Indication of Importance of Including Soil Microbial Characteristics into Biotope Valuation Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Sites and Sampling
2.2. PLFA Analyses
2.3. Other Analyses
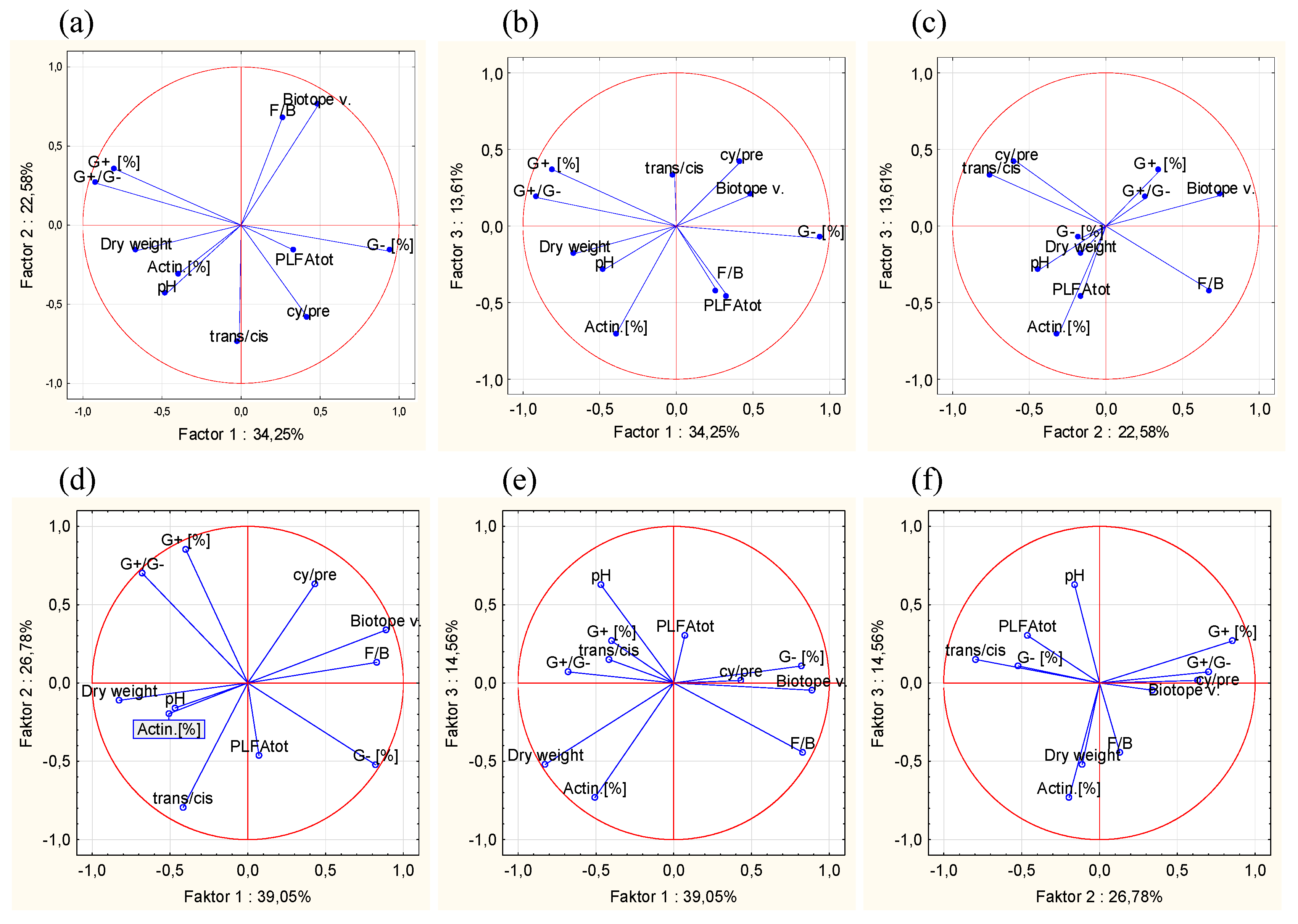
2.4. Calculation and Statistics
3. Results and Discussion
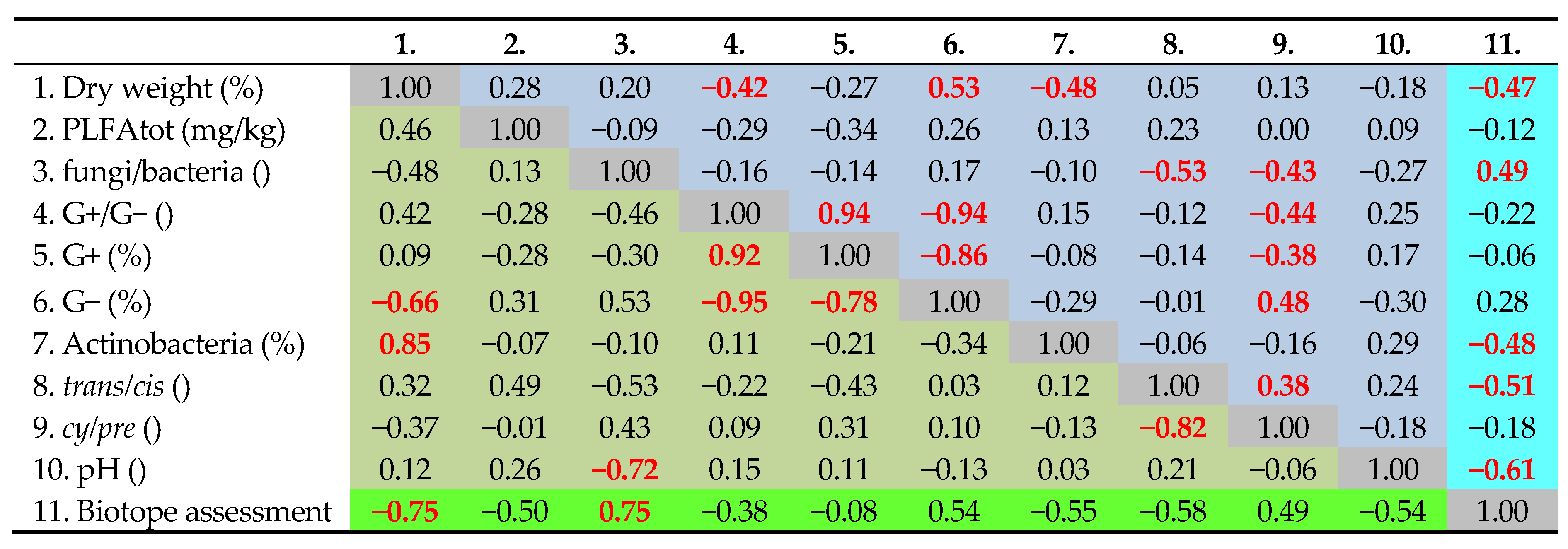
3.1. Soil Indicators in Study Sites and Their Variability
3.2. Relationships of Soil Parameters to Biotope Values
3.3. Implications for Biotope Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Cimmision. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora; European Commision: Brussels, Begium, 1992; Available online http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31992L0043 (accessed on 7 March 2016).
- Sejak, J.; Dejmal, I.; Petricek, V.; Cudlin, P.; Michal, I.; Cerny, K.; Kucera, T.; Vyskot; Strejcek, J.; Cudlinova, E.; et al. Valuation and Pricing the Biotopes of the Czech. Republic; Cesky ekologicky ustav: Prague, Czech Republic, 2003; p. 450. [Google Scholar]
- Pithart, D.; Krovakova, K.; Zaloudik, J.; Dostal, T.; Valentova, J.; Valenta, P.; Weyskrabova, J.; Dusek, J. Ecosystem services of natural floodplain segment - Luznice river, Czech Republic. Flood Recovery Innov. Response Ii 2010, 133, 129–139. [Google Scholar]
- Darbi, M.; Tausch, C. Loss-gain calculations in german impact mitigation regulation. Occasional Paper Contributed to BBOP. Available online: http://www.forest-trends.org/documents/files/doc_2404.pdf (accessed on 7 March 2016).
- European Commission. White Paper on Environmental Liability; European Commission: Luxembourg, Luxembourg, 2000; p. 52. [Google Scholar]
- Sejak, J.; Dejmal, I.; Petricek, V.; Cudlin, P.; Michal, I.; Cerny, K.; Kucera, T.; Vyskot, I.; Strejcek, J.; Cudlinova, E.; et al. Method of monetary valuation of territorial ecological functions. Available online: http://fzp.ujep.cz/projekty/bvm/bvm.pdf (accessed on 7 March 2016).
