Extinction or Survival? Behavioral Flexibility in Response to Environmental Change in the African Striped Mouse Rhabdomys
Abstract
:1. Introduction
2. Species Response to Environmental Change: Adaptation and Phenotypic Flexibility
2.1. Adaptation
| Term | Definition |
| Evolutionary adaptation | Changes in gene frequencies at the population level over multiple generations [18] that increases species survival over time. |
| Phenotypic plasticity | The ability of an individual genotype to produce alternative phenotypes (morphological, behavioral, physiological) in response to prevailing environmental conditions [4]. There are two forms of phenotypic plasticity: developmental plasticity and phenotypic flexibility. |
| Developmental plasticity | Irreversible phenotypic variation originating early in development due to organizational effects and results in variation between individuals with a similar genotype [9]. Developmental plasticity can manifest in one of two ways: inherent resilience and adaptive resilience. |
| Phenotypic flexibility | Originates during an individual’s lifetime due to activational effects and results in reversible phenotypic variation in response to changing environmental conditions [28]. |
| Behavioral flexibility | Considers phenotypic flexibility of behavioral traits. It is the ability of an individual to alter its behavior reversibly in response to changing environmental conditions [29]. |
| Adaptive resilience | The ability of an organism to modify its phenotype under stable, predictable (Table 2), but fluctuating/dynamic circumstances due to prior experience [30,31]. Here, an individual’s genotype expresses variable phenotypes, in which genes have “biased” expression [32], in response to changing environments, resulting in widening of the reaction norm [33]. Although similar to inherent resilience, in adaptive resilience, gene expression is relaxed and facultative based on prevailing environmental conditions [32]. For example, in spade foot toad Scaphiopus species, tadpoles develop into one of two morphs: carnivore morphs feeding on shrimps develop a short gut whereas omnivores feeding on detritus have longer guts [34]. |
| Inherent resilience | The ability of an organism to modify its phenotype under normal circumstances [35]. This plasticity is possible because alternative alleles, controlled by a single genetic locus, express different phenotypes that may confer different benefits at particular times (i.e., genetic polymorphism [36]). For example, in populations of Atlantic salmon Salmo salar, males develop either “bourgeois” or “sneaker” tactics, depending on the timing of sexual maturity and their body size [37]. Inherent resilience is thus a fixed attribute of an individual. |
| The definitions of various terms, in particular “stability” and “predictability”, to explain patterns of variability have often been vague and differ significantly between authors [38]. We use the following definitions: | |
| Term | Definition |
| Homogeneous environments | Maintain a constant suite of environmental and ecological characteristics with no distinguishable gradient of variation (e.g., some parts wetter or drier than others [39]) in time or space [40]. |
| Heterogeneous environments | Show variation/disturbance over a spatial and/or temporal scale [40]. |
| Stable, predictable environments | Spatially and temporally homogeneous over the course of many generations, have low levels of disturbance (e.g., species invasions), promote evolutionary adaptation and are sustainable (e.g., through species coexistence) over long time periods. |
