Evolutionary Plant Breeding in Cereals—Into a New Era
Abstract
: In evolutionary plant breeding, crop populations with a high level of genetic diversity are subjected to the forces of natural selection. In a cycle of sowing and re-sowing seed from the plant population year after year, those plants favored under prevailing growing conditions are expected to contribute more seed to the next generation than plants with lower fitness. Thus, evolving crop populations have the capability of adapting to the conditions under which they are grown. Here we review the current state of research in evolutionary plant breeding and concentrate on the ability of evolving plant populations to deal with stressful, variable, and unpredictable environments. This resilience of evolving plant populations is seen as a major advantage under the predicted threats faced by agriculture such as global climate change. We have conducted an analysis of the strengths, weaknesses, opportunities and threats of this breeding approach and suggest how its concept can be broadened and expanded. Given the current legal restrictions for realizing the potential of evolutionary plant breeding, we call for a change in legislation to allow evolving crop populations to enter agricultural practice on a larger scale.1. Introduction
The amount and nature of the food to be provided by the year 2050, when the human population is forecast to peak, is a matter of debate. Less contentious, however, is that even to maintain production of major staples such as wheat will be difficult because of serious constraints [1]. These include climate instability [2], the impact of climate change on plant pests and diseases [3] and the decline of non-renewable resources [4]. In addition, there is mounting pressure expected from increasing biotic stresses, as intensifying global trade and mobility aggravates the risks of pest, weed or pathogen species becoming invasive [5]. At the same time, resources that are needed to mitigate and counter-balance these pressure factors are predicted to get scarcer and to rise in costs [1]. Given the combination of these pressure factors, the stakes are high in the debates about which coping strategy is most appropriate for the future of agricultural production [6,7].
What is the current approach of conventional plant breeding in the attempt to tackle these problems? Since the response of plants to stress factors varies among different genotypes the prevailing strategy is to exploit this variation by selecting those genotypes that are best adapted to the stress factor in question, e.g., through heritable drought resistance [8], and to combine these traits via crossing with other desirable traits. By means of repeated backcrossings and other techniques, genetically uniform crop cultivars are produced with the appropriate combination of traits that allow the plants to perform well under stressful environmental conditions. Through their unchanging appearance over generations, these cultivars demonstrate what, under plant variety legislation, is termed ‘stability’ [9]. This genetically dominated legal meaning of stability contrasts with the use of the term in agronomy, where various statistical concepts of stability focus on how little the yield or quality of a crop varies over locations or time [10-12].
However, as we argue in this paper, the approach of creating uniform and genetically ‘stable’ cultivars that are deployed over large areas in monocultures is inappropriate for dealing with the current and predicted threats to agriculture. The response of these genetically uniform cultivars is not buffered against environmental fluctuations and novel stress factors when the direction and range of environmental changes are highly unpredictable [13]. Although well equipped to cope with one particular stress, they may not be able to cope with other stresses in a changing or fluctuating environment. In view of such variability, we argue in this paper that wider adaptation is needed, and that this can be achieved via extended intra-specific plant diversity.
If the aim is indeed to increase crop diversity in the field there are two general options: First, farmers and breeders could create diversity anew each year, e.g., by buying seed from, say, ten different varieties and sowing a mixture with the same proportions of all varieties every year. Second, in the case of cereals or grain legumes, sub-samples of harvested grain could be saved and subsequently sown in the following cropping season, year after year. In this second scenario, plants that have produced more seed will contribute more to next year's seed. Saving and re-sowing seed over several plant generations may therefore favour a gradual increase in reproductive fitness of the plant population through Darwinian selection. This approach, termed evolutionary breeding, generates plant populations that are neither uniform nor ‘stable’ (in the legislative sense); on the contrary, they are genetically highly diverse and changing in their genetic constitution over time.
In this review we use the word population (or, as synonyms, ‘plant population’ or ‘crop population’) as a general term for a (large) number of plants in one location (field), in which individual plants are not genetically identical to each other. Two special cases of populations are Composite Cross Populations (CCPs) and varietal mixtures, determined by the way in which they were created, i.e., by crossing in the case of CCPs, and by physical mixing seed of existing varieties in the case of varietal mixtures. As and when the frequencies of different genotypes in the population will change from season to season, depends on the genetic variation available and the strength and direction of environmental variables, the CCPs and varietal mixtures are evolving populations.
In this sense, CCPs and varietal mixtures are similar to traditional landraces [14,15], which are also populations of cultivated plants. Therefore CCPs have been called ‘modern landraces’ [16]. However, although there is often genetic diversity within landraces, this is not necessarily the case; also, landraces are characterised by additional properties, such as historical origin, lack of formal crop improvement, and association with traditional farming systems [15]. Here we concentrate on CCPs and varietal mixtures, although many of the considerations will also apply to genetically diverse landraces.
Since the classical study on evolutionary plant breeding was published more than half a century ago [17], and despite the prolific research conducted by Robert W. Allard and co-workers [18-20] evolutionary breeding techniques have not yet found their way into mainstream commercial breeding practice [21]. However, in recent years there has been a marked surge of interest from both plant scientists and breeders in this area (Table 1).
In this paper, we explore the potential benefits and drawbacks of evolutionary breeding and provide a summary of its current state. We look beyond the present developments and suggest how evolutionary plant breeding can expand and move on to contribute to solutions to the pressing problems of agriculture. As the instrument for evaluating the current state of affairs we use a SWOT analysis, listing and discussing the Strengths, Weaknesses, Opportunities and Threats of evolutionary plant breeding with and without the use of natural selection (evolutionary breeding), and comparing these approaches with the use of monocultures.
2. Theoretical Foundations of Evolutionary Breeding
2.1. The Stages of Evolutionary Breeding
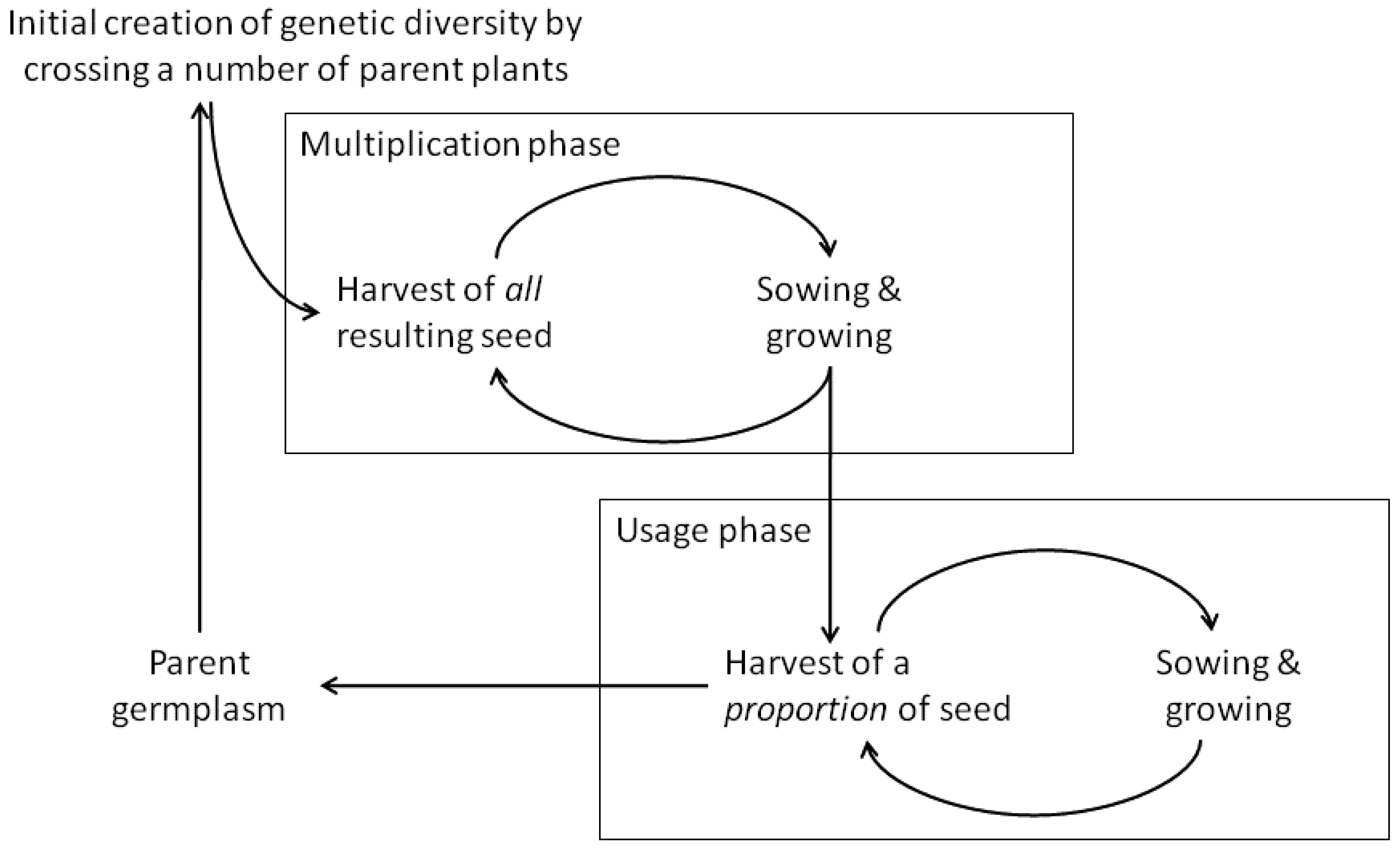
Evolutionary breeding involves four stages (Figure 1). In the first stage, which in principle is not different from conventional breeding, genetic diversity is created, e.g., by hand crossing parent plants or by mixing multiple cultivars. The second stage is a cycle of multiplication of seeds from each cross or from varieties separately; seeds of each cross are then equally mixed to produce the first generation of the CCP. After the initial crosses, the entire offspring is sown to grow and set seed. The result is then a crop population, which, in the case of generating it via crosses, is called a Composite Cross Population (CCP). In the third stage, as the number of plants in the population increases, a proportion of the harvested seed is saved for sowing, again without active selection of individual plants. The fourth stage concerns the output of the evolutionary breeding process: while the grain can be used as food or feed, it can also be used to provide input into plant breeding, via selecting single plants that can be used as new genetic material in further breeding programs or as parental material for new composite crosses or other evolving populations.
