Inclusion and Implementation of Socio-Economic Considerations in GMO Regulations: Needs and Recommendations
Abstract
:1. Introduction
2. Methodology and Information Sources
3. Results and Discussion: Inclusion of Socio-Economic Considerations in National Biosafety Decision-Making
3.1. Basis for Inclusion of Socio-Economic Considerations
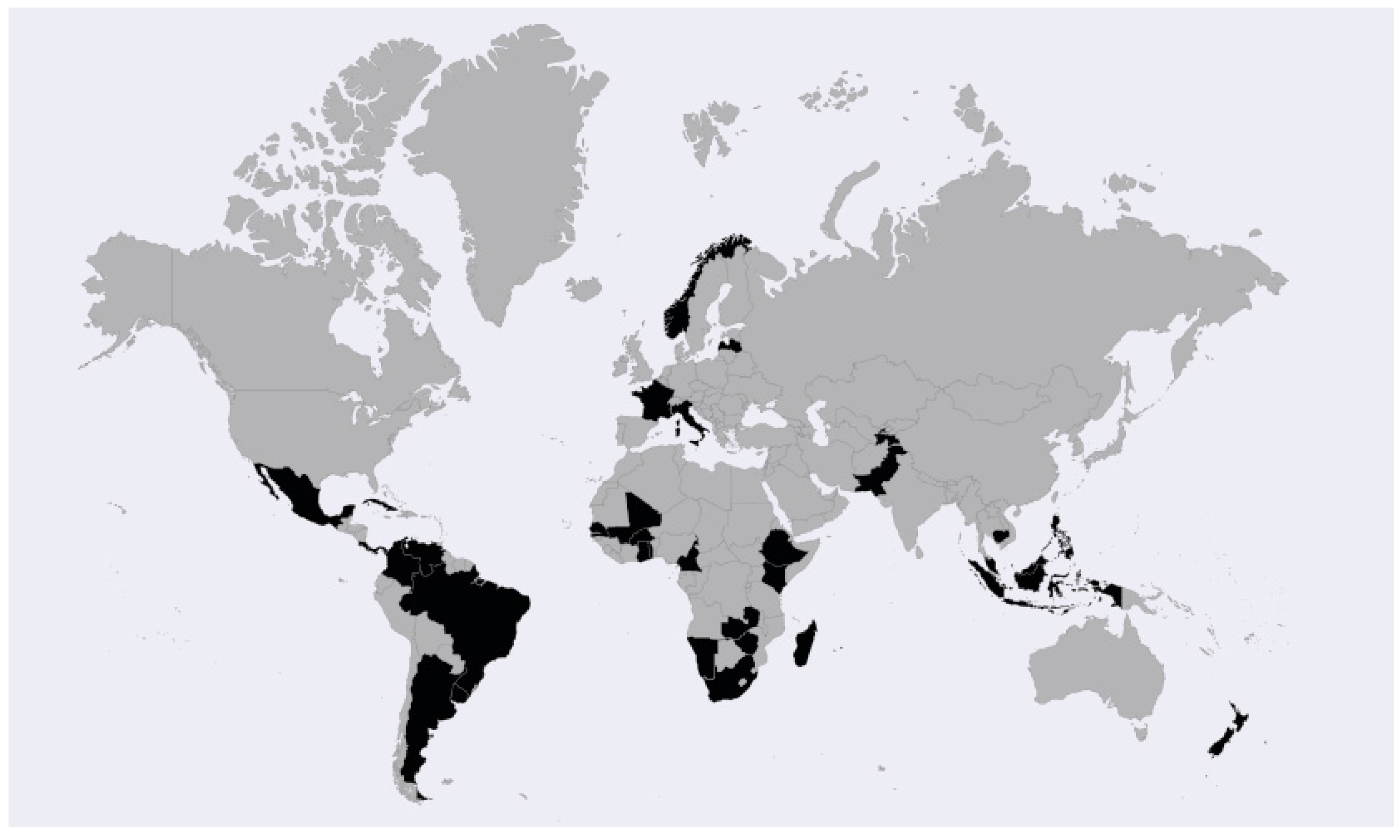
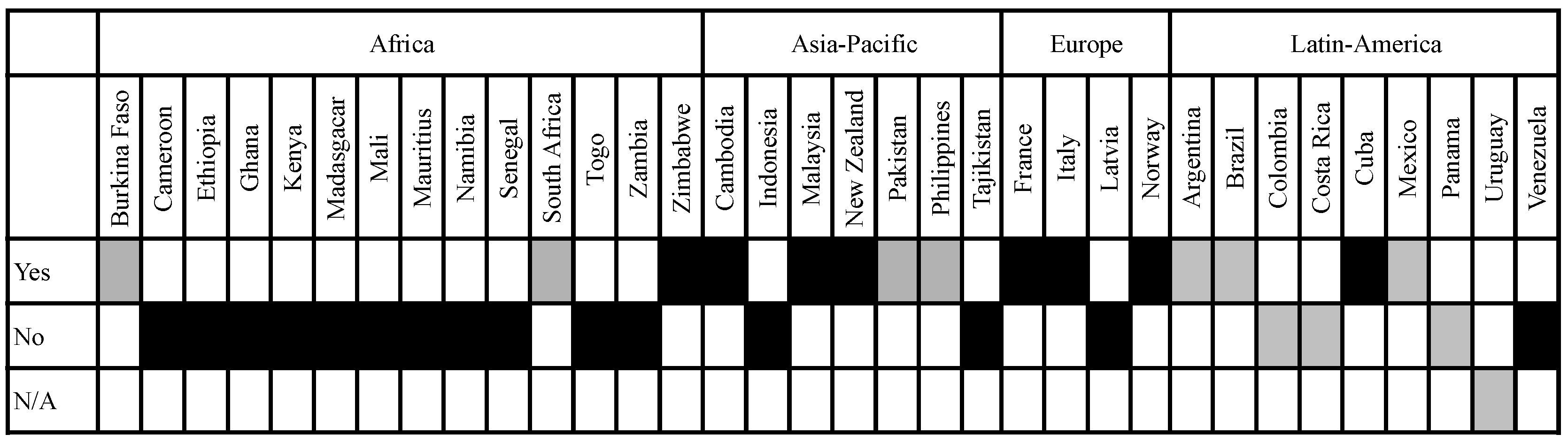
3.2. Definition of Socio-Economic Considerations in the Legislation of Analysed Countries
3.2.1. Implementation Is Based on Either a Prescriptive or a Descriptive Approach
3.2.2. Scope of the Socio-Economic Considerations Included in the Analysed Legislation: Protection Goals and Considerations Taken into Account
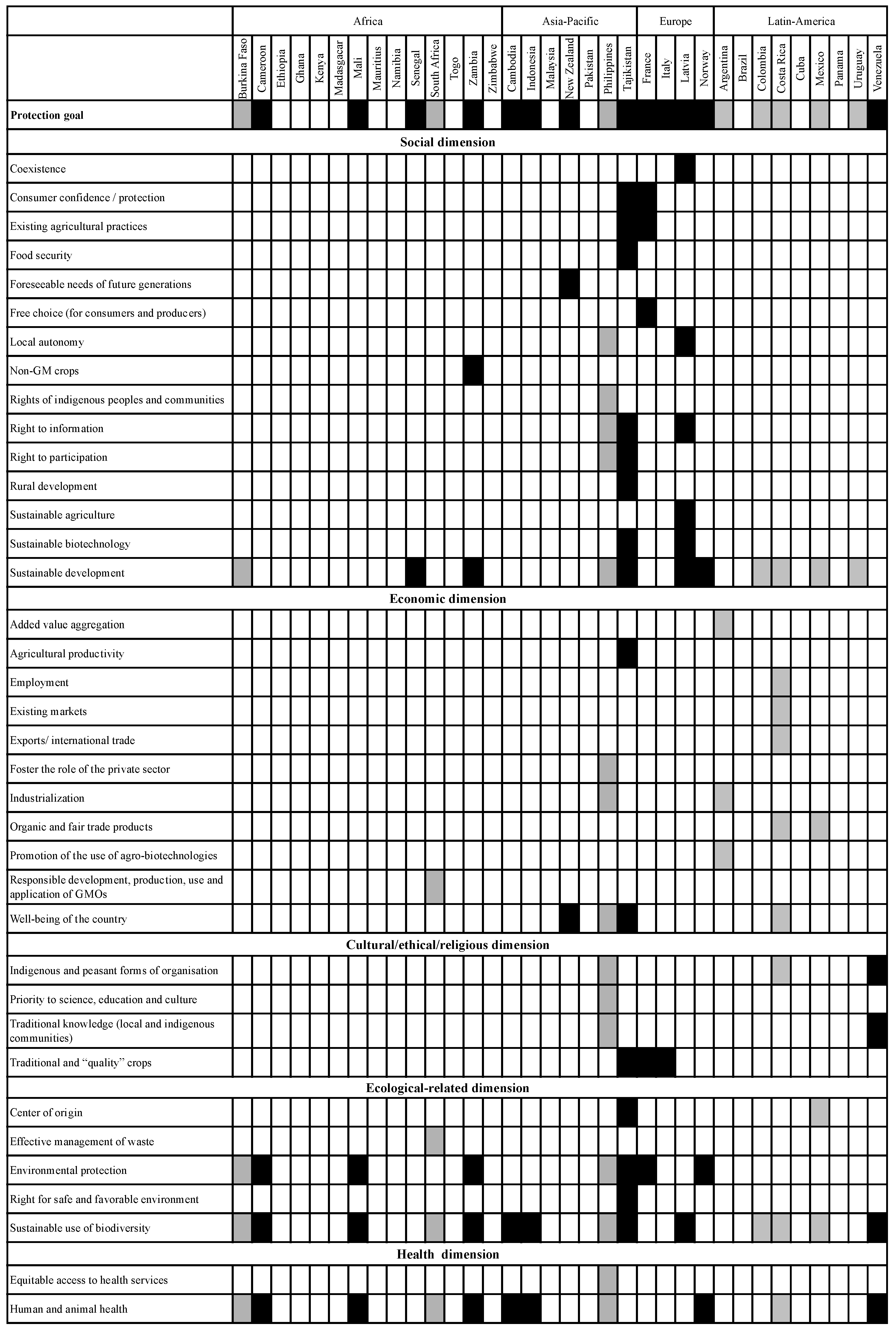
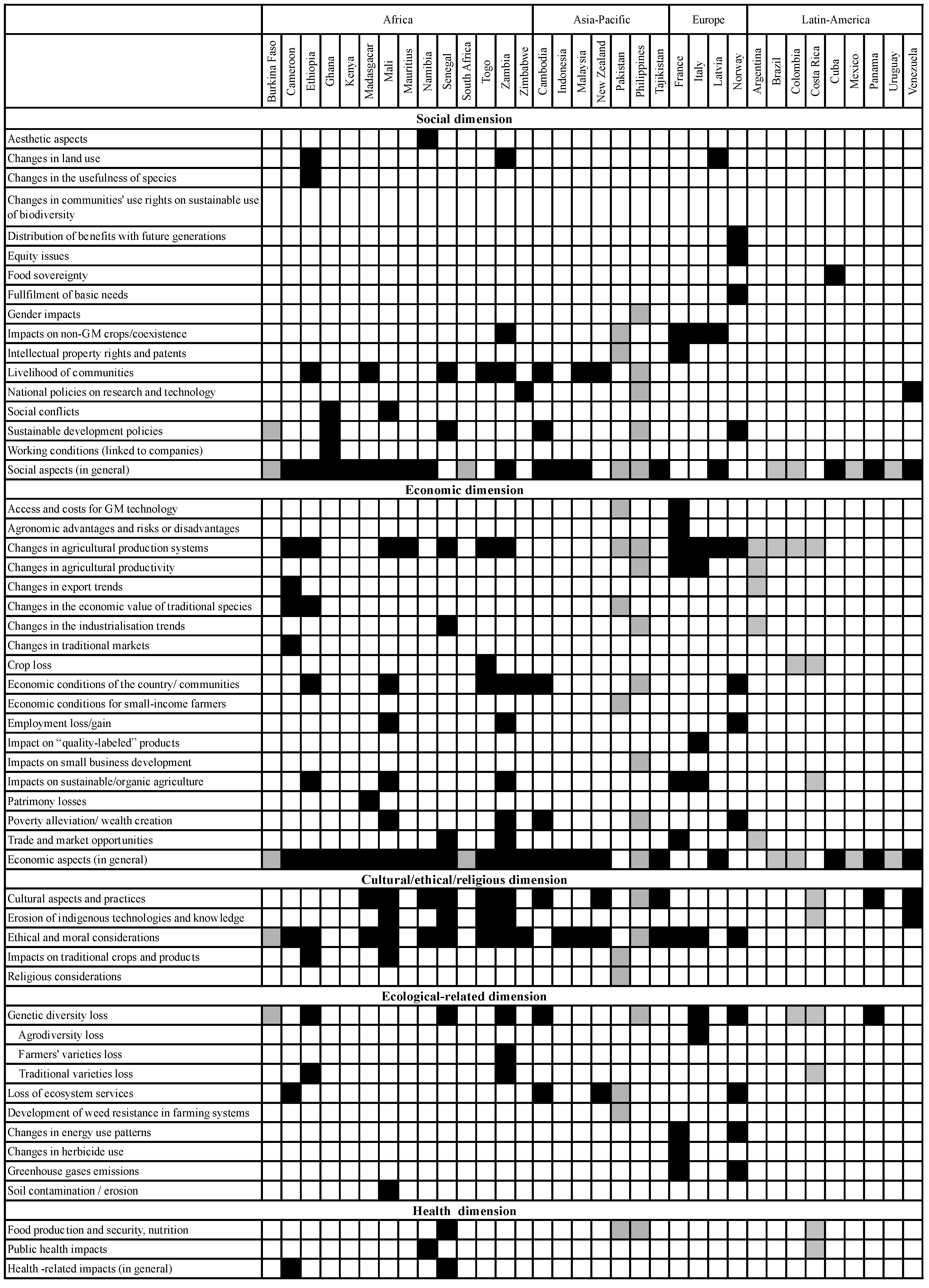
3.3. Methodological Approaches and Use of Criteria and Indicators for Assessing Socio-Economic Considerations
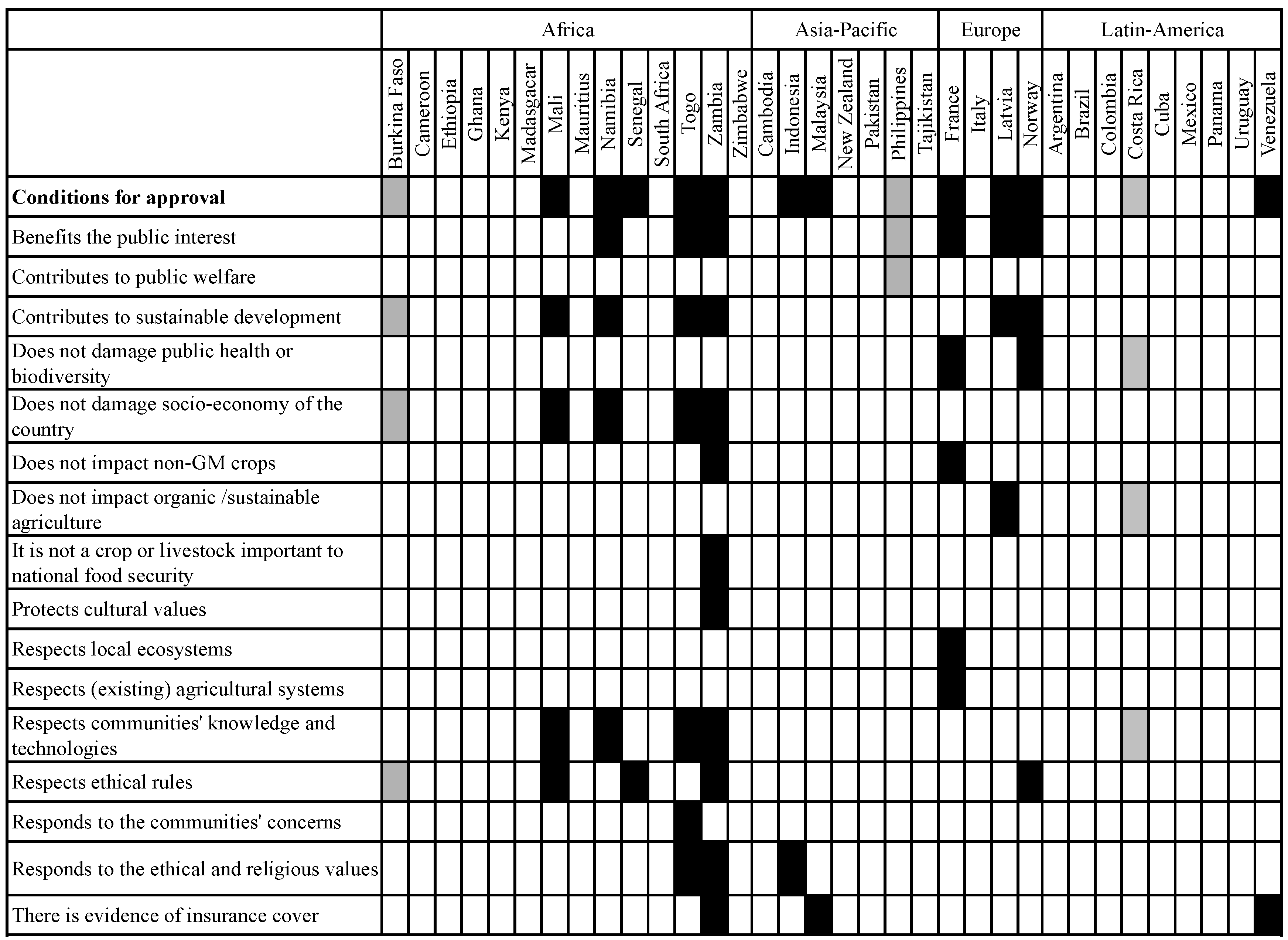
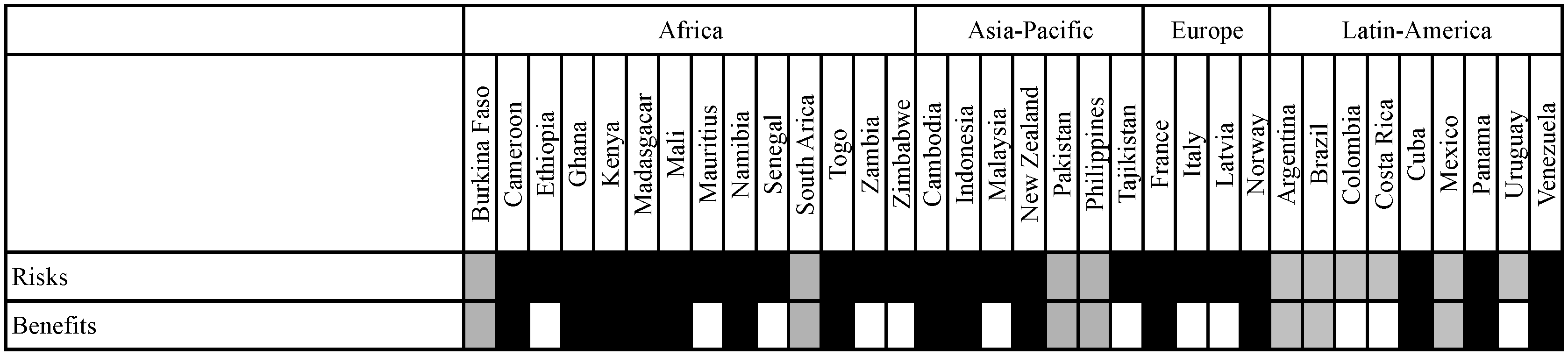
3.3.1. Differences with Regard to Inclusion of Risks and Benefits as Well as Direct and Indirect Effects
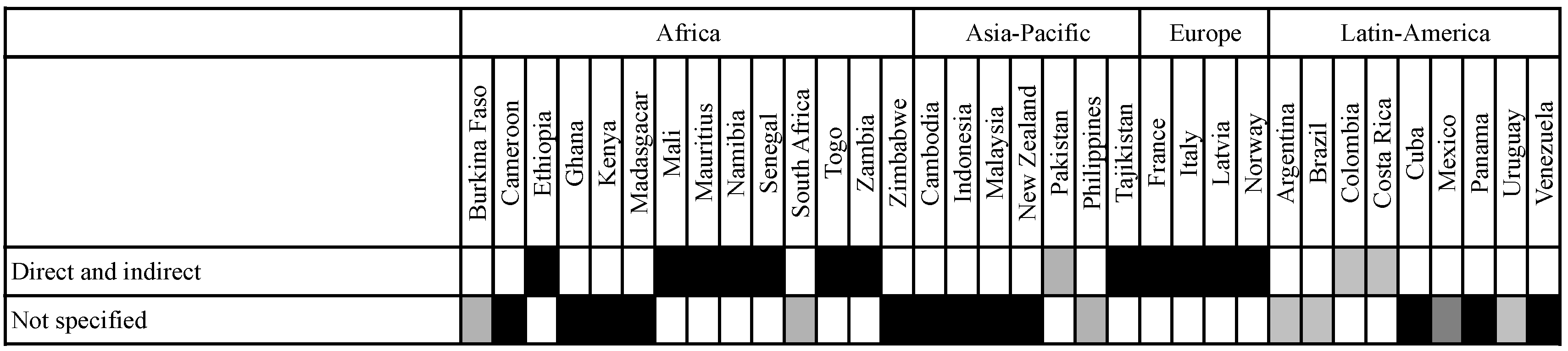
3.3.2. Relation between the Socio-Economic Impact Assessment and the Environmental and Health Risk Assessment
| Region | Country | Definition of Environment | Article or Section |
|---|---|---|---|
| Africa | Cameroon | Environment: (a) all the natural or artificial elements and the bio-geochemic equilibriums in which they are involved, as well as the economic, social and cultural factors which foster the existence, transformation and development of the environment, living organisms and human activities; (b) natural abiotic resources such as the surrounding air, surface waters, underground water, soils, land surface, wildlife and plants, and the interactions between the elements which all form an integral part of the cultural heritage and specificities of the landscape under Cameroon’s jurisdiction | Law N° 2003/006 of 21 April 2003, chapter II, sect 5(21) |
| Ethiopia | ...the environmental rights provided under Articles 44 and 92 of the Constitution of the Federal Democratic Republic of Ethiopia require that human and animal health, environmental well-being and, in general, the socio-economic conditions of the country be protected from risks that may arise from modified organisms | Proclamation 665/2009 on Biosafety, preamble | |
| Namibia | "Environment" means the complex of natural and anthropogenic factors and elements that are mutually interrelated and affect the ecological equilibrium and the quality of life, and includes: (a) the natural environment being land, water, air, all organic and inorganic material and all living organisms; and (b) the human environment being the landscape and natural, cultural, historical, aesthetic, economic and social heritage and values | Biosafety Act (Act No.7 of 2006), chapter 1, definitions | |