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. Z. Fur Pflanzenernahrung Und Bodenkunde 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Xue, D.; Yao, H.Y.; Ge, D.Y.; Huang, C.Y. Soil microbial community structure in diverse land use systems: A Comparative Study Using Biolog, DGGE, and PLFA Analyses. Pedosphere 2008, 18, 653–663. [Google Scholar] [CrossRef]
- Frostegard, A.; Tunlid, A.; Baath, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Kaur, A.; Chaudhary, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid—A bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Baldrian, P.; Trogl, J.; Frouz, J.; Snajdr, J.; Valaskova, V.; Merhautova, V.; Cajthaml, T.; Herinkova, J. Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol. Biochem. 2008, 40, 2107–2115. [Google Scholar] [CrossRef]
- Kuran, P.; Troegl, J.; Novakova, J.; Pilarova, V.; Danova, P.; Pavlorkova, J.; Kozler, J.; Novak, F.; Popelka, J. Biodegradation of spilled diesel fuel in agricultural soil: Effect of Humates, Zeolite, and Bioaugmentation. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Troegl, J.; Jirkova, I.; Zemankova, P.; Pilarova, V.; Danova, P.; Pavlorkova, J.; Kuran, P.; Popelka, J.; Kriklavova, L. Estimation of the quantity of bacteria encapsulated in lentikats biocatalyst via phospholipid fatty acids content: A Preliminary Study. Folia Microbiol. 2013, 58, 135–140. [Google Scholar] [CrossRef] [PubMed]
- The International Organization for Standardization (ISO). Soil quality—determination of soil microbial diversity—part 2: Method by Phospholipid Fatty acid Analysis (PLFA) Using the Simple PLFA Extraction Method. In International Standard Organisation; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Federici, E.; Giubilei, M.A.; Cajthaml, T.; Petruccioli, M.; D’Annibale, A. Lentinus (Panus) Tigrinus augmentation of a historically contaminated soil: Matrix Decontamination and Structure and Function of the Resident Bacterial Community. J. Hazard. Mater. 2011, 186, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Moore-Kucera, J.; Dick, R.P. PLFA profiling of microbial community structure and seasonal shifts in soils of a douglas-fir chronosequence. Microb. Ecol. 2008, 55, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. New look at statistical-model identification. IEEE Trans. Autom. Control. 1974, AC19, 716–723. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ponder, F., Jr.; Tadros, M.; Loewenstein, E.F. Microbial properties and litter and soil nutrients after two prescribed fires in developing savannas in an upland Missouri ozark forest. Forest Ecol. Manag. 2009, 257, 755–763. [Google Scholar] [CrossRef]