| Sustainable environments | Maintain their characteristic organismal diversity, biogeochemical cycling and productivity through a series of normal/cyclical environmental perturbations [41]. |
| Unstable, predictable environments | Spatially and/or temporally heterogeneous, with seasonal/cyclical changes experienced by populations over multiple generations, such that species show phenotypic plasticity in response to change. |
| Unstable, unpredictable environments | Spatially and/or temporally heterogeneous, with random, rare or sporadic environmental changes experienced by an individual over the course of its lifetime, such that species show phenotypic flexibility and, as a result, may only be sustainable in the short-term, if at all. |
2.2. Phenotypic Plasticity, Developmental Plasticity and Phenotypic Flexibility
3. Behavioral Flexibility
4. Social Flexibility: A Unique Type of Behavioral Flexibility
5. The Striped Mouse Rhabdomys: A Case Study
5.1. Taxonomy and Distribution
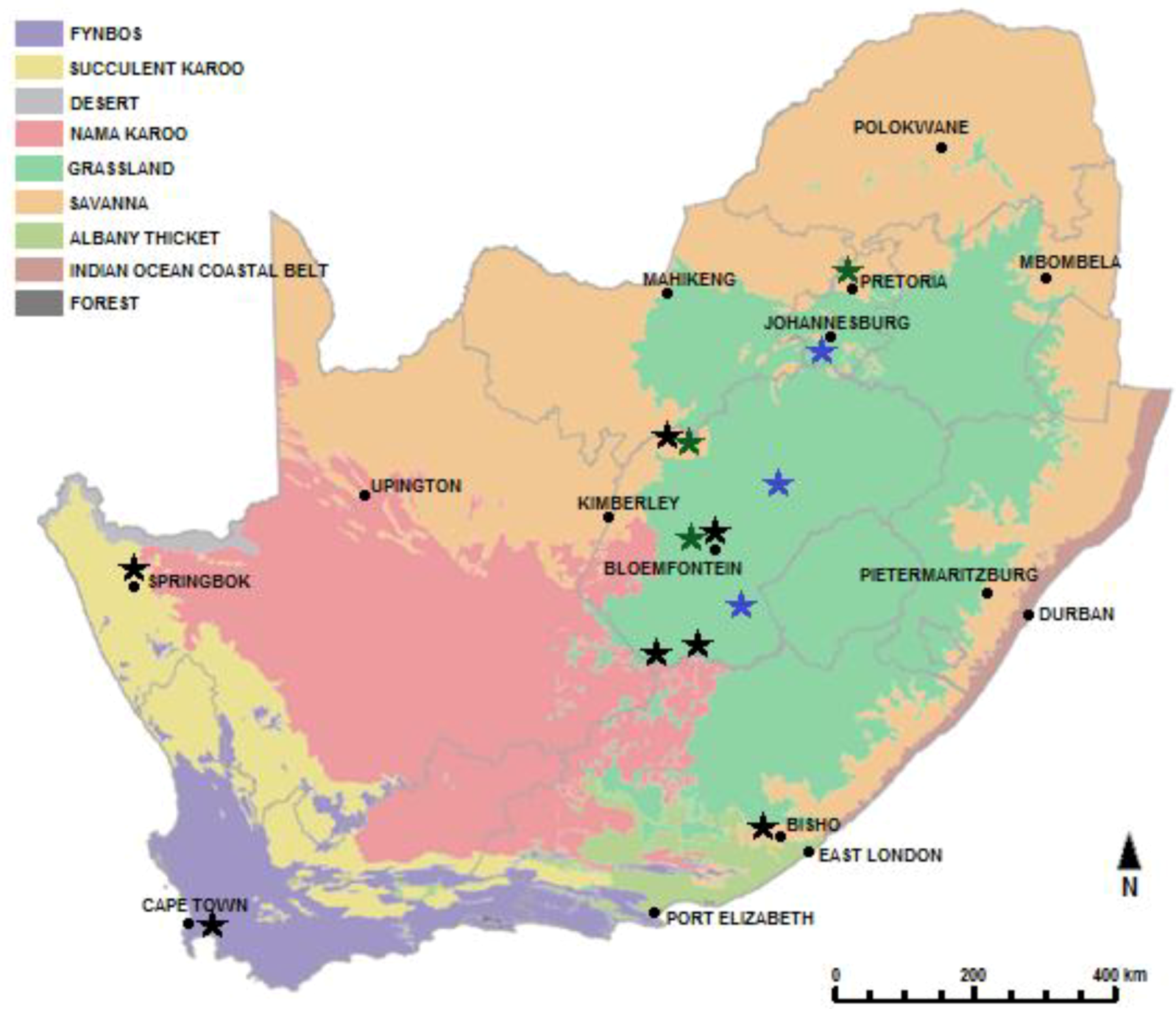
Behavior
Sociality
Social Flexibility in R. pumilio
6. The Value of Social Flexibility for Rhabdomys
7. Social Flexibility in R. dilectus?
8. Scenarios for Survival and Persistence of Rhabdomys during Impending Aridification in Southern Africa
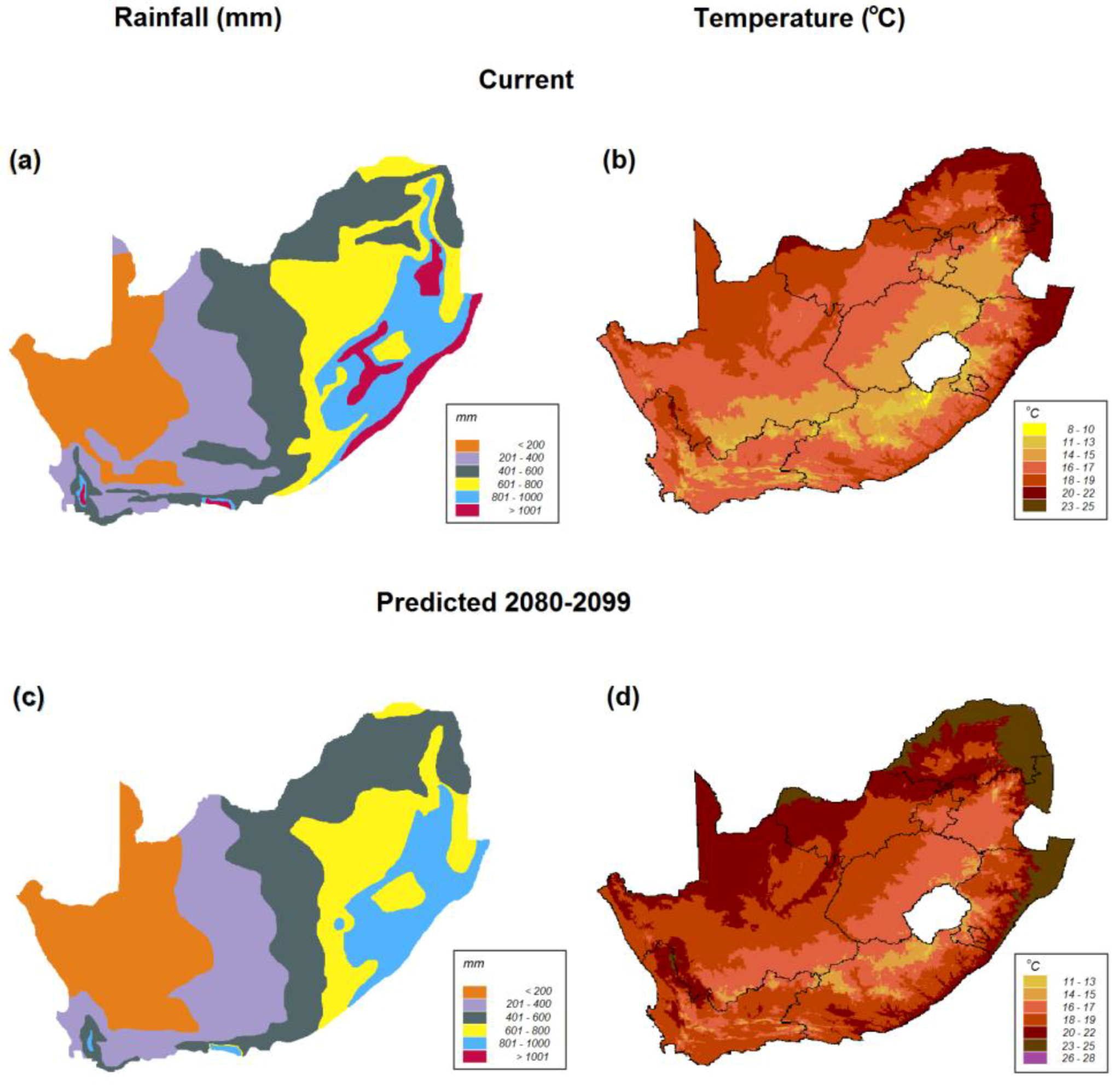
8.1. Scenario 1. R. pumilio Will become Extinct in Its Current Arid Distribution

8.2. Scenario 2. R. pumilio Will Displace R. dilectus in the East of Southern Africa
8.3. Scenario 3. R. dilectus Is Socially Flexible, Allowing for Continued Survival and Persistence
9. Conclusions
Acknowledgments
Conflict of Interest
References
- Zidanšek, A.; Blinc, R.; Jeglič, A.; Kabashi, S.; Bekteshi, S.; Šlaus, I. Climate changes, biofuels and the sustainable future. Int. J. Hydrogen Energ. 2009, 34, 6980–6983. [Google Scholar]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Sparks, T.H.; Frederiksen, M.; Burthes, S.; Bacon, P.J.; Bell, J.R.; Botham, M.C.; Brereton, T.M.; Bright, P.W.; Carvalhos, L.; et al. Trophic level asynchrony in rates of phonological change for marine, freshwater and terrestrial environments. Glob. Change Biol. 2010, 16, 3304–3313. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 1989, 20, 249–278. [Google Scholar]
- Przybylo, R.; Sheldon, B.C.; Merilä, J. Climate effects on breeding and morphology: Evidence for phenotypic plasticity. J. Anim. Ecol. 2000, 69, 395–403. [Google Scholar] [CrossRef]
- Chen, I-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Huey, R.B.; Hertz, P.E.; Sinervo, B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am. Nat. 2003, 161, 357–366. [Google Scholar] [CrossRef]
- Sinervo, B.; Losos, J.B. Walking the tight rope: arboreal sprint performance among Scleroporus occidentalis lizard populations. Ecology 1991, 72, 1225–1233. [Google Scholar] [CrossRef]
- Piersma, T.; van Gils, J.A. The Flexible Phenotype—A Body-Centred Integration of Ecology, Physiology and Behaviour; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Wilson, R.S.; Franklin, C.E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 2002, 17, 66–70. [Google Scholar] [CrossRef]
- Magistretti, P.J. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006, 209, 2304–2311. [Google Scholar] [CrossRef]
- Etterson, J.R.; Shaw, R.G. Constraint to adaptive evolution in response to global warming. Science 2001, 294, 151–154. [Google Scholar] [CrossRef]