2.2. Effects of Genetic Diversity in a Non-Evolving Crop
Genetic diversity is a central requirement for evolutionary breeding. However, not all genetically diverse crops ‘need’ to evolve. While CCPs cannot be reconstituted to the same specifications each year, this would, as pointed out above, be possible for varietal mixtures. For the sake of clarity of the argument we first consider the effects of diversity per se, in a diverse but non-evolving crop population and then move on to explore the additional effects of evolution in the next section.
Crop diversity has four major beneficial effects. The first is complementation. When genotypes grown together in a diverse population have different profiles of resource use they complement each other in the exploitation of the limiting resource and therefore are subject to smaller between-plant competition. Such effects can be realised by mixing genotypes of different rooting patterns (shallow vs. deep), or different light interception strategies. In a similar vein, mixing genotypes with complementary resistance patterns exerts less selection pressure on pathogens and thus reduces the risk of new virulent strains appearing [22].
The second effect of genetic diversity can be termed cooperation and involves phenomena such as induced resistance against plant pathogens. For example, in the relationship between cultivated barley (Hordeum vulgare L.) and the cherry-oat aphid (Rhopalosiphum padi L.), some barley genotypes can produce volatiles which induce other barley genotypes to become more resistant to pest attack [23]. Also, with greater distance between susceptible genotypes there is a reduced dispersal of pathogens through the plant stand [22].
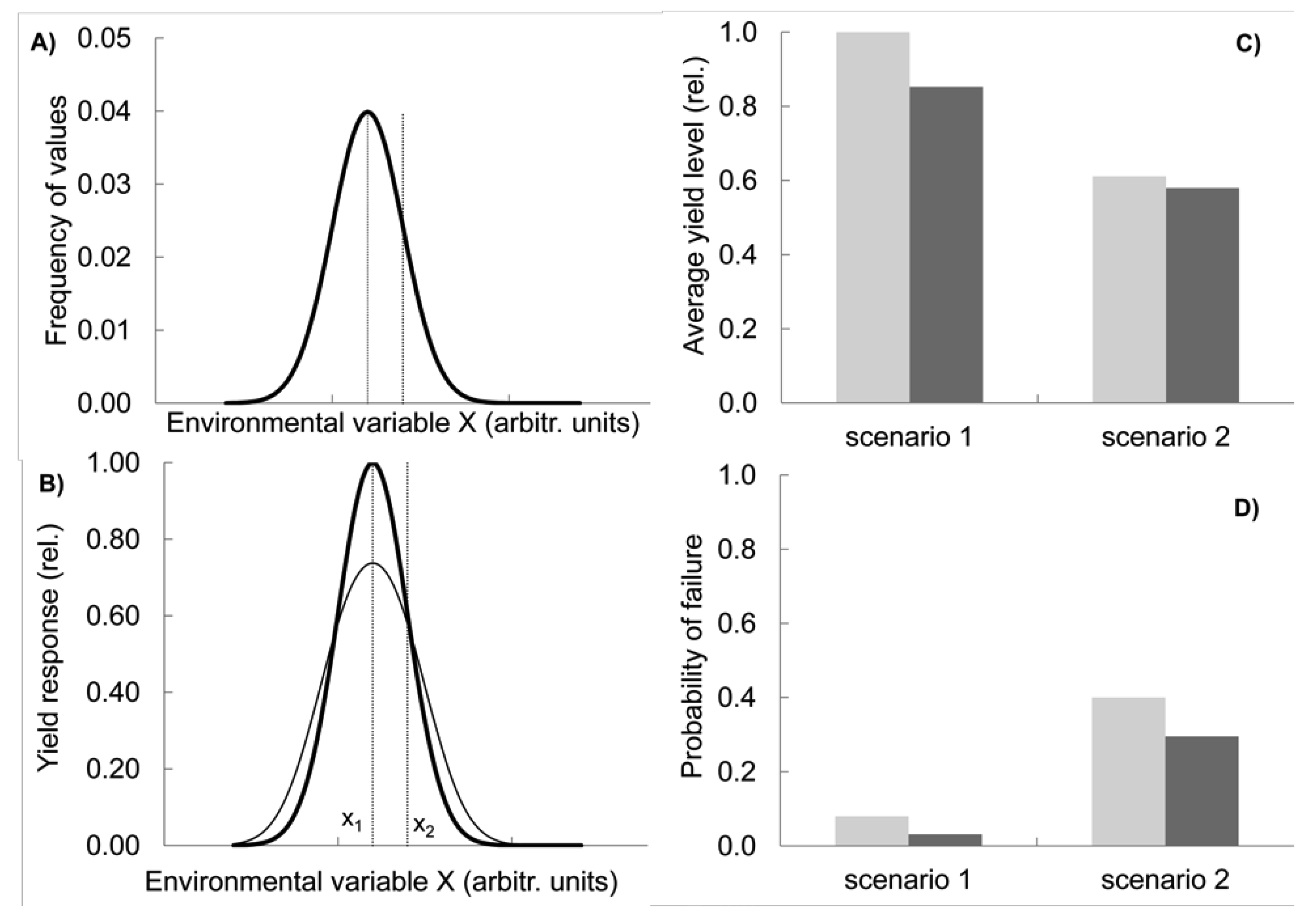
The other two effects are realised in two contrasting situations and can be summarized as compensation and capacity. Let us first consider compensatory effects. Imagine a variable environment in which a key environmental variable X (e.g., temperature at flowering) has a given frequency distribution with a mean value of x1 (scenario 1, shown by the bold line in Figure 2(A); modelling methods see Appendix, note 1). In this situation, a genotype G1 is best adapted on the long term, with its maximum performance coinciding with the value x1 at which the environmental variable peaks in frequency (bold line, Figure 2(B)).
Now consider that the environment changes, with the mean temperature shifting towards hotter, i.e., more stressful conditions (x2 in Figure 2(A)). Simultaneously, its variance increases, resulting in a broader frequency distribution function, i.e., also the frequency of cold conditions is higher than before (scenario 2, fine line in Figure 2(A)). These assumptions reflect predictions of climate change research about regionally changing means and increasing variance [24]. As a consequence of the shift and broadening of the distribution function, the overall performance of G1, measured as the average yield over several years, decreases because more extreme temperature conditions are now more prevalent for which G1 has a sub-optimum yield response (Figure 2(C), light grey columns, compare scenario 1 and 2). At the same time, the frequency of low yields in G1 increases from scenario 1 to scenario 2, i.e., yields below a set threshold, e.g., 20% of the maximal yield, become more common (Figure 2(D), light grey columns).