| Zambia | “environment” means the aggregate of surrounding objects, conditions and influences that affect the life and habits of human beings or any other organism or collection of organisms; | Biosafety Act (Act 10) of 2007, part 1, art. 1 | |
| Zimbabwe | “environment” means the aggregate of surrounding objects, conditions and influences that affect the life and habits of human beings or any other organism or collection of organisms; | National Biotechnology Authority Act of 2007, part 1, art. 2 | |
| Asia-Pacific | Pakistan | Environment: An ecosystem or habitat, including humans and animals, which is likely to come in contact with a released organism | National Biosafety Guidelines 2005, app. 12 |
| New Zealand | environment includes: (a) ecosystems and their constituent parts, including people and communities; and (b) all natural and physical resources; and (c) amenity values; and (d) the social, economic, aesthetic, and cultural conditions which affect the matters stated in paragraphs (a) to (c) or which are affected by those matters | Hazardous Substances and New Organisms Act 1996, interpretation | |
| Latin-America | Mexico | Environment: The set of natural and artificial elements or those induced by man allowing the existence and development of human beings and other living organisms that interact in a determined space and time, outside the facility areas, or outside the realms where genetically modified organisms are used in a confined manner | Law on Biosafety of GMOs 2005, art. 3(XIX) |
| Venezuela | Environmental Impact Assessment: Study oriented to predict and assess the impact of developing an activity on components of the natural and social environment and to propose preventive, mitigation and corrective measures in order to verify compliance with environmental provisions contained in current legislation in the country and to identify environmental parameters there under may be established for each program or project (own translation) | Normas sobre Evaluación Ambiental deActividades Susceptibles de Degradarel Ambiente título I, art 3 |
3.3.3. Variation in the Level of Assessment and Specifications When Assessing Socio-Economic Considerations
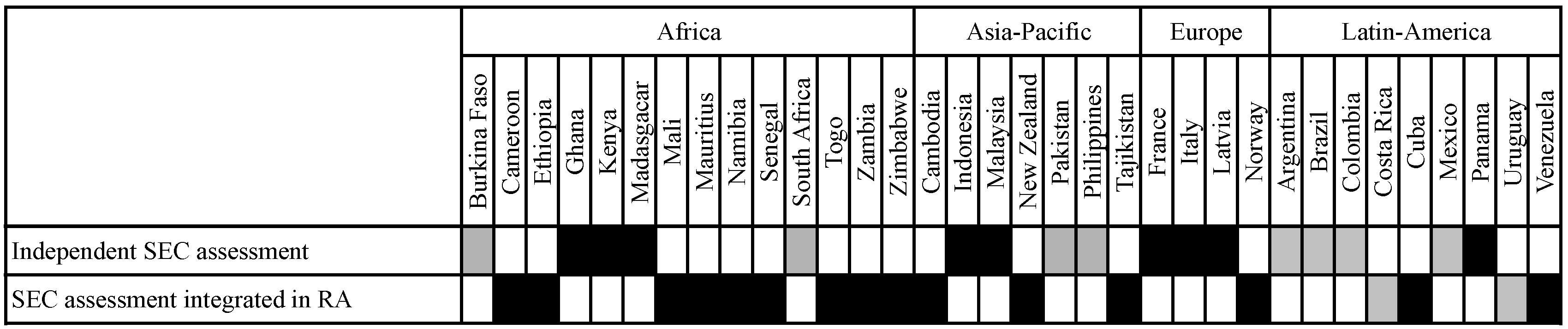
3.3.4. Socio-Economic Expertise in Decision and Advisory Bodies
3.3.5. Other Aspects Related to Socio-Economic Assessment: Inclusion of Precautionary Approaches in GMO Regulation
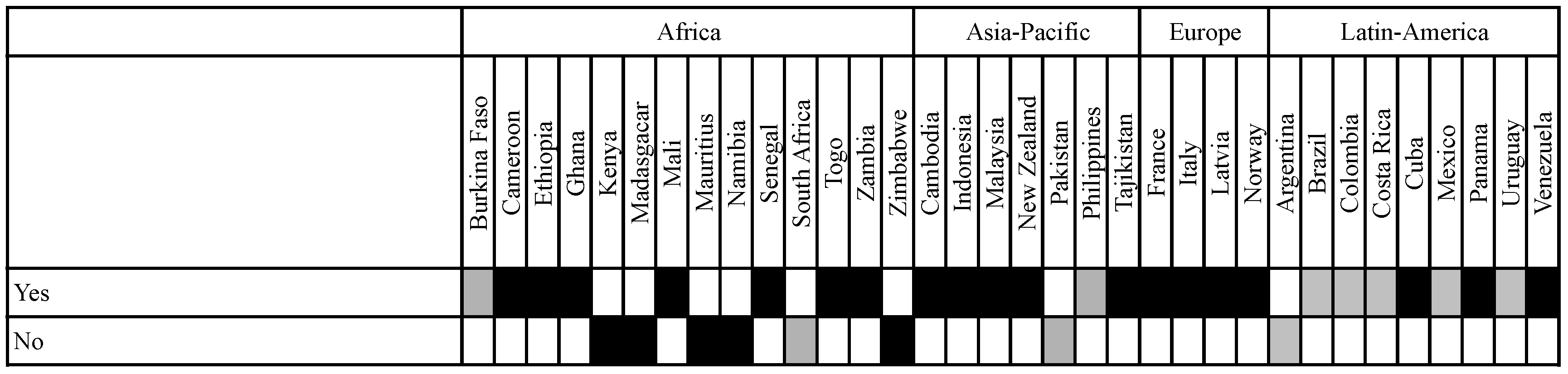
3.4. Participatory Approaches in GMO Regulation
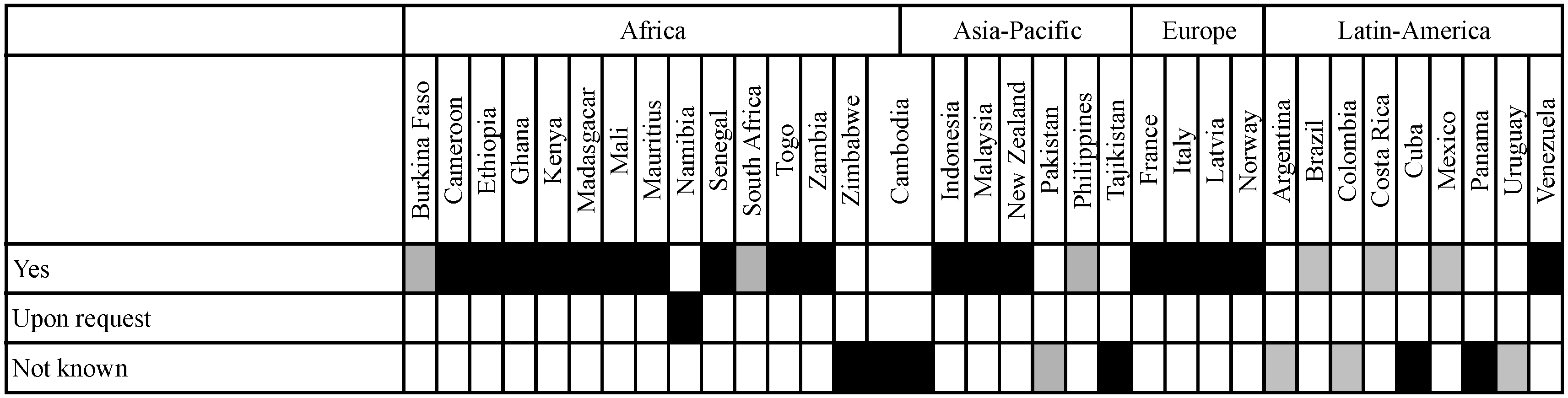
3.5. The Norwegian Approach for Assessing Broader Issues