- Alley, R.B.; Marotzke, J.; Nordhaus, W.D.; Overpeck, J.T.; Peteet, D.M.; Pielke, R.A., Jr.; Pierrehumbert, R.T.; Rhines, P.B.; Stocker, T.F.; Talley, L.D.; Wallace, J.M. Abrupt climate change. Science 2003, 299, 2005–2010. [Google Scholar]
- Friedlingstein, P. A steep road to climate stabilization. Nature 2008, 451, 297–298. [Google Scholar] [CrossRef]
- Sih, A.; Ferrari, M.C.O.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef]
- McLaughlin, J.F.; Hellmann, J.J.; Boggs, C.L.; Ehrlich, P.R. Climate change hastens population extinctions. Proc. Natl. Acad. Sci. USA 2002, 99, 6070–6074. [Google Scholar]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar]
- Rezende, E.L.; Diniz-Filho, J.A. Phylogenetic analyses: Comparing species to infer adaptations and physiological mechanisms. Compr. Physiol. 2012, 2, 639–674. [Google Scholar]
- Schluter, D. The Ecology of Adaptive Radiation; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Balmford, A.; Thomas, A.L.R.; Jones, I.L. Aerodynamics and the evolution of long tails in birds. Nature 1993, 361, 628–631. [Google Scholar] [CrossRef]
- Garland, T.Jnr.; Carter, P.A. Evolutionary Physiology. Annu. Rev. Physiol. 1994, 56, 579–621. [Google Scholar] [CrossRef]
- Huntley, B. The dynamic response of plants to environmental change and the resulting risks of extinction. In Conservation in a Changing World; Mace, G.M., Balmford, A., Ginsberg, J.R., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 69–85. [Google Scholar]
- Jaeger, R.G. Potential extinction through competition between two species of terrestrial salamanders. Evolution 1970, 24, 632–642. [Google Scholar] [CrossRef]
- Hendry, A.P.; Farrugia, T.J.; Kinnison, M.T. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 2008, 17, 20–29. [Google Scholar] [CrossRef]
- Stockwell, C.A.; Hendry, A.P.; Kinnison, M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003, 18, 94–101. [Google Scholar] [CrossRef]
- McNair, M.R. Heavy metal tolerance in plants: a model evolutionary system. Trends Ecol. Evol. 1987, 2, 354–359. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 1994, 39, 47–79. [Google Scholar] [CrossRef]
- Piersma, T.; Lindström, Å. Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol. Evol. 1997, 12, 134–138. [Google Scholar] [CrossRef]
- Gordon, D.M. Behavioral flexibility and the foraging ecology of seed-eating ants. Am. Nat. 1991, 138, 379–411. [Google Scholar]
- Stearns, S.C. The evolutionary significance of phenotypic plasticity. Bioscience 1989, 39, 436–445. [Google Scholar] [CrossRef]
- Scheiner, S.M. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 1993, 24, 35–68. [Google Scholar]
- Leichty, A.R.; Pfennig, D.W.; Jones, C.D.; Pfennig, K.S. Relaxed genetic constraint is ancestral to the evolution of phenotypic plasticity. Integr. Comp. Biol. 2012, 52, 16–30. [Google Scholar] [CrossRef]
- Via, S.; Gomulkiewicz, R.; De Jong, G.; Scheiner, S.M.; Schlichting, C.D.; van Tienderen, P.H. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 1995, 10, 212–217. [Google Scholar] [CrossRef]
- Ledón-Rettig, C.C.; Pfennig, D.W.; Nasconde-Yoder, N. Ancestral variation and the potential for genetic accommodation in larval amphibians: Implications for the evolution of novel feeding strategies. Evol. Dev. 2008, 10, 316–325. [Google Scholar] [CrossRef]
- Rose, A. Defining and measuring economic resilience to disasters. Dis. Prev. Manage. 2004, 13, 307–314. [Google Scholar]
- Potts, R. Variability selection in hominid evolution. Evol. Anthropol. 1998, 7, 81–96. [Google Scholar] [CrossRef]
