Influence of Strong Diurnal Variations in Sewage Quality on the Performance of Biological Denitrification in Small Community Wastewater Treatment Plants (WWTPs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pilot Plant Description
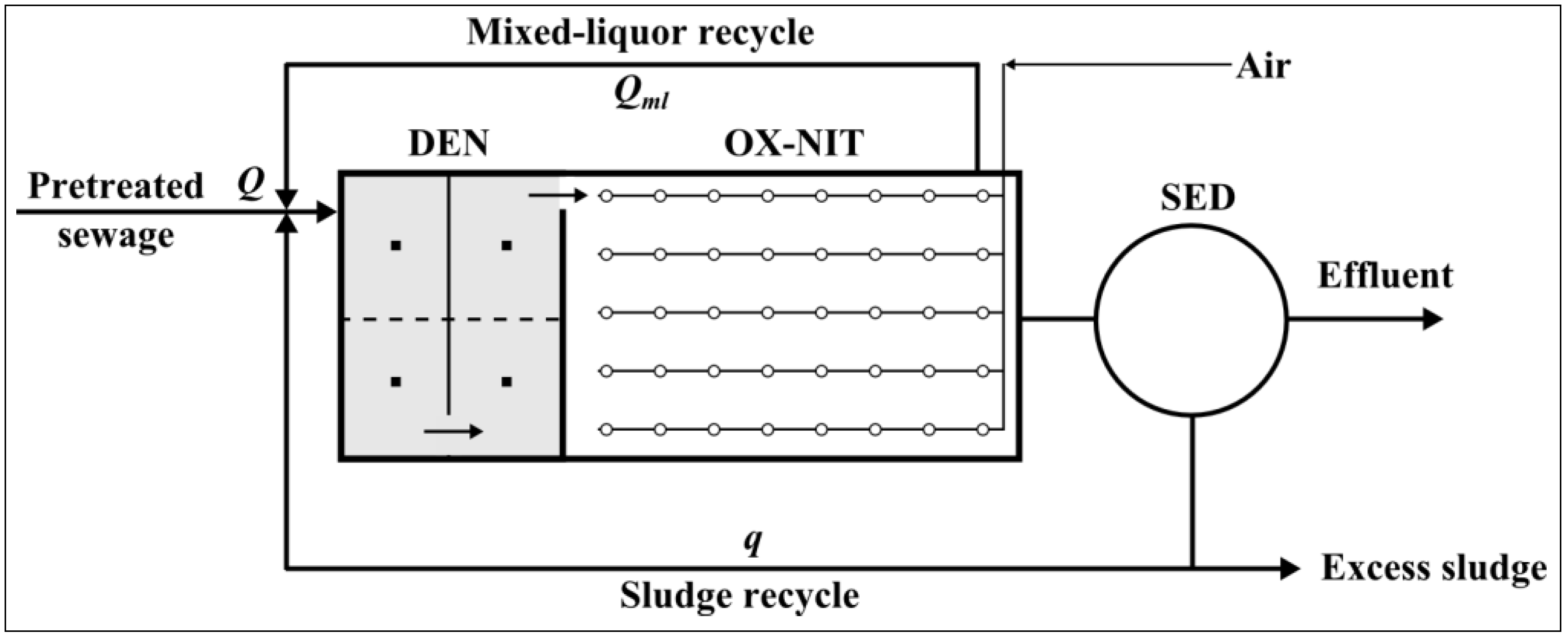
- Pre-denitrification (DEN) tank: volume 10 m3; liquid height 1.8 m;
- Biological oxidation-nitrification (OX-NIT) tank: volume 20 m3; liquid height 1.8 m;
- Final sedimentation (SED): diameter 2 m; volume 6 m3;
- Sewage flow rate, Q = 1.5 m3·h−1;
- Mixed-liquor recycle rate, Qml = 4 Q = 6 m3·h−1;
- Sludge recycle rate, q = Q = 1.5 m3·h−1.