3.5.1. Basis for the Inclusion and Implementation Experience
3.5.2. Implementation of the Socio-Economic Aspects
| Checklist in Appendix 4 of the Regulations on Impact Assessment Pursuant to the Gene Technology Act. | Available Information Found in Applications That Can be Used for Answering Questions in the Checklist. | Information Lacking in Applications that Concerns the Questions in the Checklist |
|---|---|---|
| Global impacts | Persistence, invasiveness, possible population and fitness changes introduced in the GMPPotential for gene transfer | Changes in biogeochemical processesChanges due to cultivation patternsEffects on water and energy balanceLatency/cumulative effects |
| Ecological limits | Interaction between GMP and target organismsInteractions between GMO and non-target organisms | Impacts on socio-ecological relationships |
| Basic human needs | Benefits for healthToxicity and allergenicity | Latency/cumulative effectsFood security issues |
| Distribution between generations | Not found | Latency/cumulative effectsInfluence by scientific innovationsTrade-off between utility and risk |
| Distribution between rich and poorer countries | Not found | Adequacy for meeting problems in poor countries and especially for small-scale farmers |
| Economic growth | Not found | Latency/cumulative effectsTrade off between short term economic growth versus potential long term adverse effects |
3.5.3. Methodological Approaches and Use of Criteria and Indicators
| Parameter | Questions to Applicants |
|---|---|
| Environment/Ecology | On the GM plant: characterization, gene flow, interaction between plant and the environment, preservation of biodiversity, comparison with control plants |
| On the herbicide/Bt toxin: characterization, effects of altered use, development of resistance | |
| Soil, water, energy and climate | |
| Society/Economy | The right to sufficient, safe and healthy food (food safety, security and quality) |
| Animal health and welfare (feed quality) | |
| Living conditions and profitability for the farmers who cultivate GM plants, in the short term (less than 5 years) and in the long term (more than 20 years) Health and safety, contracts and framework conditions, employment, developments of costs and incomes, agronomic factors, the right to seed, ownership rights etc. | |
| Plant genetic resources for food and agriculture | |
| Independent risk research | |
| Freedom to choose agricultural system in the future |
3.5.4. Participatory Approaches
4. Discussion: Opportunities for and Challenges to the Inclusion of Socio-Economic Considerations
4.1. Methodological Issues and Approaches Taken
4.2. Need for More Empirical Data
4.3. Values and Public Participation
4.4. The Precautionary Principle
