.; Filser, J.; Hantshcel, R.; Beese, F. The influence of soil type and cultivation system on the spatial distribution of the soil fauna and microorganisms and their interactions. Geoderma 1993, 60, 109–118. [Google Scholar] [CrossRef]
- Lamandé, M.; Hallaire, V.; Curmi, P.; Pérés, G.; Cluzeau, D. Changes of pore morphology, infiltration and earthworm community in a loamy soil under different agricultural managements. Catena 2003, 54, 637–649. [Google Scholar] [CrossRef]
- Marhan, S.; Scheu, S. The influence of mineral and organic fertilisers on the growth of the endogeic earthworm Octolasion tyrtaeum (Savigny). Pedobiologia 2005, 49, 239–249. [Google Scholar] [CrossRef]
- De Goede, R.G.M.; Brussaard, L.; Akkermans, A.D.L. On-farm impact of cattle slurry manure management on biological soil quality. NJAS Wagening. J. Life Sci. 2003, 51, 103–133. [Google Scholar] [CrossRef]
- Murchie, A.K.; Blackshaw, R.P.; Gordon, A.W.; Christie, P. Responses of earthworm species to long-term applications of slurry. Appl. Soil Ecol. 2015, 96, 60–67. [Google Scholar] [CrossRef]
- Pommeresche, R.; Loes, A.K. Relations between Agronomic Practice and earthworms in Norwegian Arable Soils. Dyn. Soil Dyn. Plant 2009, 3, 129–142. [Google Scholar]
- Crittenden, S.J.; Eswaramurthy, T.; De Goede, R.G.M.; Brussaard, L.; Pulleman, M.M. Effect of tillage on earthworms over short- and medium-term in conventional and organic farming. Appl. Soil Ecol. 2014, 83, 140–148. [Google Scholar] [CrossRef]
- Hansen, S.; Engelstad, F. Earthworm populations in a cool and wet district as affected by tractor traffic and fertilisation. Appl. Soil Ecol. 1999, 13, 237–250. [Google Scholar] [CrossRef]
- Zajonc, I. Earthworms (Lumbicidae) in Meadows of Carpathian Region in Czechoslovakia; VEDA: Bratislava, Slovakia, 1970. [Google Scholar]
- Dinter, A.; Oberwalder, C.H.; Kabouw, P.; Coulson, M.; Ernst, G.; Leicher, T.; Miles, M.; Weyman, G.; Klein, O. Occurrence and distribution of earthworms in agricultural landscapes across Europe with regard to testing for responses to plant protection products. J. Soils Sediments 2013, 13, 278–293. [Google Scholar] [CrossRef]
- Sheppard, D. Earthworms in England: Distribution, Abundance and Habitats; Natural England Commissioned Report NECR 145; Natural England: Worcester, UK, 2014.
- Ivask, M.; Kuu, A.; Sizov, E. Abundance of earthworm species in Estonian arable soils. Eur. J. Soil Biol. 2007, 43, 39–42. [Google Scholar] [CrossRef]
- Zeithaml, J.; Pižl, V.; Sklenička, P. Earthworm assemblages in an ecotone between forest and arable field and their relations with soil properties. Pesq. Agropec. Bras. Brasília 2009, 44, 922–926. [Google Scholar]