2.2. Pilot Plant Operating Conditions and Testing Methods
2.2.1. Operating Conditions and Samplings
| Parameter | Unit | Value |
|---|---|---|
| Overall biological process (DEN + OX-NIT) sludge load | kg BOD5·d−1·kg−1 VSS | 0.068 |
| kg BOD5·d−1·kg−1 SS | 0.043 | |
| Denitrification (DEN) load | kg BOD5·d−1·kg−1 VSS | 0.204 |
| kg BOD5·d−1·kg−1 SS | 0.129 | |
| DEN retention time (for the whole inlet flowrate, Q + q + Qml) | h | 1.11 |
| OX-NIT retention time (for the whole inlet flowrate, Q + q + Qml) | h | 2.22 |
| DEN + OX-NIT retention time (for the whole inlet flowrate, Q + q + Qml) | h | 3.33 |
| Mixed-liquor temperature | °C | 15 |
- Automatic daily average samplings of the raw wastewater and the treated effluent;
- Manual instantaneous samplings (at 8 AM, 12 AM and 4 PM respectively) at the input and the output of the denitrification reactor as well as on the treated effluent.
2.2.2. Testing Methods
3. Results and Discussion
3.1. Raw Sewage Quality
3.1.1. Mean Values
| Parameter | Unit | Sampling time (1) | |||
|---|---|---|---|---|---|
| Daily average | 8.00 AM | 12.00 AM | 4.00 PM | ||
| CODin | [mg·L−1] | 226.5 | 89.9 | 372.7 | 324.6 |
| σ ± 60.1 | σ ± 21.3 | σ ± 135.2 | σ ± 74.6 | ||
| CODout | [mg·L−1] | 40.5 | 38.0 | 38.8 | 38.8 |
| σ ± 18.9 | σ ± 19.1 | σ ± 19.8 | σ ± 19.6 | ||
| BOD5 in | [mg·L−1] | 125.0 | 39.9 | 208.3 | 187.7 |
| σ ± 45.0 | σ ± 13.1 | σ ± 72.0 | σ ± 37.4 | ||
