Oxygen Control and Improved Denitrification Efficiency by Means of a Post-Anoxic Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Batch Tests
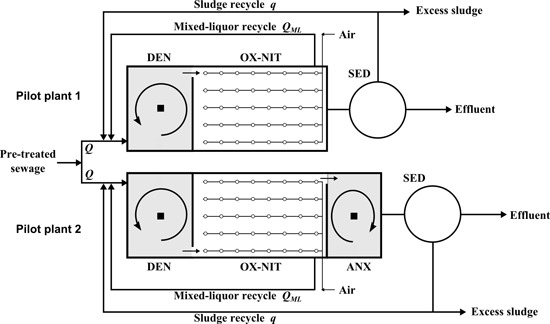
2.2. Experimental Pilot Plants
2.2.1. Description
- •
- Pilot plant 1 (standard plant with pre-denitrification reactor): biological pre-denitrification stage (DEN) followed by an oxidation-nitrification stage (OX-NIT) and a final clarifier (SED);
- •
- Pilot plant 2 (standard plant with pre-denitrification and post-anoxic reactor): biological pre-denitrification stage (DEN) followed by an oxidation-nitrification stage (OX-NIT), an anoxic stage (ANX) and a final clarifier (SED).

- DEN. Volume: 10 m3; liquid height: 1.8 m; mixing: one slow vertical-axis mixer (power input: 12 W·m−3);
- OX-NIT. Volume: 20 m3; liquid height: 1.8 m; aeration and mixing: micro-bubble aeration system;
- ANX. Volume: 6 m3; liquid height: 1.6 m; mixing: one slow vertical-axis mixer (power input: 12 W·m−3);