- Páez, D.J.; Bernatchez, L.; Dodson, J.J. Alternative life histories in the Atlantic salmon: Genetic covariances within the sneaker sexual tactic in males. Proc. Roy. Soc. (London) B 2011, 278, 2150–2158. [Google Scholar] [CrossRef]
- Colwell, R.K. Predictability, constancy, and contingency of periodic phenomena. Ecology 1974, 55, 1148–1153. [Google Scholar] [CrossRef]
- Zonneveld, I.S. The land unit—A fundamental concept in landscape ecology, and its applications. Landscape Ecol. 1989, 3, 67–86. [Google Scholar] [CrossRef]
- Wiens, J.A. Chapter 2: Ecological heterogeneity: an ontogeny of concepts and approaches. In The Ecological Consequences of Heterogeneity; Hutchings, M.J., John, E.A., Stewart, A.J.A., Eds.; Blackwell Science: Oxford, UK, 2000; pp. 9–31. [Google Scholar]
- Chapin, F.S., III.; Torn, M.S.; Tateno, M. Principles of ecosystem sustainability. Am. Nat. 1996, 148, 1016–1037. [Google Scholar]
- Breed, M.F.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Clarifying climate change adaptation responses for scattered trees in modified landscapes. J. Appl. Ecol. 2011, 48, 637–641. [Google Scholar] [CrossRef]
- Hau, M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays 2007, 29, 133–144. [Google Scholar] [CrossRef]
- Moczek, A.P.; Sultan, S.; Foster, S.; Ledón-Rettig, C.; Dworkin, I.; Nijhout, H.F.; Abouheif, E.; Pfennig, D.W. The role of developmental plasticity in evolutionary innovation. Proc. Roy. Soc. (London) B 2011, 278, 2705–2713. [Google Scholar]
- Smith-Gill, S.J. Developmental plasticity: developmental conversion versus phenotypic modulation. Am. Zool. 1983, 23, 47–55. [Google Scholar]
- West-Eberhard, M.J. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B 2005, 304, 610–618. [Google Scholar] [CrossRef]
- Gienapp, P.; Teplitsky, C.; Alho, S.; Mills, J.A.; Merilä, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Elekonich, M.M.; Robinson, G.E. Organizational and activational effects of hormones on insect behavior. J. Insect Physiol. 2000, 46, 1509–1515. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Wan, J.; Jia, H. Resilience to natural hazards: a geographic perspective. Nat. Hazards 2010, 53, 21–41. [Google Scholar] [CrossRef]
- Champagne, F.A. Epigenetic influence of social experience across the lifespan. Dev. Psychobiol. 2010, 52, 299–311. [Google Scholar] [CrossRef]
- Vasanthi, D.; Mishra, R.K. Epigenetic regulation of genes during development: A conserved theme from flies to mammals. J. Genet. Genomics 2008, 35, 413–429. [Google Scholar] [CrossRef]
- Munsky, B.; Neuert, G.; van Oudenaarden, A. Using gene expression noise to understand gene regulation. Science 2012, 336, 183–187. [Google Scholar] [CrossRef]
- Wingfield, J.C. Control of behavioural strategies for capricious environments. Anim. Behav. 2003, 66, 807–816. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Bini, L.M. Macroecology, global change and the shadow of forgotten ancestors. Global Ecol. Biogeogr. 2008, 17, 11–17. [Google Scholar]
- Poisot, T.; Bever, J.D.; Nemri, A.; Thrall, P.H.; Hochberg, M.E. A conceptual framework for the evolution of ecological specialisation. Ecol. Lett. 2011, 14, 841–851. [Google Scholar] [CrossRef]
- Rhen, T.; Crews, D. Variation in reproductive behaviour within a sex: Neural systems and endocrine activation. J. Neuroendocrinol. 2002, 14, 517–531. [Google Scholar] [CrossRef]
- Duckworth, R.A. The role of behavior in evolution: a search for mechanism. Evol. Ecol. 2009, 23, 513–531. [Google Scholar] [CrossRef]