Let us now compare this outcome with the response of a simple mix in which two new genotypes G2 and G3 are added to G1 and all three are present in equal proportions. This mix represents a simplified case, illustrating the behaviour of varietal mixtures or CCP. We assume that while G2 has its optimum yield at x2 and is therefore better adapted to the new hotter climate, the response of G3 peaks on the left hand side from x1 so that the resulting overall yield response function of the mixture (Figure 2(B), fine line) has its maximum still at x1. In this respect the mix is not different from G1. As a consequence of mixing the three genotypes with their differing optima, the performance of the mix at the extreme ends of the temperature spectrum lies above the yield level of G1 grown on its own Figure 2(B). Conversely, in the middle range of temperature, the single genotype G1 outperforms the mix because G2 and G3 are not performing optimally there and reduce the mix's yield level. Overall, in this model the mix has a lower average yield than the more specialist crop G1, although this difference is smaller in the more variable environment Figure 2(C). However, the risk of low yields decreases by using the mix and this advantage is more pronounced under the higher variability conditions of scenario 2 (Figure 2(D)). In the mix, as one genotype fails, another one compensates the failure. In other words, the genotype diversity provides insurance against environmental fluctuations [25].
It could of course be argued that the best response to the new environmental conditions (Figure 2(A)) would be to grow genotype G2 only, which performs best at x2, the new peak of the environmental frequency distribution. Unfortunately, however, there are several problems with this approach: First, the exact position of x2 is subject to uncertainty and might be difficult to establish. Because of the large variance of X, it might be some time before it is realised in which direction the mean is shifting. Second, it will be difficult to establish experimentally that G2 is now indeed better suited than G1 grown under the previous conditions, because of genotype by environment interactions. Third, if the tolerance of genotype G2 is narrow relative to the distribution function of X, G2 will still suffer from frequent poor performances when X deviates from its mean value. Indeed, the larger the environmental variance, and the larger the uncertainty about the position of x2, the higher the advantage of the mix over the specialist G2 in terms of reducing the risk of a calamitous crop failure. However, on average over a long time period, G2 would still outperform the mix.
Importantly, a genotype with the properties of G2 must be available in the first place, i.e., there must be the capacity to deal with the specific environmental condition. Thus, crop diversity (in gene banks, in the field or via new crossings) is a central requirement to provide this capacity of specialist response. The trade-off between the capacity of single genotypes and the compensatory power of diversity as illustrated in Figure 2 is one of the most fundamental issues of ecology, the specialist vs. generalist problem. Should we deploy diversity by growing specialists all targeted at different environments or by growing a generalist mix grown in several different environments? Scientists promoting the need for an increased use of crop diversity in the field have usually emphasised the importance of environmental variability. On the other hand, targeted breeding of uniform and ‘stable’ varieties for specific stress conditions has been seen by conventional breeders and researchers as the appropriate response. So far, these two views have not been reconciled.
Our simple model demonstrates that the decision on whether to use a specialist genotype or a diverse generalist mix depends on three parameters: (a) the size of the environmental variance; (b) the position of the environmental mean x2 relative to where on the environmental scale the performances of available genotypes peak; and (c) the degree of uncertainty about the position of x2. All three parameters however, are subject to uncertainty and are therefore difficult to predict. It is hard to quantify how uncertain and variable the environment will be. Therefore, finding the best strategy of deploying crop diversity is a great challenge. The relative advantage of diversity-based over specialist (e.g., stress resistant) genotypes depends critically on the unpredictability and relative strengths of different stresses, but it is unknown how much, at what spatial level, and what kind of diversity is needed in relation to specialist approaches (such as tolerance of high temperatures), for building resilient cropping systems. However, as we show in the next section, evolutionary breeding, as illustrated in Figure 1, offers an elegant solution to overcome this challenge.
2.3. The Evolving Crop in a Changing Environment
When grain from a diverse population (varietal mixture or CCP) is harvested and a proportion re-sown in the next season, the genotypes that performed well in terms of grain number and grain fitness will increase their proportion in the next generation relative to poor performers. Let us now consider the effects of a shifting environmental mean and an increasing environmental variance on the properties of such a diverse and changeable population over time, using the same two scenarios as in Figure 2(A).
Instead of three genotypes in a mix (as in the example above) we now have a very large number n of genotypes Gi with i = (1, …, n) in the population, each with the peak performance at a particular environmental value xi. Assume further, for the sake of simplicity and as an initial condition before starting the evolutionary process, that the frequency of individuals depends on x, in the way that individuals of more stress tolerant genotypes are (initially) rarer than of those with peak performances at moderate values of environmental conditions (also see note 2 in Appendix).
In addition to variation in the value of X at which their performance is highest, the different genotypes in the evolving population may also vary with respect to the breadth of their individual yield responses to X, i.e., the width of their individual ecological tolerances (compare Figure 2(B)).
Let us first consider how the diversity of genotypes in the population changes over time. Starting with the maximal degree of diversity of the population, we can expect that this diversity will decrease over time, because genotypes with optima far from the prevailing environmental condition will decrease in frequency. Conversely, those genotypes performing best in the average environmental condition will increasingly dominate the population. Confirming the theoretical expectation of decreasing diversity over time, experimental work shows the tendency of a decrease in phenotypic variability from F3 to F18, e.g., in plant height, spike length, and heading time [26].
An important question arising from these considerations is whether resistance to plant pathogens in the evolving population will disappear when there is no selection pressure, (i.e., when the pathogen is absent), so that subsequent generations are vulnerable to the re-occurrence of the pathogen. Encouragingly, there is evidence that this may not necessarily be the case. When an American barley population previously unexposed to powdery mildew was moved to England, where the pathogen was present, resistant alleles were still present and increased significantly in frequency [27].
If environmental variation is large (e.g., as indicated by the fine line in Figure 2(A)), diversity will stay in the population for a longer period of time, because the selection pressure is less severe on those genotypes performing best away from the environmental mean. Similarly, including genotypes with broader ecological tolerance will keep the diversity of genotypes longer in the population, since the differential between the performances of different genotypes is less marked than with more specialist genotypes and therefore selection forces act more slowly. The effects of broad tolerance genotypes in evolving populations are of particular importance for wheat, because wheat shows a large tolerance and plasticity already in uniform pure line varieties due to its hexaploid nature and the resulting intergenomic heterosis, providing many triple versions of genes in the overall genome [28,29].
How does the overall performance of the population develop over time? As pointed out above, we would expect a gradual increase of the yield level of the evolving population as the most successful genotypes make up an increasing proportion of the population. Again, this theoretical expectation is backed by empirical evidence [17,26]. Of course, over time this effect of yield increase will gradually decline, as the population reaches an equilibrium state in its environment–provided that the environmental mean is indeed stable. Even with a constant mean however, the process of yield increase will take longer when environmental variation is large because the selection pressure on genotypes with different response functions will be less consistent: The environmental conditions will favour some genotypes in one year, but different ones in the next. Therefore, the course of yield levels over the years will also be more erratic, i.e., yield may decrease rather than increase in a number of years.
Interestingly, the initial distribution of genotypes in the population also plays a major role for the potential improvement of the evolving population's performance over time. When considering the response to a changing environment, a more even distribution of genotypes in the population will lead to a larger and faster improvement of yield over time, because those genotypes with the best pre-adaptation to the new environment will already be present in the population at a higher frequency. On the other hand however, the stronger improvement with a more even distribution of genotypes also results from the initial low performance, as a high proportion of genotypes is grown outside their optimal conditions.
A further key parameter is stability of performance over time [30]. Naturally, larger environmental variance will result in lower stability. The evolving population will also show lower stability if it is composed of genotypes with relatively narrow environmental tolerance functions. This is because, with narrowly adapted genotypes, the selective effect of each year with its particular environmental condition leads to stronger domination of particular genotypes. Subsequent environmental changes are then met by these dominant genotypes pre-adapted to the different condition of the previous year, but less adapted to the current year. In contrast, genotypes that have a broader response to the environmental variable will change more slowly in frequency within the population, resulting in higher buffering capacity and stability across fluctuating environments.
Summarizing these effects, there are trade-offs between overall performance, diversity and stability when using an evolving population: Larger improvement over time is correlated with a higher loss of diversity and a lower stability over time. However, these very properties of the population provide the solution to the problem discussed above; the difficulty lies in how to decide on the best strategy for deploying crop diversity for contrasting situations.
When environmental variance is large, the evolving population will keep diversity longer [31], thus providing a buffer against the environmental fluctuations through compensatory effects. On the other hand, when environmental variance is small, selection forces will drive the evolving population relatively quickly to a targeted composition of genotypes that are well adapted to the (new) environmental mean. Populations in markedly different environments will diverge in their composition of genotypes [32], thus exhibiting the capacity aspect of diversity. In effect, we do not need to know the size of the environmental variance (or even the direction of where the mean is going) to decide whether to grow a more diverse ‘mixture’ or a targeted genotype, because this will be decided by the evolutionary forces acting on the population. In this sense, an evolving population could be called an ‘intelligent’ crop.