- SED. Volume: 6 m3; diameter: 2 m.
2.2.2. Samplings and Analyses
- 15 DO fixed probes (accuracy: 0.01 mgO2·L−1; automatic calibration and temperature compensation);
- 7 pH fixed probes (accuracy: 0.05);
- 5 temperature fixed probes (accuracy: 0.05 °C);
- 4 magnetic flow-meters (accuracy: 0.5% of the reading).
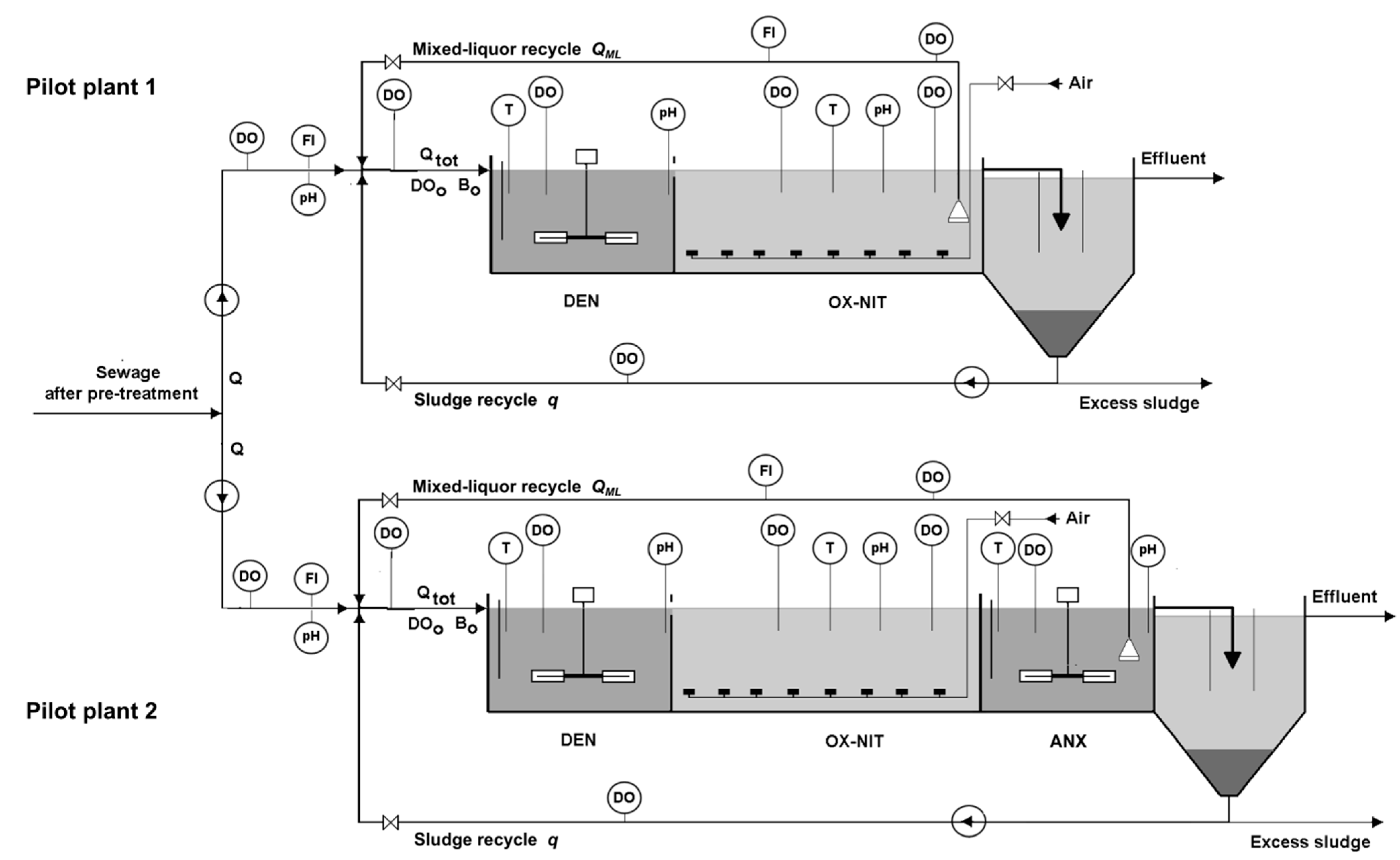
- BOD5, COD, TN, NO3-N, Ptot and Suspended Solids (SS) in both the pre-treated sewage and the pilot plants effluents (automatic daily average samplings);
- TN and NO3-N in filtered samples collected at both the inlet and the outlet of ANX (automatic daily average samplings);
- MLVSS and MLSS in DEN and OX-NIT (manual sampling);
- DO, pH and temperature at the locations shown in Figure 2 (continuous sampling and recording).
2.2.3. Operating Conditions
- •
- the effective decrease of DO concentration in ANX (DOANX);
- •