| BOD5 out | [mg·L−1] | 4.9 | 4.5 | 4.1 | 4.6 |
| σ ± 1.7 | σ ± 1.6 | σ ± 1.6 | σ ± 1.7 | ||
| TNin = TKNin | [mg·L−1] | 27.7 | 18.0 | 35.6 | 24.5 |
| σ ± 5.4 | σ ± 3.6 | σ ± 7.9 | σ ± 5.2 | ||
| TNout (*) | [mg·L−1] | 11.0 | 9.8 | 9.6 | 10.6 |
| σ ± 2.7 | σ ± 2.1 | σ ± 1.7 | σ ± 2.6 | ||
3.1.2. Daytime Variations
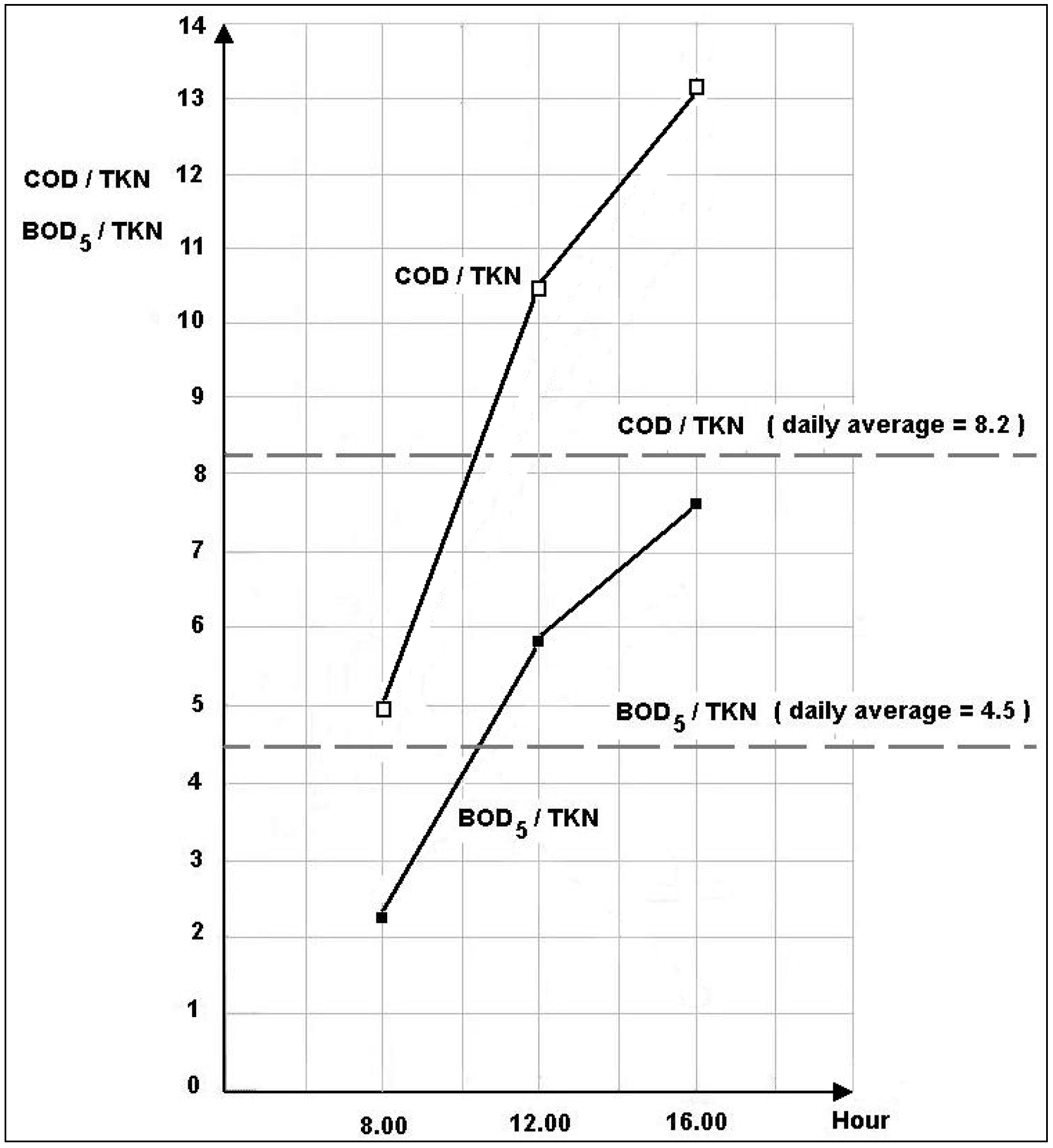
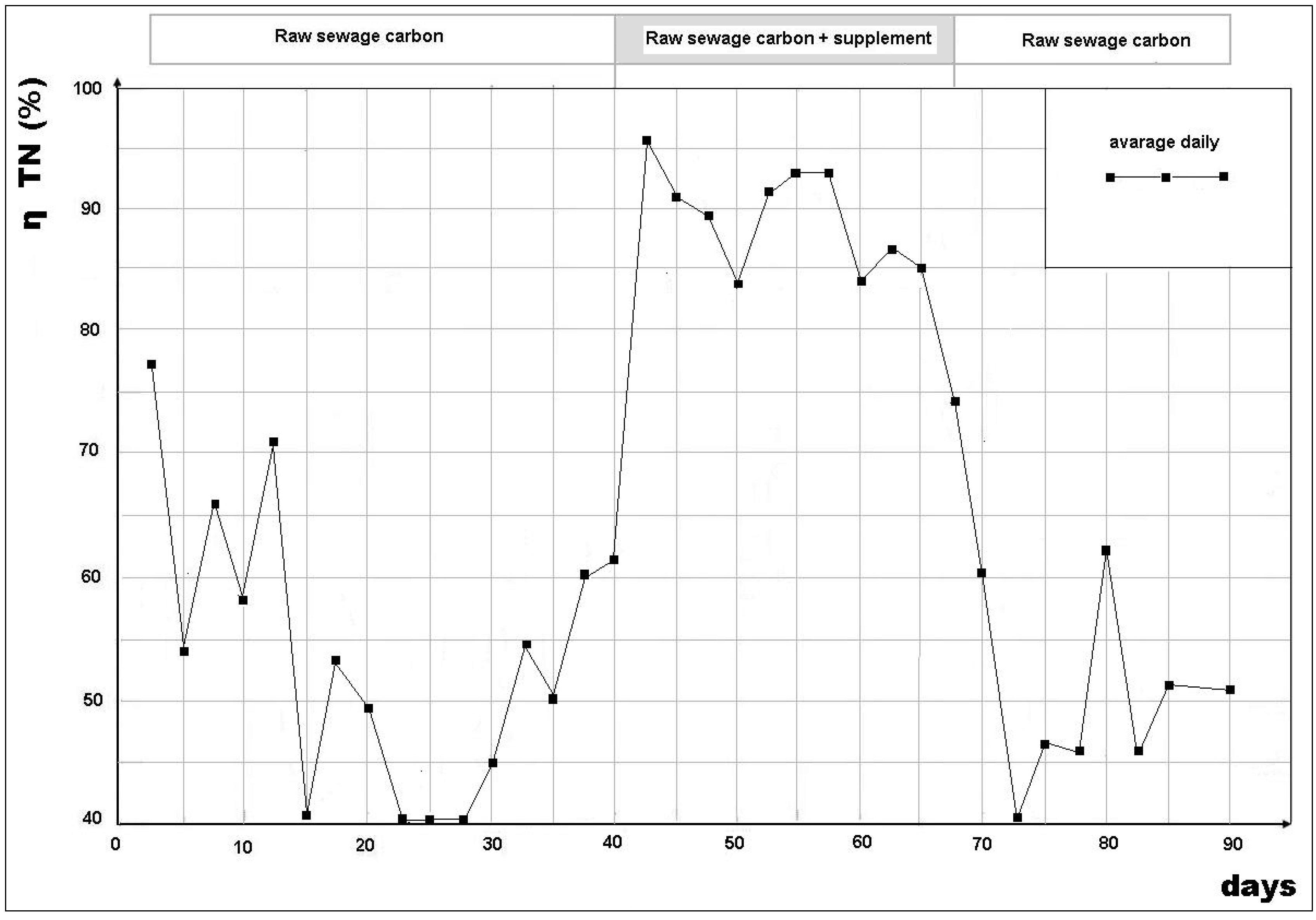
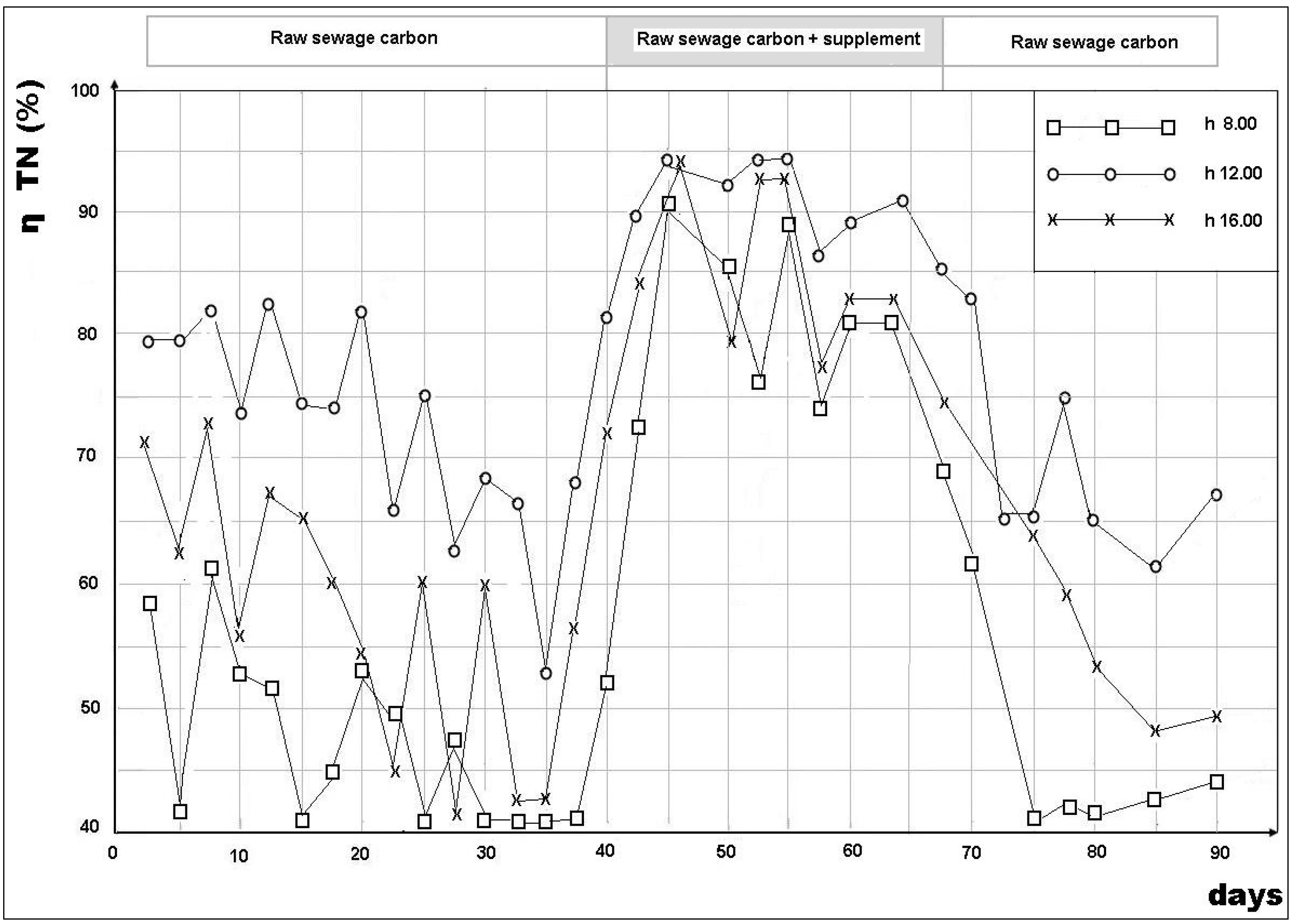
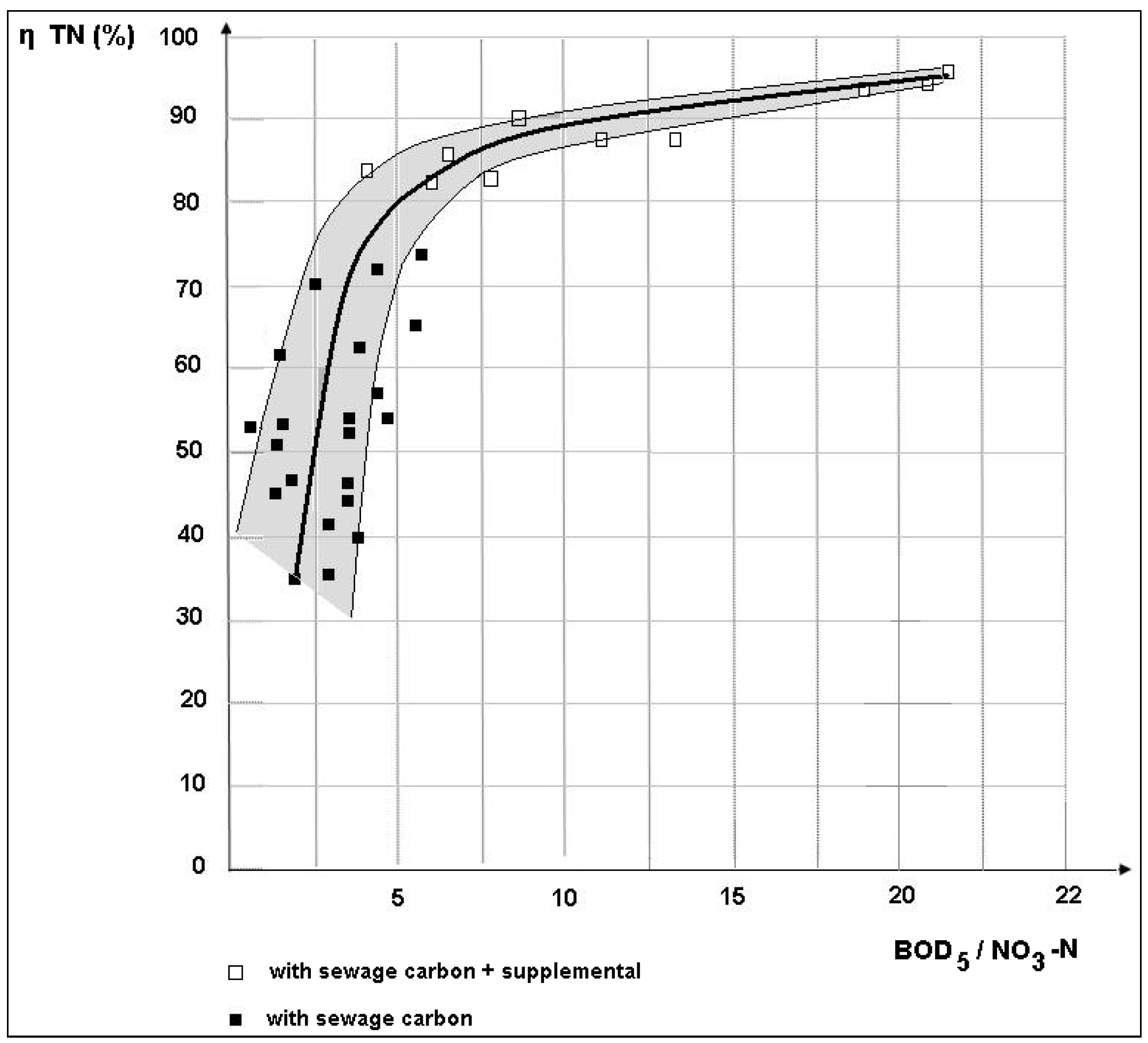
3.2. Removal Efficiency
- Daily mean BOD5/NO3-N = 3.78 (40 samples);
- 8.00 AM BOD5/NO3-N = 1.35 (40 samples);
- 12.00 AM BOD5/NO3-N = 7.2 (40 samples);
- 4.00 PM BOD5/NO3-N = 5.9 (40 samples).