So far we have only looked at the response to a single environmental variable moving towards more stressful conditions. However, plants always face a combination of different stresses varying in predictability. An ideal strategy could therefore be to deploy diversity buffering against unpredictable stress factors, but within this diverse system to use genotypes adapted to more predictable stresses.
2.4. Limitations of Evolving Populations
Within an evolving population a major force is competition between different genotypes. In addition, there is also competition for resources (e.g., photosynthates or water) within each plant. These competition effects may limit what evolving populations can achieve; in particular, three main points can be raised.
The first is the issue of grain size vs. number of grains per plant. Both parameters influence the reproductive fitness of each genotype and therefore determine its frequency in the evolving population. However, the contribution of grain size and grain number to a genotype's frequency may not be equal. In particular, a genotype A with a high number nA of small grains (weight wA) may have a higher effective fitness than a second genotype B with a smaller number nB of larger grains (weight wB). Even though the yield of genotype A might be slightly smaller than that of B (nAwA < nBwB), A's fitness could still be greater than B's. In this case, the yield of the evolving population would decrease over time, with a reduction in grain size that is not compensated by the increase in grain number. As with the potential increase of the population's yield, however, there is a limit to how far grain size can decrease as the reproductive fitness will begin to be affected.
Second, there is a trade-off between individual and population performance [33]; while the former is the target of natural selection and the latter is the target of agricultural production. When different genotypes compete against each other for abiotic resources, they may differ in the strategy of how resources within the plant are allocated to competitive ability. For example, a genotype may invest strongly in root biomass (to compete for nutrients and water) or in plant height (to compete for light), thereby gaining high competitive ability against other genotypes. The price for this investment is a loss of potential grain yield since a proportion of the photosnythates goes into competitive mechanisms (e.g., root or shoot) rather than grain biomass. Following this idea, Zhang et al. [34] argue that maximizing grain yield in the population is not an evolutionary stable strategy, as the population can be invaded by genotypes with higher investment in competitive ability but lower grain yield. Supporting Donald's concept of the crop ideotype [25], these authors recommend that uniform crops should be bred that show relatively low competitive ability, so that resources within the plant are freed up for grain biomass that would otherwise have gone into the root or shoot system [35].
A counter-argument against this analysis is that the strategy would only work in a weed-free system, where weak competitors are not penalised by the effects of weed plants that invest in competitive ability but do not produce any grain. Unless there are separately inherited mechanisms of inter- and intra-specific competitive behaviour, a population could not evolve that is strongly competitive against weeds as a whole (interspecific competition) but simultaneously consists of individuals that exert low competitive pressure on each other (intraspecific competition).
Another weakness in this analysis is that it does not consider resource use complementation between different genotypes, as indeed Zhang et al. [34] assume complete niche competition. Nevertheless it needs to be tested empirically whether an evolving plant population moves towards higher competitive ability, and, if this is indeed the case, whether this effect is linked to a loss of yield potential. One relatively easy way to test this would be to study the plant height of an evolving population over several generations. In an early study on barley populations, Allard & Jain [26] found no increase in plant height over the course of fifteen successive generations, while other studies on barley populations suggested that natural selection favoured taller plants [31,36].
A third, more applied issue is the potential trade-off between grain yield and grain quality, in particular grain protein content. A negative link between yield and protein is well known for wheat, i.e., genotypes with high grain yield potential are often characterised by low protein content and vice versa [37]. If a population is evolving towards a higher frequency of high-yielding genotypes because these show greater reproductive fitness, it could therefore be hypothesized that at the same time the overall grain protein of the population may decrease over time. Again, empirical tests are necessary to test this hypothesis; however, if the undesired effect of decreasing protein content is confirmed, it can be counteracted by appropriate mass selection or choice of high protein parent material (see below).
Finally, there is the possibility of disease (or pest) problems in the evolving population. In general, it can be expected that the proportion of susceptible genotypes decreases over time as long as their fitness is lower than in resistant genotypes. However, in case of genetic linkage, i.e., if relative fitness in the evolving population is linked to other plant traits that are highly correlated with susceptibility to disease, susceptible genotypes could be maintained in the evolving population [38], e.g., if a fitness gene and susceptibility gene are located near to each other. Such ‘genetic hitchhiking’ effects [39] could limit the potential that the evolving population can achieve, and the resulting background level of disease might therefore rise above acceptable thresholds. They are of special concern in inbreeding crop species where no recombination takes place that could break disadvantageous genetic linkage. This highlights the need for frequent recombination, e.g., through the usage of male sterile parents or similar mechanisms.
2.5. Genetic Aspects of Evolving Crop Populations
A crucial question for the ecology of an evolving population is whether, over time, novel genetic variation enters the population in the field. This can have implications for the emergence of new traits. For example, in a barley CCP Saghai-Maroof et al. showed that no scald resistant alleles were present in the original parental population, but after 45 generations of selection in a scald infested environment, resistance alleles were found [40].
A further highly interesting question with regard to evolving crop population arises from epigenetic effects, i.e., from the possibility that changes in gene expression and gene regulation acquired in response to environmental conditions may be passed on to the next generation [41-43]. The evolutionary effects of epigenetic phenomena, which are particularly prevalent in polyploid species, depend to a large extent on reversibility. If epigenetic changes are not reversible, i.e., they are fixed in response to the environment, the result is a narrowing of the tolerance in the particular genotype: While the parental generation had an initially high plasticity regarding the particular environmental conditions in one year, these conditions have led to an altered epigenetic profile. The next generation (which has inherited the acquired way of responding to the environment) will then be pre-adapted accordingly. As a consequence of such epigenetic effects, the population may be able to adapt more quickly to environmental changes. Importantly, such adaptations can only be exploited if the population is re-sown under the same conditions (e.g., at the same site), but this is not possible in common centralized breeding. At the same time, however, in highly variable environments epigenetic effects, with their resulting narrowing of tolerance will mean lower stability over time (see above).
If epigenetic changes in the population are reversible their effect could in principle mean a broadening of the tolerance of individual genotypes, which in turn would result in a slower decrease of genetic diversity over time. However, this is complicated by generational delay of the epigenetic effects. Currently, not enough is known about the evolution of epigenetic profiles in plant populations to assess the net effect on genetic diversity and stability of performance.
3. Options in the Process of Evolutionary Breeding
3.1. Options for Initial Creation of Diversity
An important question for evolutionary breeding is how to create and extend intra-specific diversity. There are six decisions to make:
3.1.1. Varietal Mixtures or Composite Crosses
The first decision is whether to use crosses or physical mixtures of pure line varieties. In principle, (reconstituted) mixtures have the advantage of being legal under current legislation (see below) whereas the trading of non-uniform crop populations is presently illegal. Also, when creating mixtures it is possible to generate much larger quantities of seed early on, whereas crop populations will need a phase of seed multiplication before they can be used on a farm-scale. However, populations have the advantage of a much larger pool of genetic diversity.
3.1.2. Number of Parents
The second choice—key for the success of an evolving population—concerns the number of parents for the establishment for CCPs. There are two extremes: On the one hand, one could use only two different parent varieties [44]. On the other, as many parents as physically possible would be crossed with each other. Here, the trade-off lies in the number of crosses per parent-combination that can be made. With a given number of offspring plants, the two-parent option would be able to generate many different F1s of a single cross. With many more parents, as in the second option, fewer F1s per parent combination would be possible. Which strategy is better will depend on the relative importance of the presence of genes versus the effect of recombination of the same genes in different ways. Thus the decision will also depend on how genetically different the parents are [45] (also see below). During the last few years some studies have been carried out to measure the necessary minimum number of parents to establish evolutionary populations; experience from Hungary with bread wheat, using different number of parents, suggest that the necessary minimum for the establishment of a successful population is around 7 parents, and the optimum is around 12 parents [45]. The same study found that under extreme or marginal environmental conditions more parents are needed than under nearly optimal conditions.