- Whalen, J.K. Spatial and temporal distribution of earthworm patches in corn field and forest systems of southwestern Quebec, Canada. Appl. Soil Ecol. 2004, 27, 143–151. [Google Scholar] [CrossRef]
- Haynes, R.J.; Dominy, C.S.; Graha, M.H. Effect of agricultural land use on soil organic matter status and the composition of earthworm communities in KwaZulu-Natal, South Africa. Agric. Ecosyst. Environ. 2002, 95, 453–464. [Google Scholar] [CrossRef]
- Edwards, C.A. The importance of earthworms as key representatives of the soil fauna. In Earthworm Ecology; Edwards, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Laossi, K.R.; Decens, T.; Jouquet, P.; Barot, S. Can we predict how earthworm effects on plant growth vary with soil properties? Appl. Environ. Soil Sci. 2010. [Google Scholar] [CrossRef]
- Butt, K.R.; Lowe, C.H.N. Presence of earthworm species within and beneath Lumbricus terrestris (L.) middens. Eur. J. Soil Biol. 2007, 43, S57–S60. [Google Scholar] [CrossRef]
- Sims, R.; Gerard, B. Earthworms: Notes for the identification of British species. In Synopses of the British Fauna 31; Barnes, R.S.K., Crothers, J., Eds.; Field Studies Council: Shrewsbury, UK, 1999. [Google Scholar]
- Zhu, X.; Lian, B.; Yang, X.; Liu, C.; Zhu, L. Biotransformation of earthworm activity on potassium-bearing mineral powder. J. Earth Sci. 2013, 24, 65–74. [Google Scholar] [CrossRef]
- Antunes, P.M.; Franken, P.; Schwarz, D.; Rillig, M.C.; Cosme, M.; Scott, M.; Hart, M.M. Linking soil diversity and human health: Do arbuscular mycorrhizal fungi contribute to food nutrition? In Soil Ecology and Ecosystem Services; Wall, D.H., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 173–232. [Google Scholar]
- Ložek, V. Nature in the Quaternary; Academia: Praha, Czech, 1973. [Google Scholar]
- Hendrix, P.F.; Callaham, M.A.; Drake, J.M.; Huang, C.; James, S.W.; Snyder, B.A.; Zhang, W. Pandor’s box contained bait: The global problem of introduced earthworms. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 593–613. [Google Scholar] [CrossRef]
- Frelich, L.E.; Hale, C.M.; Scheu, S.; Holdsworth, A.R.; Heneghan, L.; Bohlen, P.J.; Reich, P.B. Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol. Invasions 2006, 8, 1235–1245. [Google Scholar] [CrossRef]
- Wall, D.H.; Bardgett, R.D.; Behan-Pelletier, V.; Jeffrey, E.; Herrick, T.; Jones, H.; Ritz, K.; Six, J.; Strong, D.R.; van der Putten, W.H. Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Boudiaf, N.K.M.; Metheri, M.S.; Mouas, B.S.; Derridj, A. Climate impact on the abundance of soil macroinvertebrates in Algerian olive orchards. Agric. Food 2015, 3, 320–324. [Google Scholar]