- the impact of DOANX decrease on (i) the DO concentration in DEN (DODEN) and, consequently, (ii) on the denitrification performance (ηDEN, %) at different sludge loadings:
3. Results and Discussion
3.1. Mean Quality of the Raw Sewage and the Treated Effluent
| Parameter | Unit of measurement | Values (daily average samplings) | |
|---|---|---|---|
| m | sd | ||
| CODin | mg·L−1 | 281.0 | 58.6 |
| CODeff 1 | mg·L−1 | 82.7 | 17.7 |
| BOD5 in | mg·L−1 | 135.2 | 26.0 |
| BOD5 eff 1 | mg·L−1 | 13.2 | 3.8 |
| SSin | mg·L−1 | 159.0 | 41.0 |
| SSeff 1 | mg·L−1 | 19.8 | 4.1 |
| TNin = TKNin | mg·L−1 | 29.8 | 5.2 |
| TNeff 1 (*) | mg·L−1 | 7.2 | 2.7 |
| Ptot in | mg·L−1 | 4.7 | 1.6 |
| Ptot eff 1 | mg·L−1 | 3.9 | 1.1 |
3.2. Preliminary Batch Tests
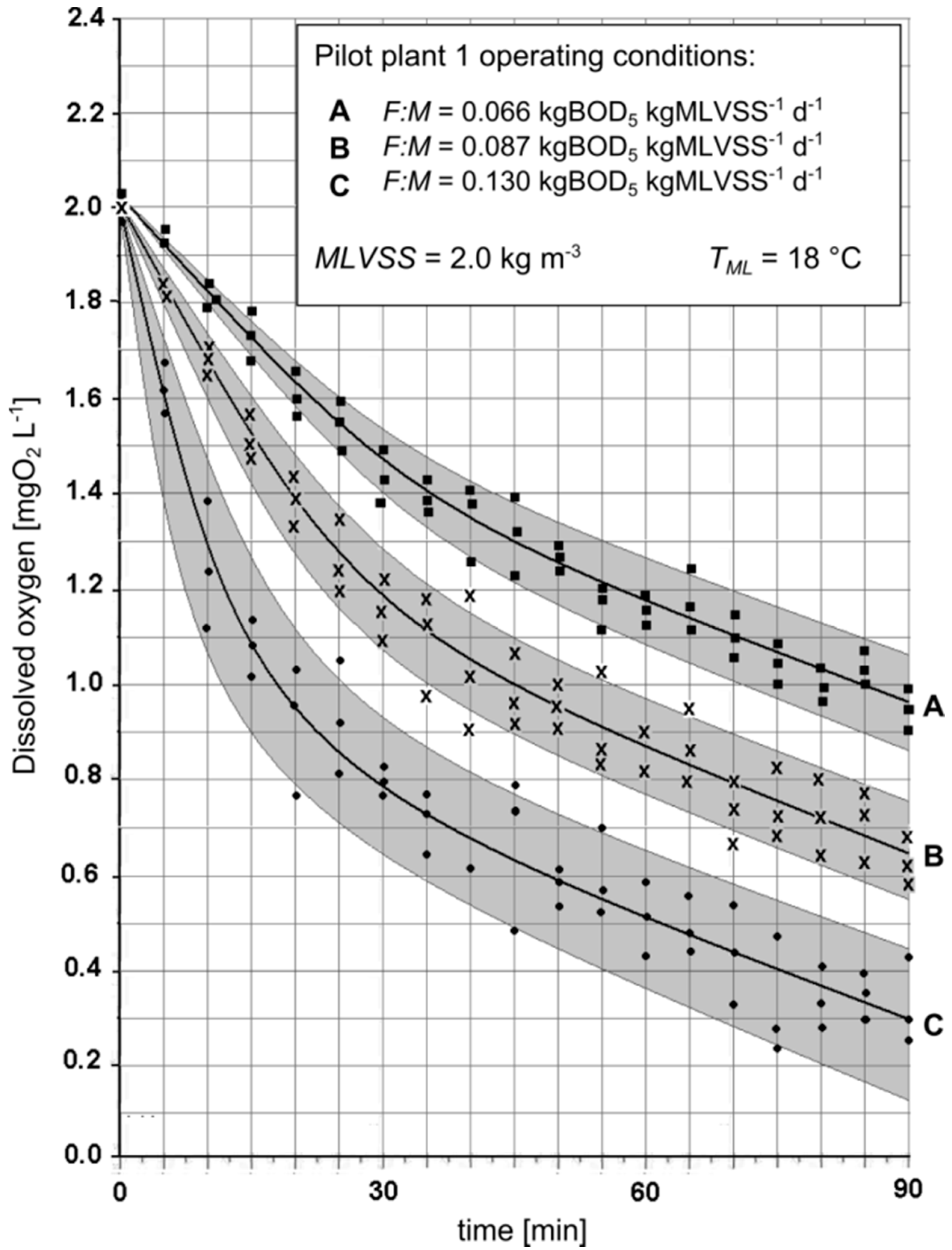
- The great presence of residual BOD at high F:M ratios that favors the kinetics of both assimilative and endogenous respiration;
- The low degree of sludge stabilization at high F:M that favors the kinetics of endogenous respiration.