3.1.3. Identity of Parents
A third question is the identity of the parents. Although it is advisable to make wide use of gene bank material or even of novel species structures (see below) observations from the USA suggest that using too many varieties unadapted to the target region can result in poor yielding CCPs with many deleterious traits (Murphy, pers. observation). The choice of parent material greatly depends on end use: with a more specific use in mind it is appropriate to constrain varieties to that use (for example, high yield potential for feed wheat, or high baking quality, or good drought resistance). As argued above, a good strategy would then be to ensure wide functional diversity outside those constraints [22]. However, because of potential complex intragenomic interactions and linkages [46] this may be difficult to achieve. In any case, the decision on which parents to include will depend on the assessment of risk and environmental variability. For example, the decision of whether or not to include a highly stress tolerant parent with an overall low yield potential will be dependent on how frequently these stress conditions are expected to occur. A useful compromise could be to adjust the proportion of seed in the initially generated bulk CCP, with the proportion of the stress-resistant parent, tuned according to the perceived risk of that stress factor (see below); however, this brings us back to the problem of unpredictable environments.
When homozygous parents are used in creating CCPs, early generations will be characterised by a heterosis effect. This may be seen as a disadvantage in the evolutionary breeding process. Some useful alleles may be selected out in the early generations when present in heterozygous genotypes, although they would have performed well when present as homozygous ones. A possible way of counteracting this effect would be the use of double haploids when creating the populations (but see section 3.1.4).
A commonly used way of parent selection is based on the success of different varieties in the production. In this case, varieties cultivated over many years in practical production under different ecological conditions are used as parents [47]. The use of such successful varieties ensures that all components of the populations carry important adaptability characters, and exhibit good agronomic performance. Theoretically, the use of these types of parents will result in a population which could have large-scale adaptability in combination with relatively high yield and yield stability under predominant farming conditions. However, in regions where the predominant agricultural system is characterized by high inputs, these populations may not be well adapted to more marginal conditions.
To create CCPs for marginal environmental conditions a parental selection system based on locally used varieties and landraces could be more effective. Here, the best way for parental selection is to explore whether locally adapted varieties and landraces are still available, or to search gene bank accessions which were collected at or close to the location.
A further concept in establishing CCPs is the establishment of elite CCPs. In this case, the parents are selected from elite breeding material namely the best adapted pure lines at the trial stage (compare Table 1). The basis of parental selection is their similar agronomic performance having different genetic background. Breeding lines having different pedigree (no common parents in the pedigree) are grown in a simple spike row, and characterised for the most important agronomic and technological characters, such as plant height, flowering and ripening time, spike characters, etc. and the offspring of the selected rows are used for crossing.
Finally, the inclusion of male sterile components in the establishment of CCPs appears to be more relevant under more extreme environmental conditions, where high rates of recombination would allow for a better adaptation, due to the constant creation of new genetic combinations (Kovács, pers. observation).
3.1.4. Initial Population Development
There are different options to generate the populations. We recommend using a half-diallel approach to generate as much F1 seed as is practically possible so as to maximise the F2 segregation for the largest possible number of different segregants, since maximising transgressive segregation is an important target of population breeding.
Normally CCPs are developed by using diallel or half diallel crosses to minimize relatedness of the components in which all parents are crossed with all the other ones. The difference between the two crossing systems is the presence or absence of reciprocal crosses, where possible maternal inheritance can cause differences. In general no maternal effects could be expected for the majority of the agronomically important traits, but in some special cases, where cytoplasmic inheritance is involved in the expression of traits, the reciprocal crosses could be of high importance. In the case of bread wheat, generally the half diallel crossing scheme is the most frequently used method to establish CCPs. However, in cases where cytoplasmic substitution parents are included the use of complete diallel crossing system is often necessary. For example, in some new composite cross populations Triticum timopheevii has been used to introduce resistance and here the maternal inheritance is significant.
If the CCPs are intended for organic agriculture, plant breeding methods for creating the CCP should follow agreed principles of organic plant breeding; in particular, some techniques, such as the use of double haploids, male sterility or interspecific crosses (see below) may be seen critically [48,49]. However, we feel that more discussion is needed on these specific issues before firm recommendations can be given to organic plant breeders.
3.1.5. Proportion of Seed
Regarding the proportions of seed from different genotypes in the initial population, there are, in principle, two options. Either seed from all crosses goes into the population in equal proportions, or the proportion is varied between different crosses. For example, there might be a parent line that provides resistance against a rare but severe stress factor but has a low yield under non-stress conditions. As indicated above, it is useful to introduce offspring from this parent in a lower proportion than from a parent line with high yield potential if the stress condition is indeed considered to occur very rarely. Conversely, the more variable the environment the more even should the initial proportions of (functionally) different genotypes be.
3.1.6. Number of Populations
Finally, it needs to be decided how many different populations should be created. As currently crop populations are not grown widely,at least not in countries with a high proportion of industrialised high-input agriculture,—it is useful to create more than one population so that more can be learned about their behaviour in comparative trials. Creating more populations (from different sets of parents) will of course increase overall diversity but the bottleneck of the multiplication phase needs to be passed by each population separately. On a given amount of land, creating more populations therefore means a delay in getting them to farm level. Again, the useful number of populations will depend on the diversity of specific end uses and target areas. In any case, starting with a highly diverse population in several locations will lead to gradual divergence, resulting in a number of more or less distinct, locally adapted crop populations.
3.2. Options for (Additional) Selection Forces
Once a population is created, there are several options of how to apply selection to them. So far we have mainly focused on natural selection, where no direct choice of genotypes by humans is done. Here, the outcome of the selection process depends entirely on the environment. Environmental selection forces can include factors that are entirely constant at a given location, such as day-length [50], or more variable factors like rainfall patterns. However, selection forces can also include measures of crop husbandry. For instance, populations can be subjected to the differential selective effects of organic or conventional management, specific soil management practices such as minimum tillage or intercropping (see below). Apart from natural selection there are two more options: mass selection and manual selection.
Mass selection is the simultaneous selection of a large number of plants or grains with similar but not entirely uniform properties. For example, by passing cereal grain through a sieve before sowing, smaller grains can be separated from larger ones. Because large seed size improves the chance of a seed to develop into a vigorous plant, sieving out the small-grain fraction before sowing and then sowing only the larger seeds should lead to higher yields [51,52]. Consequently, by continually saving seed from the large size fraction and sieving it before sowing, the population could gradually increase both in grain size and in yield over time. However, the morphology of the wheat ear sets a boundary to such a gain in yield as it literally leaves little room for limitless grain size increases. Therefore, wheat breeders have traditionally considered grain size as not holding much potential for yield improvements. However, if there is variation of grain size, sieving does offer some potential as a tool for farmer-led crop improvement.
Mass selection can also be used to improve grain protein content. Since protein content is correlated to a darker colour of the grain [52,53], protein improvements of the population can be made via colour sorting. For the process of colour separation, machines can be used that usually detect and remove ergot and stones from grain samples [52]. Thus, simple methods such as sieving or colour-separating grain enable farmers to increase the yield and quality of genetically diverse cereal populations.
The success of mass selection depends on the presence of sufficient diversity, but at the same time, mass selection leads to a potentially large decrease in diversity if only a particular selected fraction is maintained. This is particularly relevant when there is only little seed that is subjected to mass selection, because the risk is high that potentially useful genotypes are lost. For this reason it is sensible to use mass selection in early generations only when sufficient quantities of seed are available, so that maximal diversity is retained in the population.
Manual selection can be positive or negative. Positive selection is the process of singling out individual plants or ears in the population and manually collecting them for use as seed in the next season. The selection could either focus on one or several particular characters. Similarly, it could target a narrow or wider range of ideotypes. The advantage of positive selection is that it is both flexible and targeted. On the other hand, the resulting amount of seed is relatively small unless collection efforts are greatly increased. Further, the selection is necessarily based on the plant's performance in one particular year only, thereby being at risk not to be representative and being influenced by potential idiosyncrasies of the year.
Negative manual selection removes individual plants or ears of undesired habit from the population so that only the remaining plants contribute to the next year's seed. For example, very tall and very small plants could be taken from the growing crop, in order to narrow the height range of the population. A further important problem is that as negative selection eliminates all unwanted phenotypes from a given population at particular developmental stages of the plant growth, it disregards the value of the still invisible recessive characters. However, a key advantage over positive selection is that it maintains higher levels of genetic diversity in the population. Thus, normally negative selection is used for improving gene bank accessions in the phase of regeneration, in landrace improvement, and also in early CCP improvement, especially in case when male sterile components are involved in the combinations. Using negative selection, several populations have been improved during the last few years in Martonvásár (Hungary). This has resulted in one new registered emmer (Triticum dicoccum) variety Mv Hegyes (Kovács unpubl.)
While in conventional breeding, artificial selection is (or at least should be) based on information gathered over more than one year, manual and mass selection of populations are based on the phenotypic appearance in just one year. This practice therefore bears the risk of losing potentially important genetic material from the population through idiosyncratic year effects. When the aim is to keep diversity in the population, additional selection should therefore not act too strongly.