- Doube, B.M.; Styan, C. The response of Aporrectodea rosea and Aporrecctodea trapezoids (Oligocheta: Lumbriocidae) to moisture gradients in three soil types in the laboratory. Biol. Fertil. Soils 1996, 23, 166–172. [Google Scholar] [CrossRef]
- Pizl, V. Effect of soil compaction on earthworms (Lumbricidae) in apple orchard soil. Soil Biol. Biochem. 1992, 24, 1573–1575. [Google Scholar] [CrossRef]
- Söchtig, W.; Larink, O. Effects of soil compaction on activity and biomass of endogeic lumbricides in arable soils. Soil Biol. Biochem. 1992, 24, 1595–1599. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles in sustainable ariculture: A review on pest control efficacy, cultivation impacts and enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, C. The effect of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Bátary, P.; Báldi, A.; Kleijn, D.; Tscharntke, T. Landscape-moderated biodiversity effects of agri-environmental management: A meta-analysis. Proc. Biol. Sci. 2011, 278, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.J.; Maron, M.; House, A.P.N.; Zammit, C. Do arthropod assemblages display globally consistent responses to intensified agricultural land use and management? Glob. Ecol. Biogeogr. 2008, 17, 585–599. [Google Scholar] [CrossRef]
- Rodríguez, E.; Fernández-Anero, F.J.; Ruiz, P.; Campos, M. Soil arthropod abundance under conventional and no tillage in a Mediterranean climate. Soil Tillage Res. 2006, 85, 229–233. [Google Scholar] [CrossRef]
- Schon, N.L.; Mackay, A.D.; Hedley, M.J.; Minor, M.A. The soil invertebrate contribution to nitrogen mineralization differs between soils under organic and conventional dairy management. Biol. Fertil. Soils 2012, 48, 31–42. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Glen, D.M.; Erickson, M.L.; Liddell, J.E.; Langdon, C.J. Do earthworms help to sustain the slug predator Pterostichus melanarius (Coleoptera: Carabidae) within crops? Investigations using monoclonal antibodies. Mol. Ecol. 2000, 9, 1279–1292. [Google Scholar] [CrossRef]
- King, R.A.; Vaughan, I.P.; Bell, J.R.; Bohan, D.A. Prey choice by carabid beetles feeding on an earthworm community analysed using species- and lineage-specific PCR primers. Mol. Ecol. 2010, 19, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G. How do Earthworms Affect Microfloral and Faunal Community Diversity? In The Significance and Regulation of Soil Biodiversity; Collins, H.P., Robertson, G.P., Klug, M.J., Eds.; Springer Science and Business Media: Dordrecht, The Netherlands, 1995; pp. 245–269. [Google Scholar]