- Mery, F.; Burns, J.G. Behavioural plasticity: an interaction between evolution and experience. Evol. Ecol. 2010, 24, 571–583. [Google Scholar] [CrossRef]
- Reader, S.M.; Laland, K.N. Social intelligence, innovation, and enhanced brain size in primates. P. Natl. Acad. Sci. USA 2002, 99, 4436–4441. [Google Scholar] [CrossRef]
- Sol, D.; Duncan, R.P.; Blackburn, T.M.; Cassey, P.; Lefebvre, L. Big brains, enhanced cognition, and response of birds to novel environments. P. Natl. Acad. Sci. USA 2005, 102, 5460–5465. [Google Scholar]
- Sol, D.; Timmermans, S.; Lefebvre, L. Behavioural flexibility and invasion success in birds. Anim. Behav. 2002, 63, 495–502. [Google Scholar] [CrossRef]
- Berrigan, D.; Scheiner, S.M. Modeling the Evolution of Phenotypic Plasticity. In Phenotypic Plasticity: Functional and Conceptual Approaches; DeWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 82–97. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Relyea, R.A. The relationship between predation risk and antipredator responses in larval anurans. Ecology 2001, 82, 541–554. [Google Scholar] [CrossRef]
- Lucas, É.; Coderre, D.; Brodeur, J. Intraguild predation among aphid predators: characterization and influence of extraguild prey density. Ecology 1998, 79, 1084–1092. [Google Scholar] [CrossRef]
- Bürger, R.; Lynch, M. Evolution and extinction in a changing environment: A quantitative-genetic analysis. Evolution 1995, 49, 151–163. [Google Scholar] [CrossRef]
- Kinnison, M.T.; Hairston, N.G., Jnr. Eco-evolutionary conservation biology: Contemporary evolution and the dynamics of persistence. Funct. Ecol. 2007, 21, 441–454. [Google Scholar]
- West-Eberhard, M.J. Developmental Plasticity and the Origin of Species Differences; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Sol, D.; Lefebvre, L.; Rodriguez-Teijeiro, J.D. Brain size, innovative propensity and migratory behaviour in temperate Palearctic birds. Proc. Roy. Soc. (London) B 2005, 272, 1433–1441. [Google Scholar] [CrossRef]
- Schradin, C.; Lindholm, A.K.; Johannesen, J.; Schoepf, I.; Yuen, C-H.; König, B.; Pillay, N. Social flexibility and social evolution in mammals: a case study of the African striped mouse (Rhabdomys pumilio). Mol. Ecol. 2012, 21, 541–553. [Google Scholar]
- Schradin, C.; Lindholm, A.K. Relative fitness of alternative male reproductive tactics in a mammal varies between years. J. Anim. Ecol. 2011, 80, 908–917. [Google Scholar] [CrossRef]
- Eggert, A.-K. Alternative male mate-finding tactics in burying beetles. Behav. Ecol. 1992, 3, 243–254. [Google Scholar]
- Müller, J.F.; Braunisch, V.; Hwang, W.; Eggert, A.-K. Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 2006, 18, 196–203. [Google Scholar] [CrossRef]
- Davies, N.B. Dunnock Behaviour and Social Evolution; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Berry, R.J.; Tattersall, F.H.; Hurst, J. Genus Mus. In Mammals of the British Isles Handbook, 4th; Harris, S., Yalden, D.W., Eds.; The Mammal Society: Southampton, UK, 2008; pp. 141–149. [Google Scholar]
- McGuire, B.; Getz, L.L. The nature and frequency of social interactions among free-living prairie voles (Microtus ochrogaster). Behav. Ecol. Sociobiol. 1998, 43, 271–279. [Google Scholar] [CrossRef]
- Randall, J.A.; Rogovin, K.; Parker, P.G.; Eimes, J.A. Flexible social structure of a desert rodent, Rhombomys opimus: philopatry, kinship, and ecological constraints. Behav. Ecol. 2005, 16, 961–973. [Google Scholar] [CrossRef]