3.3. Options for Use in Breeding, Growing and Marketing
After having gone through several generations of natural or mass selection, diverse crop populations provide a valuable resource for both conventional and further population breeding. When single ears are selected and sown in ‘ear rows’, evaluation of individual genotypes can take place, eventually resulting in the selection of new breeding material or even ready-to-use lines. However the strength of evolutionary breeding is its compatibility with participatory approaches to plant breeding [16]. Again, there are several options. Each farmer could develop a separate population without interacting with other farmers. Alternatively, farmers in a region could all share the same population by pooling seed from each farm and re-distributing it; or, as a compromise, there could be occasional regional exchange of seed between different populations. While exchange would maintain higher levels of diversity in the population, separation would favour faster divergence and, possibly, local adaptation.
Using CCPs as a source for new material in conventional breeding programmes has been especially suggested when the goal is to breed for low input systems [54]. However, it is not correct to assume that a CCP will per se produce genotypes that have a high pure stand yielding ability in the prevailing environment.
Options for the end use of genetically diverse crop populations strongly depend on the qualities of the parent material, for example whether a wheat population is intended to be used for bread making or animal feeding purposes. However, most if not all crop populations share as a characteristic property that individual plants (and grains) within the populations display a high degree of diversity in appearance and quality. Therefore, the past experience that variety mixtures were not readily accepted by the food processing industry indicates that some (perceived) problems need to be overcome to introduce more genetic diversity into cropping systems.
Perhaps the most central feature of evolving crop populations is their adaptability to local conditions, although the extent of this adaptability in relation to defined eco-geographic regions is currently not yet established. Therefore, the potential of locally or regionally unique populations is suggestive of regional, rather than global, marketing paths for population-based products [21]. Thus, by creating locally or regionally unique crops with their own ‘terroir’, evolutionary breeding is in line with a re-connection between producer and consumer on a regional level, although some crops such as vegetables would possibly lend themselves more easily to this idea than cereal grain processed for bread making. For this link to be made, a requirement is that the evolutionary breeding process should be de-centralised, i.e., that populations are developed on-farm rather than being maintained centrally and distributed to multiple locations.
4. A SWOT Analysis of Evolutionary Breeding
In a SWOT analysis the first step is the definition of an objective after which the strengths, weaknesses, opportunities and threats of particular strategies to reach that objective can be analysed and discussed. Strengths (resp. weaknesses) are defined as internal factors, inherent to a strategy, that are favourable (resp. unfavourable) to achieve the defined objective and provide an advantage (resp. disadvantage) over other approaches. Opportunities (resp. threats), on the other hand, are external factors, coming from outside, that are favourable (resp. unfavourable) to achieve the objective.
The objective we define here is to increase the resilience of agricultural production in the face of increasing biotic and abiotic stresses. Resilience is understood as the ability of a system to remain functional when under external stress [47], for example to produce acceptable yield levels when the availability of resources decreases or when climatic conditions become more extreme. This ability can involve structural, lasting changes of the system in response to the stress factors. Thus, the notion of resilience is similar to the several concepts of stability in agronomy [30], but goes beyond these, as it stresses the adaptability of a system to changing conditions.
In Table 2 we compare three strategies: (1) pure line varieties for use in monocultures; (2) static diversity created anew each year as in varietal mixtures; and (3) evolving diversity, where genetically diverse crop populations (varietal mixtures or CCPs) are subjected to natural selection. In the left column of Table 2 we summarize the strengths, weaknesses, opportunities and threats of static diversity relative to pure line varieties. In the middle column we outline the relative advantages and disadvantages of evolving diversity when compared to static diversity. Some of the entries are direct contributors to the objective of increased resilience, others are more indirect. Here we briefly discuss what we believe are the key points in this SWOT analysis.
A main strength of evolutionary breeding that goes beyond benefits of static diversity is the large number of genotype by environment combinations that can simultaneously be tested. While conventional plant breeding is also able to create adaptation to changing conditions, in that it continuously creates new varieties to bring into the market, it is very limited in three aspects: (a) there is a limitation to the number of environments where the actual selection can be done; (b) the number of genotypes available for selection is restricted, since the vast majority of genotypes are removed from the population in the F2; and (c) breeding success is hampered in terms of achieving high stability since traits linked to stability appear to have low heritability [12]. Consequently, the within-crop potential for buffering against unpredictable change is limited. More importantly, in conventional breeding, genotypes potentially well adapted to stress conditions are likely to fall through the net and are discarded.
One of the major weaknesses of evolutionary breeding is the potential of seed borne diseases building up over time. The reason is that before fitness related changes in the population can take effect and limit any further disease increase, the infection level may already be much higher than tolerable infection thresholds. This is particularly so for pathogens against which resistance is rare or even completely absent in the population. Because conventional cereal breeding is currently giving low priority to seed borne diseases [55], resistant and agronomically appropriate genotypes to compose a population may be difficult to obtain. As a consequence, to ensure high seed quality (hygiene), measures such as seed treatments or rotational control [55] must be put in place on farm, but these will necessarily entail some costs. As a consequence, there are benefits in partly centralizing seed maintenance and multiplication in a regional cluster approach: population seed would be multiplied and maintained on one site where special care is taken to ensure seed health, and the seed is subsequently given to several farmers in the region (or, more accurately, to farms which are similar in their growing conditions as the maintenance site).
Opportunities and threats for evolutionary breeding approaches are harder to pin down. While there is an increasing realization of the importance of diversity for delivering ecosystem functions in agriculture, mainstream policies are still strongly focusing on high input and monoculture approaches to respond to the global pressure factors that affect agricultural production.
What are the consequences of the SWOT analysis for the evaluation of evolutionary breeding as a strategy to achieve more resilience of agricultural production? Clearly, evolutionary breeding has something novel to offer but its role is a complementary one: It cannot be the only strategy of breeding; as a single strategy of plant breeding it would be relatively slow and inefficient. Conventional breeding (as well as other novel breeding approaches) needs to be integrated into evolutionary breeding to maximize its benefits: Most fundamentally, evolutionary breeding should make use of the advances of modern variety breeding by using modern high performance varieties as parents.
In any case, evolutionary breeding will be restricted to annual grain crops. In plants where seeds (and reproductive fitness) are not tightly correlated with human breeding goals (e.g., root and leaf crops), evolutionary breeding is not a useful option. Even for cereal plants, there are restrictions: for example, using the whole plant for forage or as a cover crop, rather than only using the grain is not easily integrated in the process of evolutionary breeding as the plants are cut much before the grain is ripe. In this case, a part of the field would have to be left uncut to provide seed for the next generation. Also for plants with biennial life cycles or perennial species the path of evolutionary plant breeding would take too long. However, an exception might be most stressful environments with strong selection pressure, which re-establishes the link between root or leaf biomass and reproductive fitness which would prove useful for non-grain crops. Also for plants with biennial life cycles or perennial species, the path of evolutionary plant breeding would take too long.
What are the most appropriate actions emerging from the analysis? Few scientific papers forego the opportunity to emphasize that ‘more research is needed’; accordingly, we could stress the need to answer some urgent questions (e.g., regarding the relation of parent material, diversity and adaptability to stressful and variable environmental conditions). However, we believe that the most important barrier to development in this area is the outdated legislation that currently ‘prohibits evolution’ [56].
Nevertheless, caution is needed when introducing new legal rules for trading of population seed. In particular, because of the changing and diverse nature of evolving crop populations, it is difficult to establish visually whether the population identity as claimed by the trader is indeed true. For example, if a crop population is claimed to have adapted to certain conditions over several generations, accidental or deliberate dilution of the seed by an admixed pure line variety would be hard to detect. Adjusted seed certification schemes will therefore need to be developed to achieve protection of the party receiving the population seed.
5. Novel Approaches: Beyond Single Crop Populations
Evolutionary breeding is characterised by an inherently large complexity, even for a single population of one crop species in one location. This challenging situation has resulted in past research mainly focussing on relatively simple systems. However, the concept of evolutionary breeding can and should be expanded to more complex conditions. We believe that to create more resilient agro-ecosystems it will be useful to try and adopt evolutionary breeding approaches in an extended range of new environments and with species and genotypes. Regarding the interactions of evolving crop populations with their environment we see five areas for potential development.
5.1. Broadening Environmental Variation
The first area is the extension of environmental conditions in which populations are grown. Developing continuous targeted responses to climate change requires extending these environments to the more extreme ends of the spectrum. Here, the idea is to create highly diverse populations from a large number of parents with dissimilar genetic backgrounds and subject the offspring of these crosses to strong stressors (drought, salinity, pests and diseases etc.) so that selection pressure is high.