5. Conclusions: Needs and Recommendations
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Country | Analysed Documents | Type of Document |
|---|---|---|
| Argentina | Resolución SAGYP n° 510/2011 of 17th August 2011 | Regulation |
| Resolución SAGYP n° 763/2011 of 17th August 2011 | Regulation | |
| Brazil | Law No. 11105, 24th March 2005 | Law |
| Decree No. 5,591, 22nd November 2005 (revised version 18th January 2006) | Regulation | |
| Burkina Fasso | Loi N° 005-2006/AN du 4 Mai 2006 portant regime de securit en matiere de biotechnologie | Law |
| Cambodia | Law on Biosafety promulgated on 15th February 2008 (unofficial translation) | Law |
| Sub-decree on Mechanisms and Procedures for Implementing the Law on Biosafety (unofficial translation) | Regulation | |
| Cameroon | Law N° 2003/006 of 21 April 2003 to lay down safety regulations governing modern biotechnology in Cameroon | Law |
| Colombia | Decreto nª 4525 de 6 de diciembre de 2005 por el cual se reglamenta la Ley 740 de 2002 | Regulation |
| Costa Rica | Reglamento a la Ley de Protección Fitosanitaria (N° 26921-MAG), 2008 | Regulation |
| Reglamento para el Desarrollo, Promoción y Fomento de la Actividad Agropecuaria Orgánica (Decreto 35242-MAG-H-MEIC), 2008 | Regulation | |
| Cuba | Reglamento para el otorgamiento de la autorización de seguridad biologica (Resolución 180 2007) | Regulation |
| Ethiopia | Proclamation 665/2009 on Biosafety | Law |
| Directive No. 01/ 2009 issued to determine the contents of applications for undertaking transactions involving modified organisms | Regulation | |
| Directive No. 02/ 2009 issued to determine Risk Assessment Parameters for modified organisms | Regulation | |
| France | LOI n° 2008-595 du 25 juin 2008 relative aux organismes génétiquement modifiés (NOR: DEVX0771876L; version consolidée au 19 juin 2014) | Law |
| Code de l'environnement | Law | |
| Ghana | Biosafety Act (No. 831), 2011 | Law |
| Biosafety (Management of Biotechnology) Regulations, 2007 (Legislative Instrument 1887) | Regulation | |
| Indonesia | Regulation of the Government of the Republic Indonesia Number 21 on Biosafety of Genetically Engineered Product, 2005 | Regulation |
| Italy | Decreto Legislativo 8 luglio 2003, n. 224. Attuazione della direttiva 2001/18/CE concernente l'emissione deliberata nell'ambiente di organismi geneticamente modificati | Regulation |
| Decreto Legislativo 21 marzo 2005, n. 70. Disposizioni sanzionatorie per le violazioni dei regolamenti (CE) numeri 1829/2003 e 1830/2003, relativi agli alimenti ed ai mangimi geneticamente modificati | Regulation | |
| Kenya | Biosafety Act No. 2, 2009 (revised version 2012) | Law |
| Latvia | Law On Circulation of Genetically Modified Organisms, 2007 (text consolidated by Valsts valodas centrs (State Language Centre), 2013) | Law |