- Skinner, J.D.; Chimimba, C.T. The Mammals of the Southern African Subregion; Cambridge University Press: Cape Town, South Africa, 2005. [Google Scholar]
- Perrin, M.R. The feeding habits of two coexisting rodents, Rhabdomys pumilio (Sparrman, 1784) and Otomys irroratus Brants 1827 in relation to rainfall and reproduction. Acta Oecol. 1980, 1, 71–89. [Google Scholar]
- Schradin, C.; Pillay, N. The striped mouse (Rhabdomys pumilio) from the Succulent Karoo, South Africa: A territorial group-living solitary forager with communal breeding and helpers at the nest. J. Comp. Psychol. 2004, 118, 37–47. [Google Scholar] [CrossRef]
- Rambau, R.V.; Robinson, T.J.; Stanyon, R. Molecular genetics of Rhabdomys pumilio subspecies boundaries: mtDNA phylogeography and karyotypic analysis by fluorescence in situ hybridization. Mol. Phylogenet. Evol. 2003, 28, 564–575. [Google Scholar] [CrossRef]
- Du Toit, N.; van Vuuren, B.J.; Matthee, S.; Matthee, C.A. Biome specificity of distinct genetic lineages within the four-striped mouse Rhabdomys pumilio (Rodentia: Muridae) from Southern Africa with implications for taxonomy. Mol. Phylogenet. Evol. 2012, 65, 75–86. [Google Scholar] [CrossRef]
- Meynard, C.N.; Pillay, N.; Perrigault, M.; Caminade, P.; Ganem, G. Evidence of environmental niche differentiation in the striped mouse (Rhabdomys sp.): Inference from its current distribution in southern Africa. Ecol. Evol. 2012, 2, 1008–1023. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland. Strelitzia 2006, 19, 540–567. [Google Scholar]
- Jackson, T.P. The social organization and breeding system of Brants’ whistling rat (Parotomys brantsii). J. Zool. 1999, 247, 323–331. [Google Scholar] [CrossRef]
- Schradin, C.; Pillay, N. Intraspecific variation in the spatial and social organization of the African striped mouse. J. Mammal. 2005, 86, 99–107. [Google Scholar] [CrossRef]
- Lynch, C.D. The Mammals of the Orange Free State; National Museum Bloemfontein: Bloemfontein, South Africa, 1983. [Google Scholar]
- Taylor, K.D.; Green, M.G. The influence of rainfall on diet and reproduction in four African rodent species. J. Zool. 1976, 180, 367–389. [Google Scholar] [CrossRef]
- Schradin, C. When to live alone and when to live in groups: ecological determinants of sociality in the African striped mouse (Rhabdomys pumilio, Sparrman, 1784). Belg. J. Zool. 2005, 135, 77–82. [Google Scholar]
- Willan, K.; Meester, J. Life-History Styles of Southern African Mastomys natalensis, Otomys irroratus and Rhabdomys pumilio (Mammalia, Rodentia). In Alternative Life-History Styles of Animals; Bruton, M.N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 421–439. [Google Scholar]
- Schradin, C.; Schubert, M.; Pillay, N. Winter huddling groups in the striped mouse. Can. J. Zoolog. 2006, 117, 317–324. [Google Scholar]
- Rymer, T.; Schradin, C.; Pillay, N. Social transmission of information about novel food in two populations of the African striped mouse, Rhabdomys pumilio. Anim. Behav. 2008, 76, 1297–1304. [Google Scholar] [CrossRef]
- Rymer, T.L.; Pillay, N. The development of exploratory behaviour in the African striped mouse Rhabdomys reflects a gene x environment compromise. Behav. Genet. 2012, 42, 845–856. [Google Scholar] [CrossRef]
- Schradin, C.; Pillay, N. Paternal care in the social and diurnal striped mouse (Rhabdomys pumilio): Laboratory and field evidence. J. Comp. Psychol. 2003, 117, 317–324. [Google Scholar] [CrossRef]