3.3. Experimental Pilot Plants
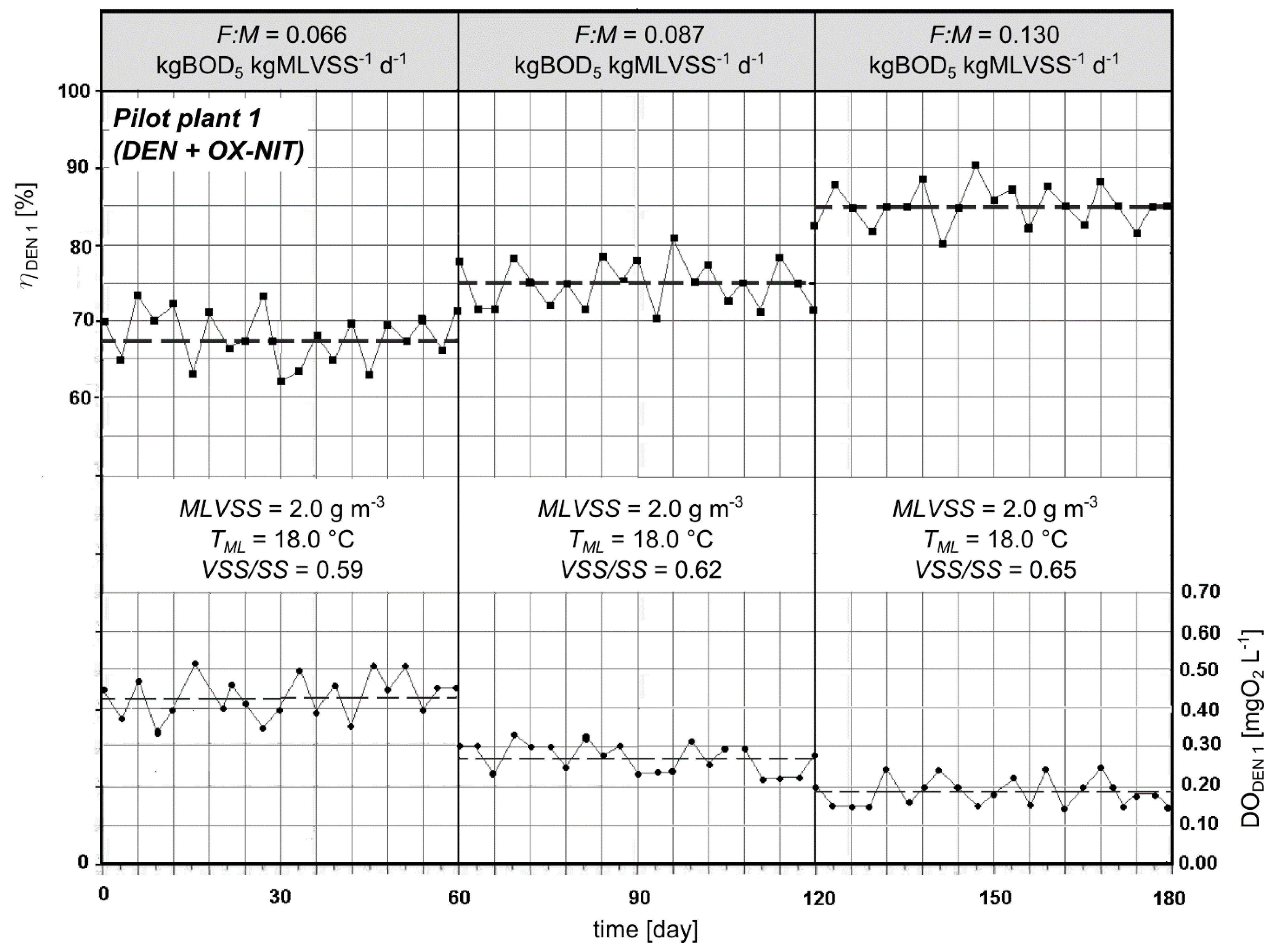
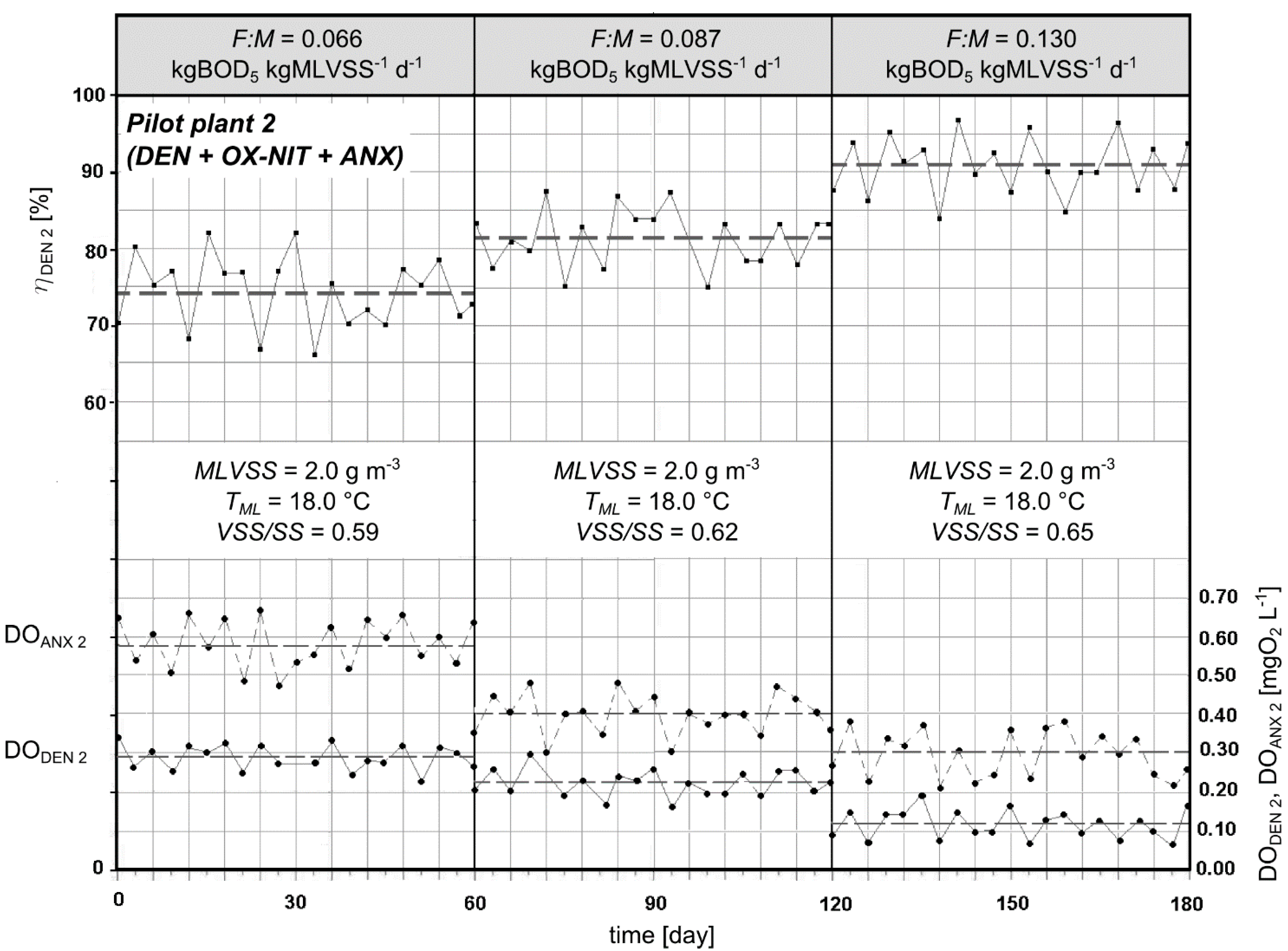
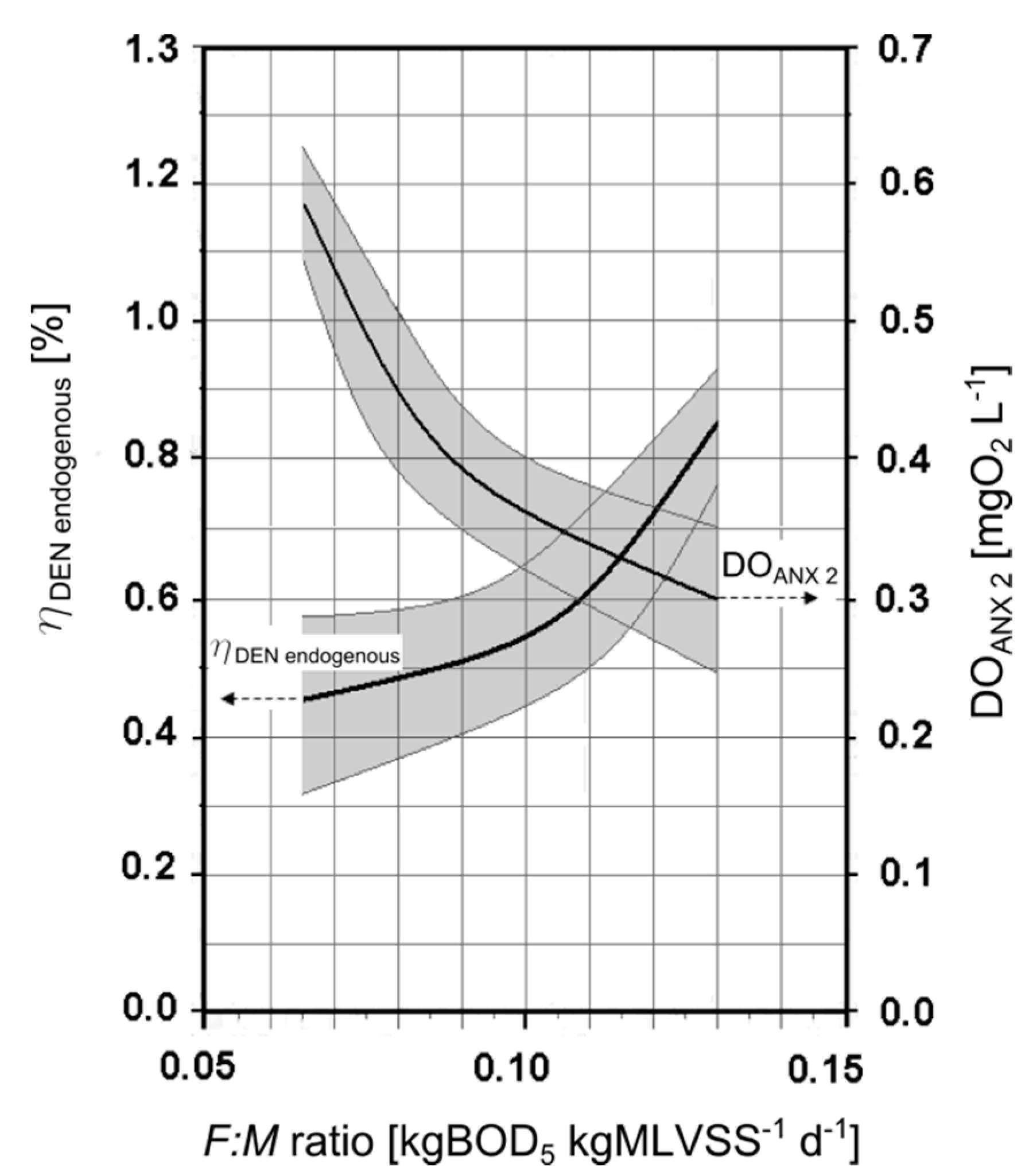
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Moghaddam, A.H.; Sargolzaei, J. A review over diverse methods used in nitrogen removal from wastewater. Recent Patents Chem. Eng. 2013, 6, 133–139. [Google Scholar] [CrossRef]
- Metcalf & Eddy Inc.; Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Raboni, M.; Torretta, V.; Viotti, P.; Urbini, G. Experimental plant for the physical-chemical treatment of groundwater polluted by municipal solid waste (MSW) leachate, with ammonia recovery. Rev. Ambient. Água. 2013, 8, 22–32. [Google Scholar]
- Zhu, G.; Peng, Y.; Li, B.; Guo, J.; Yang, Q.; Wang, S. Biological removal of nitrogen from wastewater. Rev. Environ. Contam. Toxicol. 2008, 192, 159–195. [Google Scholar] [PubMed]
- Torretta, V.; Urbini, G.; Raboni, M.; Copelli, S.; Viotti, P.; Luciano, A.; Mancini, G. Effect of powdered activated carbon to reduce fouling in membrane bioreactors: A sustainable solution. Case study. Sustainability 2013, 5, 1501–1509. [Google Scholar] [CrossRef]