5.2. Integrating with Farm Management Practices
Secondly, evolutionary breeding should be more intimately integrated with ‘packages’ of farm management practices. For example, for the integration of rotational aspects evolutionary plant breeding offers the possibility of selecting for optimized responses to given pre-crops. Similarly, options of mechanical weed control can be integrated as a factor exerting selection pressure on the evolving crop. These two simple examples highlight that in comparison to evolutionary breeding more centralised plant breeding approaches would have difficulty in responding as flexibly to the large diversity of farming practices.
5.3. Co-Breeding
A further idea of how to expand the concept of evolutionary breeding is co-breeding. Generally, growing different genotypes together results in a selection for higher ecological combining ability [18]. Rather than growing a crop population as a species-monoculture (albeit with high intraspecific diversity), it can also be grown as an intercrop or mixed crop with a second species in the same field. In this case, the intercrop (such as an undersown clover or ley mixture), exerts selection pressure on the population, e.g., by competing for commonly used resources such as water. However, positive interactions between the two crops can also have selecting effects on the population, if different genotypes within the population have differing abilities to use this beneficial relationship. It is also conceivable to grow populations of two different cereal species together, provided they have sufficiently similar harvest dates, e.g., mixtures of barley and oats.
5.4. Broadening of the Genetic Base for Initial Crossings
Modern hexaploid wheat originated by natural hybridisation of tetraploid cultivated emmer wheat with the diploid goat grass Aegilops tauschii. Cultivated emmer was domesticated from a wild progenitor T. turgidum ssp dicoccoides, which was derived by an interspecific hybridisation of two diploid progenitors, T. urartu and an unconfirmed diploid species related to Ae. speltoides. While this general pathway of the evolution of hexaploid bread wheat is well understood by cytogenetic and molecular genetic studies, the intensity of the founder effect conferred by both polyploidisation incidents remains unclear. One new pathway for introducing new genetic diversity into the bread wheat gene pool is the reconstitution of hexaploid wheat by interspecific crosses [57]. Since the early 1990s CYMMIT has focused largely on interspecific hybridisation between the diploid Ae. tauschii and tetraploid durum wheat to create synthetic hexpaloid wheat (SHW) [58]. Several hundred spring habit SHW lines have been produced, and many of them are successfully used in CYMMIT's wheat breeding programme. Thus, interspecific crosses could also be used to broaden the genetic base at the beginning of the evolutionary breeding process. Again, however, if organic agriculture is the target market, the compatibility of intraspecific or intrageneric crossing with organic plant breeding principles needs to be explored.
5.5. Pick and Mix: Combination of Populations with Mixtures and Monocultures
On a more practical level, crop populations can also be mixed with high performance pure line varieties to make simultaneous use of the variety's specific properties (e.g., high yield and/or quality) and the stabilising effects of the diverse population. However, research is needed to establish how the advantages and disadvantages of pure line cultivars, mixtures and evolving populations would combine when grown together. In any case, to realise any synergies that would emerge from combining the different approaches, changes need to be made to the legal system of trading seed.
6. Conclusions and Outlook
Benefiting from a long research tradition, evolutionary plant breeding is, in many respects, already a well understood breeding approach. With agricultural production facing the double challenge of mounting pressure from various stressors and increasing unpredictability of growing conditions, the benefits that evolutionary plant breeding has to offer are currently coming into sharper focus. Therefore it is timely that during the last decade several initiatives have (re-)introduced the concept of evolutionary breeding into larger scale on-farm research [59]. However, for two reasons a further expansion into agricultural practice is necessary.
First, when evolutionary plant breeding remains within a research framework only, its social implications cannot be explored. In particular, society's attitude towards biological diversity, its changing relationship with local food production and artisanal vs. industrial food processing, and the issues of intellectual property rights for agricultural plant material are all intricately linked with the concept of evolving and locally adapting plant populations. Only if evolving diversity is allowed to enter practice, will we be able to realise its potential. Second, as our SWOT analysis has shown, evolutionary plant breeding has some problematic aspects too. To assess the true potential and the limitations of this breeding approach it is necessary to expose it to the litmus test of farming practice.
Finally, evolutionary and conventional (‘non-evolutionary’) plant breeding approaches need to collaborate more closely with each other, by exchanging both ideas and plant material, while continuing to critically assess breeding techniques. One area for potential collaboration is seed borne diseases such as bunts and smuts. Evolutionary plant breeding depends on progress in control of seed borne diseases which might otherwise become its Achilles heel. In turn, however, we predict that in a rapidly changing and increasingly fluctuating environment conventional plant breeding approaches will also depend more and more on advances made by evolutionary plant breeding.
| Crop species | Country of origin | Name of population | Year of creation | Nr of parents | Nr of trial sites | Grown where (country code) | Notes on parents |
|---|---|---|---|---|---|---|---|
| Bread wheat | UK | YQ | 2001 | 20 | >30 | UK, F, DE, HU, IT, CH, A | high yielding and high baking quality varieties, both recent and older, from across Europe and Russia |
| Y | 2001 | 9 | >10 | UK, DE, HU | Subset of high yielding YQ parents | ||
| Q | 2001 | 12 | >10 | UK, DE, HU | Subset of high baking quality YQ parents | ||
| NIAB Elite CCP | 2008 | 8 | 3 | UK | set of high yield and high quality modern varieties | ||
| NIAB Diversity CCP | 2009 | 16 | 2 | UK | set of parents from diverse European wheat breeding programmes | ||
| HU | Kompozit 1 | 2003 | 12 | 5 | HU | old and modern varieties | |
| Kompozit 2 | 2004 | 9 | 5 | HU | old and modern varieties | ||
| Kompozit 3 | 2004 | 7 | 5 | HU | old and modern varieties | ||
| Kompozit 4 | 2004 | 5 | 5 | HU | old and modern varieties | ||
| Elite kompozit | 2004 | 7 | 7 | HU, UK, IT | high yielding and high quality modern varieties | ||
| USA | 101 | 2002 | 2 | Washington State | high yielding and high quality modern varieties | ||
| 201 | 2002 | 4 | Washington State | high yielding and high quality modern varieties | |||
| 206 | 2002 | 6 | Washington State | high yielding and high quality modern varieties | |||
| LL101 | 2002 | 18 | Washington State | high yielding and high quality modern varieties | |||
| Durum wheat | HU | Durum kompozit | 2009 | 7 | 2 | HU | high yielding and high quality modern varieties |
| Emmer wheat | HU | Dicocomp | 2008 | 7 | 2 | HU | modern varieties, landraces and selected gene bank accessions |
| Einkorn | HU | Landeinkorn kompozit | 2004 | 7 | 3 | HU | modern varieties, landraces and selected gene bank accessions |
| Einkorn dwarf kompozit | 2004 | 8 | 2 | HU | high yielding and high quality modern varieties | ||
| Maize | HU | PC kompozit | 2006 | 13 | 3 | HU, IT | local landrace originated inbred lines |
| Kompozit 1 | 2005 | 17 | 3 | HU, IT | inbred lines | ||
| Kompozit Gyula | 2006 | 13 | 3 | HU, IT | inbred lines | ||
| Interspecific CCP | HU | Kamdur (tetraploid with AABB genome) | 2005 | 7 | 3 | HU | 3 durum wheat varieties, Kamut wheat, and 3 selected Triticum turgidum ssp turgidum gene bank accessions |
| HU | Trigen (tetraploid with AABB genome) | 2006 | 7 | 3 | HU | 2 durum wheat modern varieties, 2 pre-bred accessions of wild emmer (Triticum dicoccoides) and 1 breeding line and 1 variety of cultivated emmer (Triticum turgidum ssp. dicoccon) | |
| HU | Synthetic hexa (hexaploid with AABBAA genome) | 2008 | 12 | 3 | HU, UK | 2 bread wheat varieties (Triticum aestivum ssp. aestivum), 2 spelt varieties (T. aestivum ssp. spelta), 2 macha wheat selected gene bank accession (T. aestivum ssp. macha) and 2 selected sphaerococcum gene bank accessions (T. aestivum ssp. sphaerococcum) | |
| Static diversity (in comparison to pure line varieties) | Evolving diversity (in comparison to static diversity) | Action/comments |
|---|---|---|
| Strengths | ||
|
|
|
| Weaknesses | ||
|
|
|
| Opportunities | ||
|
|
|
| Threats | ||
|
|
|
Acknowledgments
We thank Arron Carter, Oliver Crowley, Maria Finckh, Isabelle Goldringer, Meike Grosse, Peter Hoebe, Sally Howlett, Hannah E. Jones, Stephen Jones, Bruce Pearce, Helen Pearce, John Snape, Steve Wagner, Jacob Weiner, and Lawrence Woodward for contributing important ideas in discussions on evolutionary plant breeding. We are also grateful for research grants from DEFRA, UK through the Sustainable Arable LINK programme, and from the European Commission through the European FP7 Research Programme.