- Ganem, G.; Meynard, C.N.; Perigault, M.; Lancaster, J.; Edwards, S.; Caminade, P.; Watson, J.; Pillay, N. Environmental correlates and co-occurrence of three mitochondrial lineages of striped mice (Rhabdomys) in the Free State Province (South Africa). Acta Oecol. 2012, 42, 30–40. [Google Scholar] [CrossRef]
- Schradin, C. Nest-site competition in two diurnal rodents from the Succulent Karoo of South Africa. J. Mammal. 2005, 86, 757–762. [Google Scholar] [CrossRef]
- Scantlebury, M.; Bennett, N.C.; Speakman, J.R.; Pillay, N.; Schradin, C. Huddling in groups leads to daily energy savings in free-living African four-striped grass mice, Rhabdomys pumilio. Funct. Ecol. 2006, 20, 166–173. [Google Scholar] [CrossRef]
- Schradin, C. Territorial defense in a group living solitary forager: who, where, against whom? Beha. Ecol. Sociobiol. 2004, 55, 439–446. [Google Scholar] [CrossRef]
- Schradin, C.; Pillay, N. The influence of the father on offspring development in the striped mouse. Behav. Ecol. 2005, 16, 450–455. [Google Scholar] [CrossRef]
- Brooks, P.M. The Ecology of the Four-Striped Field Mouse, Rhabdomys pumilio (Sparrman, 1784), with Particular Reference to a Population on the Van Riebeeck Nature Reserve, Pretoria. PhD dissertation, University of Pretoria, South Africa, 1974. [Google Scholar]
- Brooks, P.M. Aspects of the reproduction, growth and development of the four-striped mouse, Rhabdomys pumilio (Sparrman, 1784). Mammalia 1982, 46, 53–64. [Google Scholar]
- Schradin, C.; Pillay, N. Demography of the striped mouse (Rhabdomys pumilio) in the Succulent Karoo. Mamm. Biol. 2005, 70, 84–92. [Google Scholar] [CrossRef]
- Schradin, C.; König, B.; Pillay, N. Reproductive competition favours solitary living while ecological constraints impose group-living in African striped mice. J. Anim. Ecol. 2010, 79, 515–521. [Google Scholar] [CrossRef]
- Schubert, M.; Pillay, N.; Schradin, C. Parental and alloparental care in a polygynous mammal. J. Mammal. 2009, 90, 724–731. [Google Scholar] [CrossRef]
- Schradin, C.; Scantlebury, M.; Pillay, N.; König, B. Testosterone levels in dominant sociable males are lower than in solitary roamers: Physiological differences between three male reproductive tactics in a socially flexible mammal. Am. Nat. 2009, 173, 376–388. [Google Scholar] [CrossRef]
- Schradin, C.; Schneider, C.; Yuen, C.H. Age at puberty in male African striped mice: the impact of food, population density and the presence of the father. Funct. Ecol. 2009, 23, 1004–1013. [Google Scholar] [CrossRef]
- Schradin, C.; Schneider, C.; Lindholm, A.K. The nasty neighbour in the striped mouse (Rhabdomys pumilio) steals paternity and elicits aggression. Front. Zool. 2010, 7, 19. [Google Scholar] [CrossRef]
- Meylan, S.; Miles, D.B.; Clobert, J. Hormonally mediated maternal effects, individual strategy and global change. Phil. Trans. R. Soc. B 2012, 367, 1647–1664. [Google Scholar] [CrossRef]
- Schradin, C. Differences in prolactin levels between three alternative male reproductive tactics in striped mice (Rhabdomys pumilio). P. Roy. Soc. Lond. B Bio. 2008, 275, 1047–1052. [Google Scholar] [CrossRef]
- Schradin, C.; Yuen, C.-H. Hormone levels of male African striped mice change as they switch between alternative reproductive tactics. Horm. Behav. 2011, 60, 676–680. [Google Scholar] [CrossRef]
- Rymer, T.L.; Pillay, N. The influence of the early rearing environment on the development of paternal care in African striped mice. Ethology 2011, 117, 284–293. [Google Scholar] [CrossRef]
- Mackay, M.K. The Behaviour of two Sub-Species of the Striped Mouse Rhabdomys: The Role of Phylogeny and the Environment. MSc dissertation, University of the Witwatersrand, South Africa, 2011. [Google Scholar]