| By-law of the Supervision Council for Genetically Modified Organisms (text consolidated by Valsts valodas centrs (State Language Centre), 2013) | Law | |
| Regulation No. 453 adopted 19 May 2009. Regulations Regarding the State Fee for Preparation of the Risk Assessment’s Opinion of Genetically Modified Organisms | Regulation | |
| Regulation No. 457, adopted 26 May 2009. Regulations Regarding the Procedures for the Release into the Environment or Placing on the Market of Genetically Modified Organisms, the Procedures for Monitoring and Issuance of a Permit, as well as the Procedures for Providing Information Regarding Circulation of Genetically Modified Organisms and Public Involvement in the Decision Taking Process | Regulation | |
| Regulation No. 1078, adopted 22 December 2008. Methodology for the Risk Assessment of Genetically Modified Organisms | Regulation | |
| Madagascar | Decret N° 99-954 du 15 decembre 1999 modifié par le décret n° 2004-167 du 03 février 2004 relatif à la mise en compatibilité des investissements avec l’environnement (MECIE) | Regulation |
| Malaysia | Biosafety Act, 28th 28 August 2007 | Law |
| Biosafety (Approval and Notifcation) Regulations 2010 | Regulation | |
| Mali | Loi n°08-042/AN – RM du 1er decembre 2008 relative à la sécurité en Biotechnologie en République du Mali | Law |
| Mauritius | The Genetically Modified Organisms Act 2004; Act No. 3 of 2004 | Law |
| México | Ley de Bioseguridad de Organismos Genéticamente Modificados, de 18 de marzo de 2005 | Law |
| Reglamento de la Ley de Bioseguridad de Organismos Genéticamente Modificados, de 19 de marzo de 2008 | Regulation | |
| Namibia | Biosafety Act (Act No.7 of 2006), 30th December 2006 | Law |
| New Zealand | Hazardous Substances and New Organisms Act, 10th June 1996 (reprint as at 1st January 2014) | Law |
| Biosecurity Law Reform Act No 73 of 17th September 2012 | Law | |
| Norway | Act relating to the production and use of genetically modified organisms (Gene Technology Act)Act of 2 April 1993 No. 38 with subsequent amendments, most recently by Act of 17 June 2005 No. 79 | Law |
| Regulations relating to impact assessment pursuant to the Gene Technology Act laid down by Royal Decree of 16 December 2005 and annexes 1 to 4 | Regulation | |
| Sustainability, Benefit to the Community and Ethics in the Assessment of Genetically Modified Organisms: Implementation of the Concepts set out in Sections 1 and 10 of the Norwegian Gene Technology Act. Opinion by the Norwegian Biotechnology Advisory Board. | Guidelines | |
| Pakistan | Pakistan Biosafety Rules, of 26th April 2005 | Law |
| National Biosafety Guidelines 2005, Notification No. F.2 (7)95-Bio | Guidelines | |