3.3. Effect of Dissolved Oxygen in the Denitrification Reactor

- Kc = inhibition constant of dissolved oxygen (typical value: 0.02 mg·L−1)
- C = concentration of dissolved oxygen in denitrification (mg·L−1).
4. Conclusions
- The great variability in the BOD5/NO3-N ratio in the denitrification reactor, such that, at certain times of the day, there was a strong shortage of BOD5 for denitrification (BOD5/NO3-N = 1.75, recorded, on average, in the early morning) and, at other times, an excess of BOD5 with respect to the availability of NO3-N (BOD5/NO3-N = 7.2 achieved, on average, in the middle of the day);
- The considerable accumulation of oxygen in denitrification, mainly in the periods of lower BOD5 input at the beginning of the day and at night time (peak values of 1.2 mg·L−1) which induced inhibitory effects on the denitrification rate, which were significant at concentrations over 0.2 mg·L−1.
Conflicts of Interest
References
- United States Environmental Protection Agency, Process Design Manual for Upgrading Existing Wastewater Treatment Plant; Water Resource Center: Jefferson, MO, USA, 1974.
- United States Environmental Protection Agency, Wastewater Treatment Facilities for Sewered Small Communities—Process Design Manual; Water Resource Center: Jefferson, MO, USA, 1977.
- United States Environmental Protection Agency, Process Design Manual for Nitrogen Control; Water Resource Center: Jefferson, MO, USA, 1975.