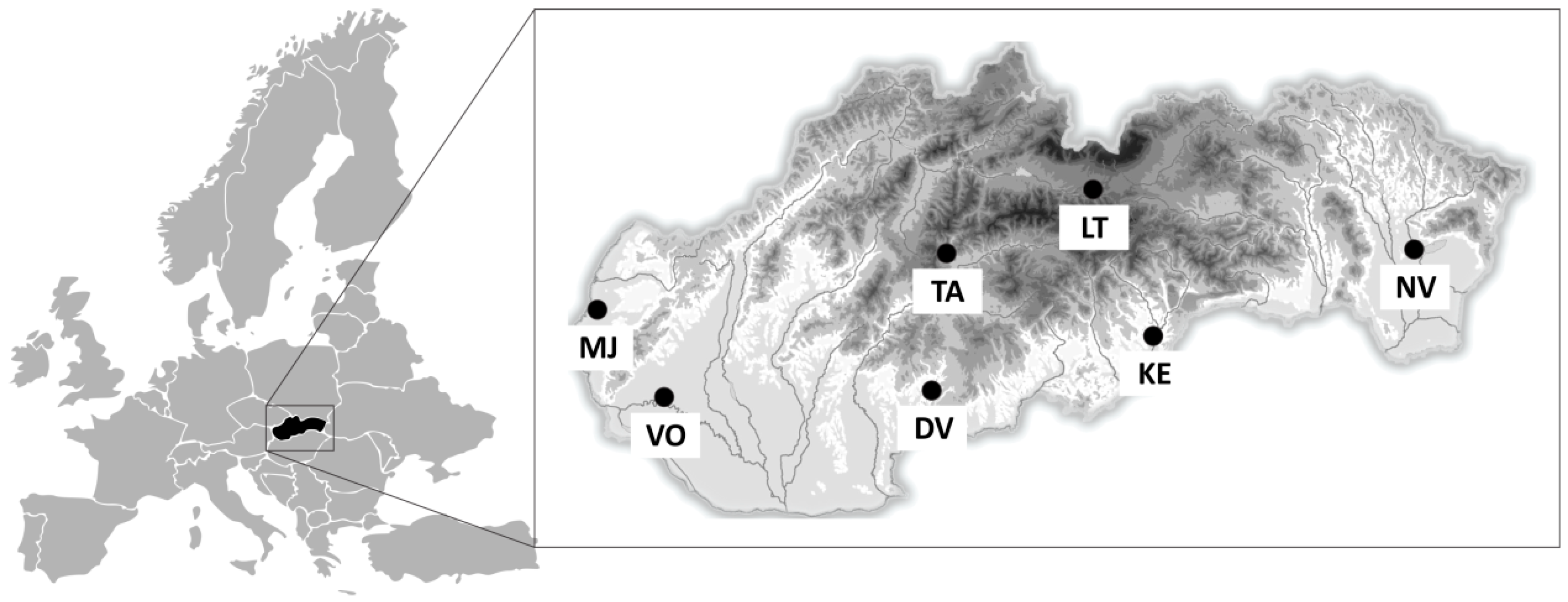
| Study Site | Geographical Location | Soil Type | Soil Texture | Altitude (m) | Land Management |
|---|---|---|---|---|---|
| NV | Eastern Slovak Upland | Humic Regosol (Fluvisol) | Clayey | AL-121 PG-123 | AL-intensive farming PG-cattle pasture |
| VO | Danubian Upland | Black Chernozem (Chernozem) | Loamy | AL-137 | AL-intensive farming |
| DV | Krupina Plain | Humic Regosol (Fluvisol) | Sandy-loam | AL-157 PG-155 | AL-intensive farming PG-alluvial meadow |
| MJ | Borská Lowland | AL-Regosol (Regosol) PG-Humic Regosol (Fluvisol) | Sandy Sandy | AL-157 PG-160 | AL-intensive farming PG-meadow |
| KE | Slovak Karst | Eutric Cambisol (Cambisol) | Loamy | AL-360 PG-344 | AL-extensive farming PG-cattle pasture |
| TA | Kremnica Mountain | Dystric Cambisol (Cambisol) | Loamy | AL-595 PG-597 | AL-extensive farming PG-sheep pasture |
| LT | Low Tatras | Regosol (Rendzina) | Loamy | AL-950 PG-931 | AL-organic farming PG-meadow |
| Study Site | Meteorological Station | Long-Term Average Air Temperature (°C) | Two Months Average Air Temperature before Sampling (°C) | Long-Term Average Rainfall (mm) | Two Months Rainfall before Sampling (mm) |
|---|---|---|---|---|---|
| NV | Michalovce | 8.9 | 4.3 | 559 | 28 |
| VO | Kráľová pri Senci | 9.5 | 4.1 | 560 | 57 |
| DV | Dudince | 8.7 | 3.5 | 606 | 67 |
| MJ | Moravský Ján | 9.2 | 4.1 | 525 | 53 |
| KE | Rožňava | 8.6 | 3.4 | 620 | 33 |
| TA | Banská Bystrica | 8.1 | 7.2 | 795 | 106 |
| LT | Poprad | 6.2 | 4.7 | 950 | 41 |
| Study Site | AL | PG | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Median | Min | Max | Mean ± SD | Median | |
| NV | 0.0 | 101.6 | 40.8 ± 31.10 | 32.6 | 64.8 | 235.6 | 134.5 ± 57.49 | 132.7 |
| VO | 0.0 | 93.3 | 28.2 ± 30.26 | 23.2 | - | - | - | - |
| DV | 13.3 | 143.5 | 66.4 ± 49.76 | 46.8 | 84.9 | 194.0 | 122.0 ± 39.11 | 129.4 |
| MJ | 0.0 | 0.0 | 0.0 | 0.0 | 15.2 | 171.0 | 84.4 ± 64.54 | 78.0 |
| KE | 42.7 | 90.4 | 63.3 ± 17.82 | 58.3 | 7.3 | 188.5 | 67.8 ± 63.42 | 51.4 |
| TA | 21.6 | 39.5 | 27.4 ± 6.25 | 25.1 | 19.2 | 69.1 | 40.8 ± 19.00 | 37.5 |
| LT | 0.0 | 21.39 | 7.23 ± 8.35 | 2.12 | 8.7 | 49.8 | 28.2 ± 16.35 | 28.8 |
| Study Site | AL | PG | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Median | Min | Max | Mean ± SD | Median | |
| NV | 0.0 | 57.1 | 29.2 ± 17.54 | 24.5 | 114.3 | 261.2 | 197.1 ± 53.20 | 220.4 |
| VO | 0.0 | 122.4 | 45.5 ± 38.25 | 40.8 | - | - | - | - |