| Panama | Ley 48 de 2002 que crea la Comisión Nacional de Bioseguridad para los Organismos Modificados Genéticamente y dicta otras disposiciones | Law |
| Decreto-Ley 11 de 2006 que crea la autoridad panameña de seguridad de alimentos y dicta otras disposiciones | Law | |
| Philippines | Executive Order No. 514 establishing the national biosafety framework, prescribing guidelines for its implementation, strengthening the national committee on biosafety of The Philippines, and for other purposes | Regulation |
| Senegal | Loi n° 2009-27 du 8 juillet 2009 portant sur la Biosécurité | Law |
| Décret n° 2009-1408 du 23 décembre 2009 portant missions, organisation et fonctionnement du Comité National de Biosécurité (CNB) | Regulation | |
| Décret n° 2009-1409 du 23 décembre 2009 portant missions, organisation et fonctionnement de l’Autorité Nationale de Biosécurité (ANB) | Regulation | |
| South Africa | Genetically Modified Organisms Act 1997 (Act No. 15, 1997) | Law |
| Genetically Modified Organisms Amendment (Act No. 23 of 2006) | Law | |
| Tajikistan | Law on Biological Safety | Law |
| National Biosafety Framework of the Republic of Tajikistan. Safarov N., Novikova T.,Idrisova A. et al. Dushanbe: National Biodiversity and Biosafety Center. 2004. - P.66 | Guidelines | |
| Togo | Loi n° 2009-001sur la prévention des risques biotechnologiques | Law |
| Uruguay | Decreto N° 353/008, 2008 | Regulation |
| Venezuela | Ley de Gestión de la Diversidad Biológica, de 1 de diciembre de 2008 | Law |
| Normas sobre Evaluacion Ambiental de Actividades Susceptibles de Degradar el Ambiente | Regulation | |
| Decreto nº 4334, mediante el cual se dispone que la Comisión Nacional de Bioseguridad, como organismo técnico-científico, asesorará al Ejecutivo Nacional en las actividades que en él se señalan. Decreto No. 1.257, de 13 de marzo de 1996 | Regulation | |
| Zambia | Biosafety Act (Act 10) of 24th April 2007 | Law |
| Zimbabwe | National Biotechnology Authority Act [Chapter 14:31], Act 3/2006 | Law |
References
- Devos, Y.; Maeseele, P.; Reheul, D.; Van Speybroeck, L.; De Waele, D. Ethics in the societal debate on genetically modified organisms: A (re) quest for sense and sensibility. J. Agric. Environ. Ethics 2008, 21, 29–61. [Google Scholar] [CrossRef]
- Dowd-Uribe, B. Engineering yields and inequality? How institutions and agro-ecology shape Bt cotton outcomes in Burkina Faso. Geoforum 2014, 53, 161–171. [Google Scholar]