- United States Environmental Protection Agency, Manual: Nitrogen Control; Water Resource Center: Jefferson, MO, USA, 1993.
- Cadmus Group, Nutrient Control Design Manual: State of Technology Review Report; The Cadmus Group, Inc.: Watertown, MA, USA; p. 2009.
- Cadmus Group, Nutrient Control Design Manual; The Cadmus Group, Inc.: Watertown, MA, USA, 2010.
- Tchobanoglous, F.L.; Burton, H.D.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; Metcalf & Eddy, Inc., Ed.; McGraw-Hill Professional: New York, NY, USA, 2003. [Google Scholar]
- Urbini, G. Advanced biological treatments for small communities. In Proceedings of the conference on WWTP for sewered small communities, Capri, Italy, 22–23 October 1992.
- Watanabe, Y.; Matsuda, S.; Ishiguro, M. Simultaneous nitrification and denitrification in micro-aerobic biofilms. Wat. Sci. Tech. 1992, 26, 511–522. [Google Scholar] [CrossRef]
- Yang, P.Y.; Zhang, Z. Nitrification and denitrification in the wastewater treatment system. In Proceedings of the UNESCO—University of Tsukuba International Seminar on Traditional Technology for Environmental Conservation and Sustainable Development in the Asian-Pacific Region, Tsukuba Science City, Japan, 11–14 December 1995.
- Wastewater treatment improvement and efficiency in small communities. Available online: http://www.wise-rtd.info/en/info/waste-water-treatment-improvement-and-efficiency-small-communities/ (accessed on 10 July 2013).
- Gernaey, K.V.; van Loosdrecht, M.C.M.; Henze, M.; Lind, M.; Jørgensen, S. Activated sludge wastewater treatment plant modelling and simulation: State of the art. Environ. Modell. Softw. 2004, 19, 763–783. [Google Scholar] [CrossRef]
- Joss, A.; Siegrist, H.; Ternes, T.A. Are we about to upgrade wastewater treatment for removing organic micropollutants? Water Sci. Technol. 2008, 57, 251–255. [Google Scholar] [CrossRef]
- Luciano, A.; Viotti, P.; Mancini, G.; Torretta, V. An integrated wastewater treatment system using a BAS reactor with biomass attached to tubular supports. J. Environ. Manage. 2012, 113, 51–60. [Google Scholar] [CrossRef]
- Hao, R.; Li, S.; Li, J.; Meng, C. Denitrification of simulated municipal wastewater treatment plant effluent using a three-dimensional biofilm-electrode reactor: Operating performance and bacterial community. Bioresource Technol. 2013, 143, 178–186. [Google Scholar] [CrossRef]
- Torti, E.; Sibilla, S.; Raboni, M. An Eulerian–Lagrangian method for the simulation of the oxygen concentration dissolved by a two-phase turbulent jet system. Comput. Struct 2013, in press. [Google Scholar]
- Ye, F.; Li, Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol. Eng. 2009, 35, 1043–1050. [Google Scholar] [CrossRef]
- Bonomo, L.; Pastorelli, G.; Zambon, N. Advantages and limitations of duckweed-based wastewater treatment systems. Water Sci. Technol. 1997, 35, 239–246. [Google Scholar]
- Pasquini, L.; Munoz, J.F.; Rimlinger, N.; Dauchy, X.; France, X.; Pons, M.N.; Görner, T. Assessment of the fate of some household in urban wastewater treatment plant micropollutants. Chem. Pap. 2013, 67, 601–612. [Google Scholar] [CrossRef]
- Torretta, V. PAHs in wastewater: Removal efficiency in a conventional wastewater treatment plant and comparison with model predictions. Environ. Technol. 2012, 33, 851–855. [Google Scholar] [CrossRef]
- Torretta, V.; Katsoyiannis, A. Occurrence of polycyclic aromatic hydrocarbons in sludges from different stages of a wastewater treatment plant in Italy. Environ. Technol. 2013, 34, 937–943. [Google Scholar] [CrossRef]
- Torretta, V.; Urbini, G.; Raboni, M.; Copelli, S.; Viotti, P.; Luciano, A.; Mancini, G. Effect of powdered activated carbon to reduce fouling in membrane bioreactors: A sustainable solution. Case study. Sustainability 2013, 5, 1501–1509. [Google Scholar] [CrossRef]
- Morris, M.W.; Su, S.K. Social psychological obstacles in environmental conflict resolution. Am. Behav. Sci. 1999, 42, 1322–1349. [Google Scholar] [CrossRef]
- Di Mauro, C.; Bouchon, S.; Torretta, V. Industrial risk in the Lombardy Region (Italy): What peopleperceive and what are the gaps to improve the risk communication and the partecipatory processes. Chem. Eng. Trans. 2012, 26, 297–302. [Google Scholar]
- IRSA-CNR. Available online: http://www.irsa.cnr.it/ShPage.php?lang=it&pag=metod/ (accessed on 10 July 2013).