| DV | 32.7 | 114.3 | 53.6 ± 27.43 | 49.0 | 228.6 | 383.7 | 277.6 ± 54.15 | 253.1 |
| MJ | 0.0 | 0.0 | 0.0 | 0.0 | 16.3 | 179.6 | 100.3 ± 59.37 | 98.0 |
| KE | 146.9 | 351.0 | 226.2 ± 69.54 | 195.9 | 32.7 | 146.9 | 84.0 ± 48.23 | 65.3 |
| TA | 32.7 | 81.6 | 56.0 ± 21.82 | 40.8 | 40.8 | 179.6 | 108.5 ± 49.37 | 89.8 |
| LT | 0.0 | 57.14 | 20.99 ± 18.77 | 24.49 | 16.3 | 163.3 | 74.6 ± 50.86 | 65.3 |
| NV | VO | DV | MJ | KE | TA | LT | Total | |
|---|---|---|---|---|---|---|---|---|
| Aporrectodea caliginosa | 7.00 | 5.83 | 1.17 | 0.00 | 11.70 | 0.00 | 2.30 | 27.99 |
| Aporrectodea rosea | 3.50 | 1.17 | 1.17 | 0.00 | 0.00 | 0.00 | 2.33 | 8.16 |
| Eiseniella tetraedra | 0.00 | 1.17 | 0.00 | 0.00 | 1.17 | 0.00 | 0.00 | 2.33 |
| Octolasion cyaneum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 16.32 | 0.00 | 16.32 |
| Octolasion lacteum | 0.00 | 0.00 | 0.00 | 0.00 | 1.17 | 0.00 | 1.17 | 2.34 |
| Sum of mature endogeic species | 10.50 | 8.17 | 2.34 | 0.00 | 14.04 | 16.32 | 5.80 | 57.17 |
| % of mature endogeic species | 64.30 | 22.26 | 70.01 | 0.00 | 66.86 | 77.79 | 99.49 | 66.23 |
| Dendrobaena octaedra | 0.00 | 1.17 | 0.00 | 0.00 | 7.00 | 0.00 | 0.00 | 8.17 |
| Dendrodrilus rubidus | 0.00 | 0.00 | 0.00 | 0.00 | 00.00 | 4.66 | 0.00 | 4.66 |
| Lumbricus rubellus | 0.00 | 2.33 | 1.17 | 0.00 | 0.00 | 0.00 | 0.00 | 3.50 |
| Sum of mature epigeic species | 0.00 | 3.50 | 1.17 | 0.00 | 7.00 | 4.66 | 0.00 | 16.33 |
| % of mature epigeic species | 0.00 | 29.99 | 11.13 | 0.00 | 33.33 | 22.21 | 0.00 | 18.92 |
| Lumbricus terrestris | 5.83 | 0.00 | 7.00 | 0.00 | 0.00 | 0.00 | 0.00 | 12.83 |
| Sum of mature anecic species | 5.83 | 0.00 | 7.00 | 0.00 | 0.00 | 0.00 | 0.00 | 12.83 |
| % of mature anecic species | 35.70 | 0.00 | 66.60 | 0.00 | 0.00 | 0.00 | 0.00 | 14.86 |
| Sum of mature species | 16.33 | 11.67 | 10.51 | 0.00 | 21.00 | 20.98 | 5.83 | 86.30 |
| Sum of juveniles | 12.83 | 33.82 | 43.15 | 0.00 | 205.25 | 34.99 | 15.16 | 345.20 |
| Total sum | 29.16 | 45.49 | 53.65 | 0.00 | 226.25 | 55.97 | 20.99 | 431.50 |
| H′ | 1.50 | 1.90 | 1.36 | 0.00 | 1.41 | 0.79 | 1.52 | 2,72 |
| NV | DV | MJ | KE | TA | LT | Total | |
|---|---|---|---|---|---|---|---|
| Allolobophora chlorotica | 3.50 | 1.17 | 0.00 | 0.00 | 0.00 | 0.00 | 4.66 |
| Aporrectodea caliginosa | 39.65 | 30.32 | 48.98 | 13.99 | 23.32 | 3.50 | 159.77 |
| Aporectodea rosea | 5.83 | 7.00 | 0.00 | 0.00 | 3.50 | 8.16 | 24.49 |
| Eiseniella tetraedra | 1.17 | 0.00 | 1.17 | 0.00 | 0.00 | 0.00 | 2.34 |
| Octolasion cyaneum | 0.00 | 0.00 | 0.00 | 0.00 | 1.17 | 4.66 | 5.83 |