- Pavone, V.; Goven, J.; Guarino, R. From risk assessment to in-context trajectory evaluation - GMOs and their social implications. Environ. Sci. Eur. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- Daño, E.C. Potential socio-economic, cultural and ethical impacts of GMOs: Prospects for socio-economic impact assessment. In Biosafety First—Holistic Approaches to Risk and Uncertainty in Genetic Engineering and GMOs; Traavik, T., Ching, L.L., Eds.; Tapic Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Eckerstorfer, M.; Gaugitsch, H. Summary report for the Workshop “Framing socio-economic assessment in GMO & chemicals regulation”; European Environment Agency: Copenhagen, Denmark, 6–7 December 2012. Available online: http://www.umweltbundesamt.at/fileadmin/site/umweltthemen/gentechnik/EEA-SEA_Dec2012/Summary-report_EEA-Workshop-SEA_6–7Dec2012.pdf (accessed on 9 May 2015).
- Interorganizational committee on principles and guidelines for social impact assessment. Principles and guidelines for social impact assessment in the USA. Impact Assess. Proj. Appraisal 2003, 21, 231–250. [Google Scholar]
- AdHoc Technical Expert Group on Socioeconomic considerations. Report of the AdHoc Technical Expert Group on Socioeconomic Considerations. UNEP/CBD/BS/AHTEG-SEC/1/3. 2014. Available online: https://www.cbd.int/doc/meetings/bs/bs-ahteg-sec-01/official/bs-ahteg-sec-01-03-en.pdf (accessed on 28 september 2015).
- Dalli, J. Commissioner for Health and Consumer Politics Full picture of GMO cultivation is now shaping up. Hearing at the European Parliament on the socio-economic dimensions of GMO cultivation Brussels, 18 October 2011; SPEECH/11/674. [Google Scholar]
- European Commission. New EU approach on GMOs. 2014. Available online: http://ec.europa.eu/food/plant/gmo/legislation/future_rules_en.htm (accessed on 9 May 2015).
- European Group on Ethics in Science and New Technologies. Ethics of modern developments in agriculture technologies. Available online: http://bookshop.europa.eu/en/ethics-of-modern-developments-in-agricultural-technologies-pbKAAJ08024/ (accessed on 26 December 2015).
- COGEM. Socio-economic aspects of GMOs. Building blocks for an EU sustainability assessment of genetically modified crops. Available online: http://ec.europa.eu/food/food/biotechnology/reports_studies/docs/Netherlands_annex_Cogem_report_en.pdf (accessed on 9 May 2015).
- Spök, A. Assessing Socio-Economic Impacts of GMOs. Issues to Consider for Policy Development; Lebensministerium/Bundensministerium für Gesundheit: Vienna, Austria, 2010. [Google Scholar]
- Greiter, A.; Miklau, M.; Heissenberger, A.; Gaugistsch, H. Socio-Economic Aspects in the Assessment of GMOs —Options for Action; Report 0345; Environment Agency Austria (Umweltbundesamt): Vienna, Austria, 2011. [Google Scholar]
- CBD. UN meeting agrees on decisions to advance the implementation of the International Agreement on the safe use of living modified organisms. Available online: http://www.cbd.int/doc/press/2014/pr-2014-10-03-bscopmop7-en.pdf (accessed on 9 October 2014).