- Lee, N.M.; Welander, T. The effect of different carbon sources on respiratory denitrification in biological wastewater treatment. J. Ferment. Bioeng. 1996, 82, 277–285. [Google Scholar] [CrossRef]
- Bonomo, L. Trattamento Delle Acque Reflue (Wastewater Treatment); McGraw Hill: Milan, Italy, 2008. [Google Scholar]
- Sutton, P.M.; Murphy, K.L.; Jank, B.E. Nitrogen control: A basis for design with activated sludges systems. In Proceedings of IAWPR Conference, Copenhagen, Denmark, 18–20 August 1975.
- Sutton, P.M.; Murphy, K.L.; Dawson, R.N. Low temperature biological denitrification of waste water. Water Pollut. Control Fed. 1975, 47, 122–134. [Google Scholar]
- Barnard, J.L. Biological denitrification. Water Pollut. Control 1975, 74, 178–186. [Google Scholar]
- Christensen, M.H.; Harremoes, P. A literature review of biological denitrification of sewage. In Proceedings of the International Nitrogen as a Water Pollutant, Copenhagen, Denmark, 18–20 August 1975.
- Rössle, W.H.; Pretorius, W.A. A review of characterization requirements for in-line prefermenters: Paper 1: Wastewater characterization. Water SA 2001, 27, 405–412. [Google Scholar]
- Cadmus Group US-EPA, Nutrient Control Design Manual Environmental Protection Development; The Cadmus Group, Inc.: Watertown, MA, USA, 2010.
- Song, S.H.; Choi, S.S.; Park, K.; Yoo, Y.J. Novel hybrid immobilization of microorganisms and its applications to biological denitrification. Enzyme Microb. Tech. 2005, 37, 567–573. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Raboni, M.; Torretta, V.; Urbini, G. Influence of Strong Diurnal Variations in Sewage Quality on the Performance of Biological Denitrification in Small Community Wastewater Treatment Plants (WWTPs). Sustainability 2013, 5, 3679-3689. https://doi.org/10.3390/su5093679
Raboni M, Torretta V, Urbini G. Influence of Strong Diurnal Variations in Sewage Quality on the Performance of Biological Denitrification in Small Community Wastewater Treatment Plants (WWTPs). Sustainability. 2013; 5(9):3679-3689. https://doi.org/10.3390/su5093679
Chicago/Turabian StyleRaboni, Massimo, Vincenzo Torretta, and Giordano Urbini. 2013. "Influence of Strong Diurnal Variations in Sewage Quality on the Performance of Biological Denitrification in Small Community Wastewater Treatment Plants (WWTPs)" Sustainability 5, no. 9: 3679-3689. https://doi.org/10.3390/su5093679
APA StyleRaboni, M., Torretta, V., & Urbini, G. (2013). Influence of Strong Diurnal Variations in Sewage Quality on the Performance of Biological Denitrification in Small Community Wastewater Treatment Plants (WWTPs). Sustainability, 5(9), 3679-3689. https://doi.org/10.3390/su5093679