| Octolasion lacteum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.33 | 2.33 |
| Sum of mature endogeic species | 50.15 | 38.49 | 50.15 | 13.99 | 27.99 | 18.65 | 199.42 |
| % of mature endogeic species | 82.69 | 80.49 | 87.74 | 70.59 | 100.00 | 99.95 | 85.92 |
| Dendrobaena octaedra | 1.17 | 1.17 | 1.17 | 1.17 | 0.00 | 0.00 | 4.67 |
| Lumbricus castaneus | 0.00 | 0.00 | 2.33 | 0.00 | 0.00 | 0.00 | 2.33 |
| Lumbricus rubellus | 0.00 | 2.33 | 1.17 | 0.00 | 0.00 | 0.00 | 3.50 |
| Sum of mature epigeic species | 1.17 | 3.50 | 4.67 | 1.17 | 0.00 | 0.00 | 10.51 |
| % of mature epigeic species | 1.93 | 7.32 | 8.17 | 5.90 | 0.00 | 0.00 | 4.53 |
| Aporrectodea longa | 0.00 | 0.00 | 1.17 | 0.00 | 0.00 | 0.00 | 1.17 |
| Lumbricus terrestris | 9.33 | 5.83 | 1.17 | 4.66 | 0.00 | 0.00 | 20.99 |
| Sum of mature anecic species | 9.33 | 5.83 | 1.17 | 4.66 | 0.00 | 0.00 | 20.99 |
| % of mature anecic species | 15.38 | 12.19 | 4.09 | 23.51 | 0.00 | 0.00 | 9.55 |
| Sum of mature species | 60.65 | 47.82 | 57.16 | 19.82 | 27.99 | 18.66 | 232.08 |
| Sum of juveniles | 136.44 | 228.57 | 43.15 | 64.14 | 80.47 | 55.98 | 608.75 |
| Total sum | 197.09 | 276.39 | 100.31 | 83.96 | 108.45 | 74.64 | 840.83 |
| H′ | 1.04 | 1.24 | 0.85 | 1.08 | 0.80 | 1.85 | 1.42 |
| pH KCl | TOC (g·kg−1) | Nt (g·kg−1) | P (mg·kg−1) | K (mg·kg−1) | Mg (mg·kg−1) | |
|---|---|---|---|---|---|---|
| NV | 5.51 | 16.67 | 2.51 | 10.16 | 245.59 | 672.27 |
| VO | 7.18 | 15.89 | 1.99 | 18.74 | 256.53 | 762.78 |
| DV | 6.04 | 13.54 | 1.61 | 48.35 | 310.84 | 318.49 |
| MJ | 4.58 | 7.43 | 0.96 | 65.77 | 132.16 | 39.32 |
| KE | 5.46 | 13.09 | 1.58 | 4.84 | 221.93 | 184.84 |
| TA | 4.84 | 15.45 | 1.62 | 33.56 | 221.90 | 127.36 |
| LT | 6.70 | 34.00 | 3.05 | 38.23 | 199.36 | 949.12 |
| pH KCl | TOC (g·kg−1) | Nt (g·kg−1) | P (mg·kg−1) | K (mg·kg−1) | Mg (mg·kg−1) | |
|---|---|---|---|---|---|---|
| NV | 7.18 | 23.18 | 2.78 | 14.52 | 293.56 | 721.78 |
| DV | 5.08 | 13.88 | 1.91 | 3.02 | 140.20 | 466.68 |
| MJ | 6.75 | 4.80 | 1.02 | 31.42 | 82.33 | 113.11 |
| KE | 5.89 | 28.20 | 3.13 | 22.83 | 349.30 | 260.85 |
| TA | 4.19 | 42.30 | 4.14 | 0.52 | 136.22 | 631.80 |
| LT | 6.94 | 51.30 | 5.16 | 3.82 | 300.33 | 1233.15 |
| AL | PG | |||||
|---|---|---|---|---|---|---|
| ED | EB | H′ | ED | EB | EH′ | |
| pH | −0.036 | 0.286 | 0.893 ** | −0.257 | 0.257 | −0.200 |
| TOC | −0.214 | −0.071 | 0.750 | −0.543 | −0.771 | −0.029 |
| Nt | −0.214 | −0.071 | 0.750 | −0.543 | −0.771 | −0.029 |
| P | −0.571 | −0.464 | −0.500 | −0.371 | 0.257 | 0.371 |
| K | 0.464 | 0.857 * | 0.393 | −0.429 | −0.200 | −0.429 |