- Farabegoli, G.; Gavasci, R.; Lombardi, F.; Romani, F. Denitrification in tertiary filtration: Application of an up-flow filter. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2003, 38, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Raboni, M.; Torretta, V.; Urbini, G. Influence of strong diurnal variations in sewage quality on the performance of biological denitrification in small community wastewater treatment plants (WWTPs). Sustainability 2013, 5, 3679–3689. [Google Scholar] [CrossRef]
- Raboni, M.; Torretta, V.; Viotti, P.; Urbini, G. Pilot experimentation with complete mixing anoxic reactors to improve sewage denitrification in treatment plants in small communities. Sustainability 2014, 6, 112–122. [Google Scholar] [CrossRef]
- United States-Environmental Protection Agency (US-EPA). Process Design Manual for Nitrogen Control; US-EPA Office of Technology Transfer: Washington, DC, USA, 1975. [Google Scholar]
- Torretta, V.; Ragazzi, M.; Trulli, E.; de Feo, G.; Urbini, G.; Raboni, M.; Rada, E.C. Assessment of biological kinetics in a conventional municipal WWTP by means of the oxygen uptake rate method. Sustainability 2014, 6, 1833–1847. [Google Scholar] [CrossRef]
- Ekama, G.A.; Wentzel, M.C. Denitrification kinetics in biological N and P removal activated sludge systems treating municipal wastewaters. Water Sci. Technol. 1999, 39, 69–77. [Google Scholar] [CrossRef]