- Fischer, K.; Ekener-Petersen, E.; Rydhmer, L.; Björnberg, K.E. Social Impacts of GM Crops in Agriculture: A Systematic Literature Review. Sustainability 2015, 7, 8598–8620. [Google Scholar] [CrossRef]
- Myhr, A.I.; Rosendal, G.K. GMO Assessment in Norway as Compared to EU Procedures: Societal Utility and Sustainable Development; The Directorate for Nature Management: Trondheim, Norway, 2009. [Google Scholar]
- Ludlow, K.; Smyth, S.; Falck-Zepeda, J.B. (Eds.) Socio-Economic Considerations in Biotechnology Regulation; Natural Resource Management and Policy Series; Springer: New York, NY, USA; Dordrecht, The Netherlands; Heidelberg, Germany; London, UK, 2014. [CrossRef]
- Catacora-Vargas, G. Socio-economic considerations under the Cartagena Protocol on Biosafety: Insights for effective implementation. Asian Biotechnol. Dev. Rev. 2012, 14, 1–17. [Google Scholar]
- Falck-Zepeda, J.B.; Zambrano, P. Socio-economic Considerations in Biosafety and Biotechnology Decision Making: The Cartagena Protocol and National Biosafety Frameworks. Rev. Policy Res. 2011, 28, 171–195. [Google Scholar] [CrossRef]
- Kleinman, D.L.; Kinchy, A.J. Against the neoliberal steamroller? The Biosafety Protocol and the social regulation of agricultural biotechnologies. Agric. Hum. Values 2007, 24, 195–206. [Google Scholar] [CrossRef]
- Stabinsky, D. Bringing Social Analysis Into a Multilateral Environmental Agreement: Social Impact Assessment and the Biosafety Protocol. J. Environ. Dev. 2000, 9, 260–283. [Google Scholar] [CrossRef]
- Falck-Zepeda, J.B. Socio-economic Considerations, Article 26.1 of the Cartagena Protocol on Biosafety: What are the Issues and What is at Stake. AgBioForum 2009, 12, 90–107. [Google Scholar]
- CBD. Global overview of information on socioeconomic considerations arising from the impact of living modified organisms on the conservation and sustainable use of biological diversity. AdHoc Technical Expert Group on Socioeconomic Considerations. Report UNEP/CBD/BS/AHTEG-SEC/1/2. 2014. [Google Scholar]
- CBD. National Reports. Available online: http://bch.cbd.int/protocol/cpb_natreports.shtml (accessed on 9 August 2015).
- Sanvido, O.; Bachmann, A.; Romeis, J.; Rippe, K.P.; Bigler, F. Valuating Environmental Impacts of Genetically Modified Crops—Ecological and Ethical Criteria for Regulatory Decision-making. Available online: https://www.researchgate.net/publication/234091426_Evaluating_environmental_risks_of_genetically_modified_crops_Ecological_harm_criteria_for_regulatory_decision-making (accessed on 26 December 2015).
- Norwegian Biotechnology Advisory Board. Herbicide-resistant genetically modified plants and sustainability. Available online: http://www.bioteknologiradet.no/filarkiv/2014/09/Herbicide-resistant_genetically_modified_plants_and_sustainability_NBAB.pdf (accessed on 3 March 2015).
- Norwegian Biotechnology Advisory Board. Insect-resistant genetically modified plants and sustainability. Available online: http://www.bioteknologiradet.no/filarkiv/2011/06/rapport_baerekraft_110627_web.pdf (accessed on 3 March 2015).
- De Melo-Martin, I.; Meghani, Z. Beyond risk. A more realistic risk-benefit analysis of agricultural biotechnologies. EMBO Rep. 2008, 9, 302–206. [Google Scholar] [CrossRef] [PubMed]
- Marris, C.; Rose, N. Open Engagement: Exploring Public Participation in the Biosciences. PLoS Biol. 2010, 8, e1000549. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Biotechnology Advisory Board. Sustainability, benefit to the community and ethics in the assessment of genetically modified organisms: Implementation of the concepts set out in Section 1 and 10 of the Norwegian Gene Technology Act. Available online: http://www.bioteknologiradet.no/filarkiv/2010/07/1999_04_11_baerekraft_samfunnsnytte_og_etikk_temahefte.pdf (accessed on 3 March 2015).
- Rosendal, G.K. Interpreting Sustainable Development and Societal Utility in Norwegian GMO Assessments. Eur. Environ. 2008, 18, 243–256. [Google Scholar] [CrossRef]
- Rosendal, G.K.; Myhr, A.I. GMO assessment in Norway: Societal utility and sustainable development. EMBO Rep. 2009, 10, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Stirling, A. Risk, precaution and science: Towards a more constructive policy debate. Talking point on the precautionary principle. EMBO Rep. 2007, 8, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Walls, J.; Rogers-Hayden, T.; Mohr, A.; O’Riordan, T. Seeking Citizens’ Views on GM Crops: Experiences from the United Kingdom, Australia, and New Zealand. Environ. Sci. Policy Sustain. Dev. 2005, 47, 22–37. [Google Scholar] [CrossRef]
- Jensen, K.K.; Gamborg, C.; Madsen, K.H.; Jørgensen, R.B.; von Krauss, M.K.; Folker, A.P.; Sandøe, P. Making the EU “Risk Window” transparent: The normative foundations of the environmental risk assessment of GMOs. Environ. Biosaf. Res. 2003, 2, 161–171. [Google Scholar] [CrossRef]
- Devos, Y.; Sanvido, O.; Tait, J.; Raybould, A. Towards a more open debate about values in decision-making on agricultural biotechnology. Transgenic Res. 2014, 23, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Gee, D. More or less precaution? In Late Lessons from Early Warnings: Science, Precaution, Innovation; Gee, D., Grandjean, P., Hansen, S.F., van denHove, S., MacGarvin, M., Martin, J., Stanners, D., Eds.; European Environment Agency: Copenhagen, Denmark, 2013; pp. 675–701. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binimelis, R.; Myhr, A.I. Inclusion and Implementation of Socio-Economic Considerations in GMO Regulations: Needs and Recommendations. Sustainability 2016, 8, 62. https://doi.org/10.3390/su8010062
Binimelis R, Myhr AI. Inclusion and Implementation of Socio-Economic Considerations in GMO Regulations: Needs and Recommendations. Sustainability. 2016; 8(1):62. https://doi.org/10.3390/su8010062
Chicago/Turabian StyleBinimelis, Rosa, and Anne Ingeborg Myhr. 2016. "Inclusion and Implementation of Socio-Economic Considerations in GMO Regulations: Needs and Recommendations" Sustainability 8, no. 1: 62. https://doi.org/10.3390/su8010062
APA StyleBinimelis, R., & Myhr, A. I. (2016). Inclusion and Implementation of Socio-Economic Considerations in GMO Regulations: Needs and Recommendations. Sustainability, 8(1), 62. https://doi.org/10.3390/su8010062