| Mg | −0.179 | 0.143 | 0.929 ** | −0.086 | −0.257 | −0.486 |
| Study Site | Soil Temperature (°C) | Soil Moisture (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| AL | PG | AL | PG | |||||
| 5 cm | 20 cm | 5 cm | 20 cm | 5 cm | 20 cm | 5 cm | 20 cm | |
| NV | 10.4 | 5.2 | 7.7 | 5.2 | 10.4 | 27.0 | 23.0 | 22.9 |
| VO | 9.1 | 6.2 | - | - | 21.1 | 20.6 | - | - |
| DV | 8.9 | 7.9 | 7.9 | 7.7 | 39.0 | 31.9 | 47.6 | 29.9 |
| MJ | 7.4 | 5.6 | 7.9 | 5.2 | 12.9 | 13.3 | 34.9 | 35.3 |
| KE | 6.0 | 4.1 | 7.2 | 5.1 | 22.0 | 19.7 | 35.3 | 18.6 |
| TA | 5.0 | 6.9 | 6.9 | 6.6 | 12.4 | 30.7 | 34.7 | 33.0 |
| LT | 4.6 | 4.3 | 4.3 | 4.4 | 22.7 | - | 40.4 | 16.4 |
| AL | PG | |||
|---|---|---|---|---|
| ED | EB | ED | EB | |
| ST05 | −0.118 | 0.185 | 0.371 * | 0.457 ** |
| ST20 | −0.055 | 0.034 | 0.481 ** | 0.309 * |
| SM05 | 0.309 * | 0.242 | 0.107 | −0.113 |
| SM20 | 0.387 ** | 0.528 ** | 0.250 | 0.225 |
| PR80 | −0.137 | −0.307 * | 0.133 | 0.072 |
| NV | VO | DV | MJ | KE | TA | LT | ||
|---|---|---|---|---|---|---|---|---|
| AL | Arthropods | 15.86 | 29.43 | 20.29 | 40.00 | 45.00 | 39.43 | 32.71 |
| of which Ground beetles | 12.43 | 17.00 | 12.00 | 7.14 | 13.29 | 23.14 | 28.43 | |
| of which Carabids | 11.6 | 3.1 | 0.4 | 1.1 | 3.1 | 21.3 | 24.4 | |
| PG | Arthropods | 36.57 | - | 43.86 | 102.57 | 78.14 | 31.43 | 23.29 |
| of which Ground beetles | 19.57 | - | 9.71 | 34.57 | 20.00 | 8.00 | 10.14 | |
| of which Carabids | 13.9 | - | 0.1 | 17.7 | 8.0 | 2.0 | 4.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanianska, R.; Jaďuďová, J.; Makovníková, J.; Kizeková, M. Assessment of Relationships between Earthworms and Soil Abiotic and Biotic Factors as a Tool in Sustainable Agricultural. Sustainability 2016, 8, 906. https://doi.org/10.3390/su8090906
Kanianska R, Jaďuďová J, Makovníková J, Kizeková M. Assessment of Relationships between Earthworms and Soil Abiotic and Biotic Factors as a Tool in Sustainable Agricultural. Sustainability. 2016; 8(9):906. https://doi.org/10.3390/su8090906
Chicago/Turabian StyleKanianska, Radoslava, Jana Jaďuďová, Jarmila Makovníková, and Miriam Kizeková. 2016. "Assessment of Relationships between Earthworms and Soil Abiotic and Biotic Factors as a Tool in Sustainable Agricultural" Sustainability 8, no. 9: 906. https://doi.org/10.3390/su8090906
APA StyleKanianska, R., Jaďuďová, J., Makovníková, J., & Kizeková, M. (2016). Assessment of Relationships between Earthworms and Soil Abiotic and Biotic Factors as a Tool in Sustainable Agricultural. Sustainability, 8(9), 906. https://doi.org/10.3390/su8090906