- Jaatinen, K.; Fritze, H.; Laine, J.; Laiho, R. Effects of short- and long-term water-level drawdown on the populations and activity of aerobic decomposers in a boreal peatland. Glob. Change Biol. 2007, 13, 491–510. [Google Scholar] [CrossRef]
- Bailey, V.L.; Peacock, A.D.; Smith, J.L.; Bolton, H. Relationships between soil microbial biomass determined by chloroform fumigation-extraction, substrate-induced respiration, and phospholipid fatty acid analysis. Soil Biol. Biochem. 2002, 34, 1385–1389. [Google Scholar] [CrossRef]
- Wu, Y.P.; Yu, X.S.; Wang, H.Z.; Ding, N.; Xu, J.M. Does history matter? Temperature effects on soil microbial biomass and community structure based on the phospholipid fatty acid (PLFA) analysis. J. Soils Sediments 2010, 10, 223–230. [Google Scholar] [CrossRef]
- Stevenson, B.A.; Hunter, D.W.F.; Rhodes, P.L. Temporal and seasonal change in microbial community structure of an undisturbed, disturbed, and carbon-amended pasture soil. Soil Biol. Biochem. 2014, 75, 175–185. [Google Scholar] [CrossRef]
- Birgander, J.; Rousk, J.; Olsson, P.A. Comparison of fertility and seasonal effects on grassland microbial communities. Soil Biol. Biochem. 2014, 76, 80–89. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar]
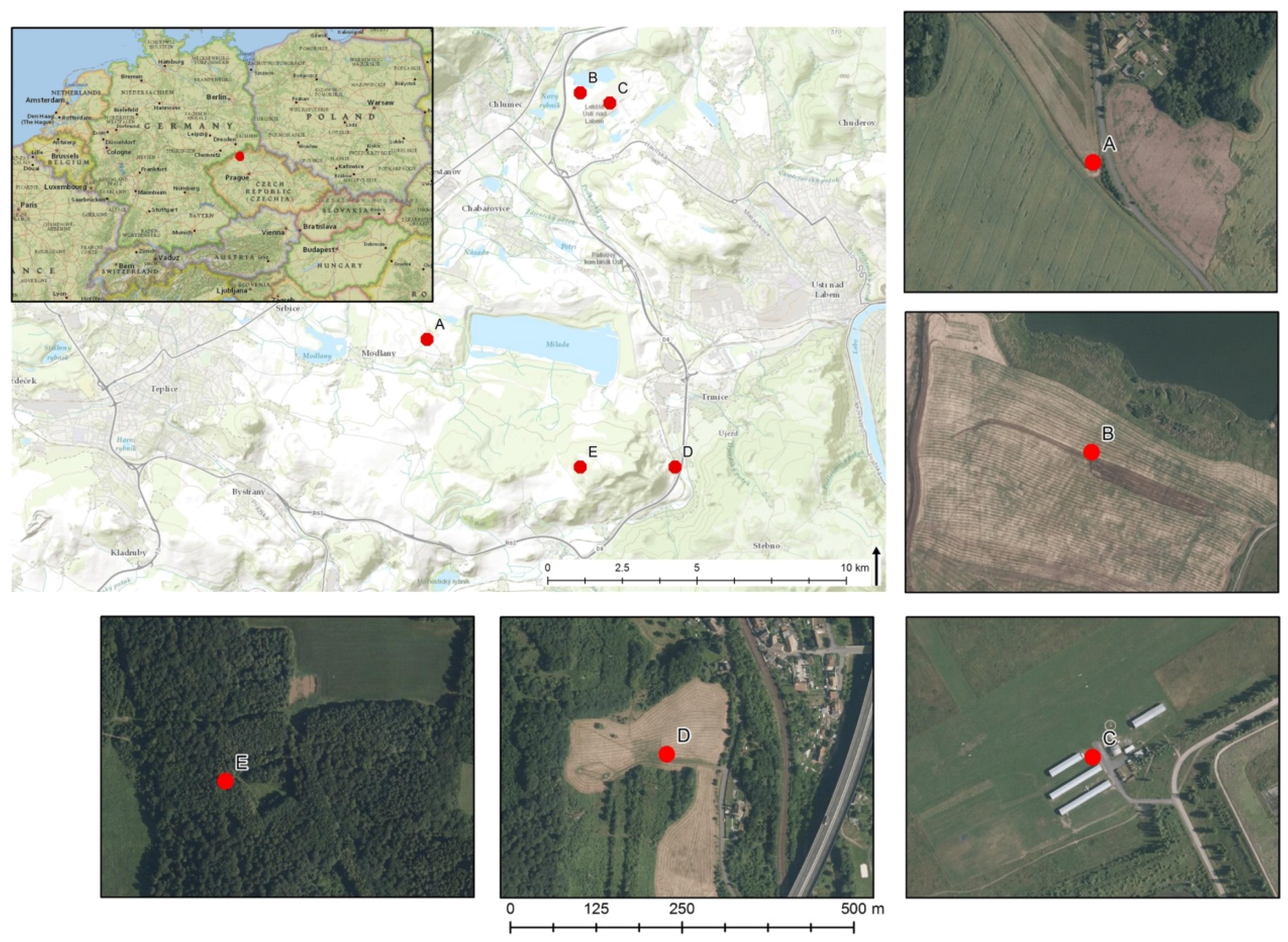

| Study Site | Characterization | BVM Value (Points) | Localization |
|---|---|---|---|
| A-Roudníky–arable | Arable natural field, intensive farming | 10 | 50°39′15′′N, 13°54′54′′E |
| B-Habrovice–wet fallow land | Primary succession on former spoil heap | 17 | 50°42′02′′N, 13°57′39′′E |
| C-Airport Ústí n.L.–grassland | Reclaimed spoil dump, regular mowing | 19 | 50°41′56′′N, 13°58′12′′E |
| D-Koštov–meadow | Natural meadow with seasonal mowing | 33 | 50°37′49′′N, 13°59′16′′E |
| E-Rovný hill–wood | Natural wood with Quercus dominance | 47 | 50°37′46′′N, 13°57′33′′E |
| Microbial Subgroup | Indicator PLFA |
|---|---|
| Microbial groups | |
| All bacteria (PLFAbac) | i14:0, i15:0, a15:0, 16:1ω7t, 16:1ω9, 16:1ω7, 18:1ω7, 10Me-16:0, i17:0, a17:0, cy17:0, 17:0, 10Me-17:0, 10Me-18:0, cy19:0 |
| i14:0, i15:0, a15:0, i17:0, a17:0 |
| cy17:0, cy19:0, 18:1ω7 |
| 10Me-16:0, 10Me-17:0, 10Me-18:0 |