- Ekama, G.A.; Wilderer, P. Biological nutrient removal. In Treatise on Water Science; Elsevier: Oxford, UK, 2011; Chapter 4.14; pp. 409–526. [Google Scholar]
- Eramo, B.; Gavasci, R.; Misiti, A.; Viotti, P. Validation of a multisubstrate mathematical model for the simulation of the denitrification process in fluidized bed biofilm reactors. Water Sci. Technol. 1994, 29, 401–408. [Google Scholar]
- Vismara, R. Depurazione Biologica, 3rd ed.; Hoepli: Milan, Italy, 1998. [Google Scholar]
- United States-Environmental Protection Agency (US-EPA). Nutrient Control Design Manual; US-EPA, National Risk Management Research Laboratory: Washington, DC, USA, 2010. [Google Scholar]
- Oh, J.; Silverstein, J. Oxygen inhibition of activated sludge denitrification. Water Res. 1999, 33, 1925–1937. [Google Scholar] [CrossRef]
- Plósz, B.G.; Jobbágy, A.; Grady, C.P.L., Jr. Factors influencing deterioration of denitrification by oxygen entering an anoxic reactor through the surface. Water Res. 2003, 37, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Raboni, M.; Torretta, V.; Viotti, P.; Urbini, G. Calculating specific denitrification rates in pre-denitrification by assessing the influence of dissolved oxygen, sludge loading and mixed-liquor recycle. Environ. Technol. 