- Schradin, C.; Kinahan, A.A.; Pillay, N. Cooperative breeding in groups of synchroneously mating females and evolution of large testes to avoid sperm depletion in African striped mice. Biol. Reprod. 2009, 81, 111–117. [Google Scholar] [CrossRef]
- Kinahan, A.A.; Pillay, N. Dominance status influences female reproductive strategy in a territorial African rodent Rhabdomys pumilio. Behav. Ecol. Sociobiol. 2008, 62, 579–587. [Google Scholar] [CrossRef]
- Collier, P.; Conway, G.; Venables, T. Climate change and Africa. Oxford Rev. Econ. Pol. 2008, 24, 337–353. [Google Scholar] [CrossRef]
- Hudson, D.A.; Jones, R.G. Simulations of Present-Day and Future Climate over Southern Africa using HadAM3H; Hadley Cent. Tech. Note 38; The Meteorological Office: Exeter, UK, 2002. [Google Scholar]
- Schär, C.; Vidale, P.L.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar]
- Tropical Data Hub. Wallace Initiative. Available online: http://wallaceinitiative.org/wallace/demo/taxonomies (accessed on 19 October 2012).
- Uploader, S. Settlement on Agricultural Ground. Available online: http://cnx.org/content/m22344/1 (accessed on 14 October 2012).
- Department of Environmental Affairs and Tourism. About South Africa. Available online: http://www.calflora.net/southafrica/temperature.html (accessed on 19 October 2012).
- Green, M.W.; Rogers, P.J.; Ellman, N.A.; Gatenby, S.J. Impairment of cognitive performance associated with dieting and high levels of dietary restraint. Physiol. Behav. 1994, 55, 447–452. [Google Scholar] [CrossRef]
- Midgley, G.F.; Hannah, L.; Millar, D.; Thuiller, W.; Booth, A. Developing regional and species-level assessments of climate change impacts on biodiversity in the Cape Floristic Region. Biol. Conserv. 2003, 112, 87–97. [Google Scholar] [CrossRef]
- Van Jaarsveld, A.S.; Chown, S.L. Climate change and its impacts in South Africa. Trends Ecol. Evol. 2001, 16, 13–14. [Google Scholar] [CrossRef]
- Dukas, R. Costs of memory: Ideas and predictions. J. Theor. Biol. 1999, 197, 41–50. [Google Scholar] [CrossRef]
- Gonzalez, A.; Ronce, O.; Ferriere, R.; Hochberg, M.E. Evolutionary rescue: an emerging focus at the intersection between ecology and evolution. Phil. Trans. R. Soc. B 2012, 368. [Google Scholar]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. P. Roy. Soc. Lond. B Bio. 2000, 267, 1947–1952. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Ferreira de Siqueira, M.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rymer, T.L.; Pillay, N.; Schradin, C. Extinction or Survival? Behavioral Flexibility in Response to Environmental Change in the African Striped Mouse Rhabdomys. Sustainability 2013, 5, 163-186. https://doi.org/10.3390/su5010163
Rymer TL, Pillay N, Schradin C. Extinction or Survival? Behavioral Flexibility in Response to Environmental Change in the African Striped Mouse Rhabdomys. Sustainability. 2013; 5(1):163-186. https://doi.org/10.3390/su5010163
Chicago/Turabian StyleRymer, Tasmin L., Neville Pillay, and Carsten Schradin. 2013. "Extinction or Survival? Behavioral Flexibility in Response to Environmental Change in the African Striped Mouse Rhabdomys" Sustainability 5, no. 1: 163-186. https://doi.org/10.3390/su5010163
APA StyleRymer, T. L., Pillay, N., & Schradin, C. (2013). Extinction or Survival? Behavioral Flexibility in Response to Environmental Change in the African Striped Mouse Rhabdomys. Sustainability, 5(1), 163-186. https://doi.org/10.3390/su5010163