| Fungi (PLFAfun) | 18:2ω6,9 |
| Ratios of microbial groups | |
| Fungi to bacteria (F/B) | PLFAfun/PLFAbac |
| Gram-positive to Gram-negative bacteria (G+/G−) | PLFAG+/PLFAG− |
| Percentage of bacterial groups | PLFAG+/PLFAbac, PLFAG−/PLFAbac, PLFAAct/PLFAbac |
| Physiological indicators | |
| General stress (trans/cis) [10] | (16:1ω7t + 18:1ω7t)/(16:1ω7 + 18:1ω7) |
| Nutrition stress (cy/pre) [16] | (cy17:0 + cy19:0)/(16:1ω7 + 18:1ω7) |
| Effects | dw | PLFAtot | F/B | G+/G− | G+ (%) | G− (%) | Act. (%) | trans/cis | cy/pre | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| Locality | 16.34 *** | 8.19 * | 7.43 | 5.00 | 7.95 * | 5.08 | 11.65 ** | 11.34 ** | 4.19 | 10.93 ** |
| Season | 1.16 | 0.02 | 0.53 | 3.34 * | 3.26 * | 3.58 * | 4.02 * | 4.87 ** | 12.02 *** | 0.94 |
| Num. of Parameters | Model | R2 | AIC | p |
|---|---|---|---|---|
| All variables | ||||
| 1 | BV=−17.9 × pH+136.2 | 0.37 | 145.2 | 3.9 × 10−4 |
| 2 | BV=−14.4 × pH − 132.0 × tc + 129.2 | 0.48 | 131.0 | 2.9 × 10−4 |
| 3 | BV=−13.6 × p − 141.4 × tc − 70.4dw + 129.2 | 0.63 | 123.1 | 1.9 × 10−5 |
| PLFA parameters only | ||||
| 1 | BV=−171.0 × tc + 44.6 | 0.26 | 138.8 | 0.0054 |
| 2 | BV=−180.8 × tc − 760.0 × Act + 57.2 | 0.50 | 129.6 | 1.6 × 10−4 |
| 3 | BV=−187.0 × tc − 732.9 × Act − 11.2 × gg + 67.8 | 0.53 | 130.3 | 3.8 × 10−4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trögl, J.; Pavlorková, J.; Packová, P.; Seják, J.; Kuráň, P.; Popelka, J.; Pacina, J. Indication of Importance of Including Soil Microbial Characteristics into Biotope Valuation Method. Sustainability 2016, 8, 253. https://doi.org/10.3390/su8030253
Trögl J, Pavlorková J, Packová P, Seják J, Kuráň P, Popelka J, Pacina J. Indication of Importance of Including Soil Microbial Characteristics into Biotope Valuation Method. Sustainability. 2016; 8(3):253. https://doi.org/10.3390/su8030253
Chicago/Turabian StyleTrögl, Josef, Jana Pavlorková, Pavla Packová, Josef Seják, Pavel Kuráň, Jan Popelka, and Jan Pacina. 2016. "Indication of Importance of Including Soil Microbial Characteristics into Biotope Valuation Method" Sustainability 8, no. 3: 253. https://doi.org/10.3390/su8030253
APA StyleTrögl, J., Pavlorková, J., Packová, P., Seják, J., Kuráň, P., Popelka, J., & Pacina, J. (2016). Indication of Importance of Including Soil Microbial Characteristics into Biotope Valuation Method. Sustainability, 8(3), 253. https://doi.org/10.3390/su8030253