2014, 35, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- United States-Environmental Protection Agency (US-EPA). Manual: Nitrogen Control; US-EPA Office of Research and Development: Washington, DC, USA, 1993. [Google Scholar]
- Henze, M. Capabilities of biological nitrogen removal processes from wastewater. Water Sci. Technol. 1991, 23, 669–679. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbini, G.; Gavasci, R.; Viotti, P. Oxygen Control and Improved Denitrification Efficiency by Means of a Post-Anoxic Reactor. Sustainability 2015, 7, 1201-1212. https://doi.org/10.3390/su7021201
Urbini G, Gavasci R, Viotti P. Oxygen Control and Improved Denitrification Efficiency by Means of a Post-Anoxic Reactor. Sustainability. 2015; 7(2):1201-1212. https://doi.org/10.3390/su7021201
Chicago/Turabian StyleUrbini, Giordano, Renato Gavasci, and Paolo Viotti. 2015. "Oxygen Control and Improved Denitrification Efficiency by Means of a Post-Anoxic Reactor" Sustainability 7, no. 2: 1201-1212. https://doi.org/10.3390/su7021201
APA StyleUrbini, G., Gavasci, R., & Viotti, P. (2015). Oxygen Control and Improved Denitrification Efficiency by Means of a Post-Anoxic Reactor. Sustainability, 7(2), 1201-1212. https://doi.org/10.3390/su7021201