Appendix
Note 1
Scenario S1 and S2 (shown in Figure 2(A)) are based on normal distributions for the environmental variable X, with means of xS1 = −1 and xS2 = 0, respectively. Standard deviations are SDS1 = 1 and SDS2 = 2, respectively. The response function of the genotypes G1, G2 and G3 (presented for G1 and the mixture of G1, G2 and G3, in Figure 2(B)) follow normal distributions as well, with peaks at xG1 = −1, xG2 = 0, and xG3 = −2, and identical standard deviations of SDG1 = SDG2 = SDG3 = 1. The performance functions of G1, G2 and G3 are normalized with the peak performance set to 1. For each value of X, the performance of the mixture of the three genotypes is calculated as the average performance of all three genotypes.
The average performance of any genotype over all environmental conditions (presented in Figure 2(C)) is calculated as the sum of individual performances for each value of X weighted by the frequency of that value of X. For Figure 2(D), the frequency of low performance was determined by summing up the frequencies of environmental values X for which a given genotype or the mixture would perform less than 0.2 (i.e., 20% of the maximum performance). All values in Figure 2(C) are expressed as a proportion of the performance of G1 in Scenario 1, i.e., the value for the light grey column for scenario 1 in Figure 2(C) is 1. The maximum frequency in Figure 2(D) (light grey column in Scenario 2) is 0.4.
Note 2
We assume that before starting the evolutionary process, the frequency of individuals depends on x, in the way that individuals of more stress tolerant genotypes are rarer than those with peak performances at moderate values of environmental conditions. The frequency is assumed to be normally distributed with x1 being the mean. The degree to which this condition holds will depend on the choice of parents and the crossing regime; an alternative assumption would be that the frequency of individuals is independent of x. In this case, however, the evolution of the population over time will result in a situation similar to the previous assumption.
References
- Pimentel, D. Food for thought: A review of the role of energy in current and evolving agriculture. Crit. Rev. Plant Sci. 2011, 30, 35–44. [Google Scholar]
- Nelson, G.C.; Rosegrant, M.W.; Koo, J.; Robertson, R.; Sulser, T.; Zhu, T.; Ringler, C.; Msangi, S.; Palazzo, A.; Batka, M.; et al. Climate Change: Impact on Agriculture and Costs of Adaptation; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2009; p. 20. [Google Scholar]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar]
- Østergård, H.; Finckh, M.R.; Fontaine, L.; Goldringer, I.; Hoad, S.P.; Kristensen, K.; Lammerts van Bueren, E.T.; Mascher, F.; Munk, L.; Wolfe, M.S. Time for a shift in crop production: Embracing complexity through diversity at all levels. J. Sci. Food Agric. 2009, 89, 1439–1445. [Google Scholar]
- Spedding, C.; Frape, D.; Cook, R. The ‘Organics’ debate. World Agric. 2011, 2, 5. [Google Scholar]
- Cattivelli, L.; Rizza, F.; Badeck, F.; Mazzucotelli, E.; Mastrangelo, A.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar]
- Smith, S. Intellectual property protection for plant varieties in the 21st century. Crop Sci. 2008, 48, 1277–1290. [Google Scholar]
- Piepho, H.P. Methods for comparing the yield stability of cropping systems—A review. J. Agron. Crop Sci. 1998, 180, 193–213. [Google Scholar]
- Robert, N. Comparison of stability statistics for yield and quality traits in bread wheat. Euphytica 2002, 128, 333–341. [Google Scholar]
- Becker, H.C.; Léon, J. Stability analysis in plant breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar]
- Verboom, J.; Schippers, P.; Cormont, A.; Sterk, M.; Vos, C.; Opdam, P. Population dynamics under increasing environmental variability: Implications of climate change for ecological network design criteria. Landsc. Ecol. 2010, 25, 1289–1298. [Google Scholar]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar]
- Carolina, T.; Villa, C.; Maxteda, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. Charact. Util. 2005, 3, 373–384. [Google Scholar]
- Murphy, K.; Lammer, D.; Lyon, S.; Carter, B.; Jones, S. Breeding for organic and low-input farming systems: An evolutionary-participatory breeding method for inbred cereal grains. Renew. Agric. Food Syst. 2005, 20, 48–55. [Google Scholar]
- Suneson, C.A. An evolutionary plant breeding method. Agron. J. 1956, 48, 188–191. [Google Scholar]
- Allard, R.W.; Adams, J. Population studies in predominantly self-pollinating species. XIII. Intergenotypic competition and population structure in barley and wheat. Am. Nat. 1969, 103, 621–645. [Google Scholar]
- Allard, R.W. Genetic changes associated with the evolution of adaptedness in cultivated plants and their wild progenitors. J. Hered. 1988, 79, 225–238. [Google Scholar]
- Jain, S.K.; Allard, R.W. The effects of linkage, epistasis, and inbreeding on population changes under selection. Genetics 1966, 53, 633–659. [Google Scholar]
- Phillips, S.L.; Wolfe, M.S. Centenary review: Evolutionary plant breeding for low input systems. J. Agric. Sci. 2005, 140, 1–10. [Google Scholar]
- Finckh, M.R.; Wolfe, M.S. The Use of Biodiversity to Restrict Plant Diseases and Some Consequences for Farmers and Society. In Ecology in Agriculture; Jackson, L.E., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 203–237. [Google Scholar]
- Ninkovic, V.; Åman, I. Aphid acceptance of Hordeum genotypes is affected by plant volatile exposure and is correlated with aphid growth. Euphytica 2009, 169, 177–185. [Google Scholar]
- Schär, C.; Vidale, P.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 1463–1468. [Google Scholar]
- Allard, R.W.; Jain, S.K. Population studies in predominantly self-pollinated species. II. Analysis of quantitative genetic changes in a bulk-hybrid population of barley. Evolution 1962, 16, 90–101. [Google Scholar]
- Ibrahim, K.; Barrett, J. Evolution of mildew resistance in a hybrid bulk population of barley. Heredity 1991, 67, 247–256. [Google Scholar]
- Meimberg, H.; Rice, K.J.; Milan, N.F.; Njoku, C.C.; McKay, J.K. Multiple origins promote the ecological amplitude of allopolyploid Aegilops (Poaceae). Am. J. Bot. 2009, 96, 1262–1273. [Google Scholar]
- Paterson, A. Polyploidy, evolutionary opportunity, and crop adaptation. Genetica 2005, 123, 191–196. [Google Scholar]
- Annicchiarico, P. Coping with and exploiting genotype × environment interactions. In Plant Breeding and Farmer Participation; Ceccarelli, S., Guimarães, E.P., Weltzien, E., Eds.; Food and Agricultural Organization: Rome, Italy, 2009; pp. 519–564. [Google Scholar]
- Patel, J.D.; Reinbergs, E.; Mather, D.E.; Choo, T.M.; Sterling, J.D.E. Natural selection in a doubled-haploid mixture and a composite cross of barley. Crop Sci. 1987, 27, 474–479. [Google Scholar]
- Nichols, P.G.H.; Cocks, P.S.; Francis, C.M. Evolution over 16 years in a bulk-hybrid population of subterranean clover (Trifolium subterraneum L.) at two contrasting sites in south-western Australia. Euphytica 2009, 169, 31–48. [Google Scholar]
- Weiner, J.; Andersen, S.B.; Wille, W.K.-M.; Griepentrog, H.-W.; Olsen, J.M. Evolutionary Agroecology—The potential for cooperative, high density, weed suppressing cereals. Evol. Appl. 2010, 3, 473–479. [Google Scholar]
- Zhang, D.-Y.; Sun, G.-J.; Jiang, X.-H. Donald's ideotype and growth redundancy: A game theoretical analysis. Field Crops Res. 1999, 61, 179–187. [Google Scholar]
- Donald, C.M. The breeding of crop ideotype. Euphytica 1968, 17, 385–403. [Google Scholar]
- Hensleigh, P.F.; Blake, T.K.; Welty, L.E. Natural selection on winter barley composite cross XXVI affects winter survival and associated traits. Crop Sci. 1992, 32, 57–62. [Google Scholar]
- Simmonds, N.W. The relation between yield and protein in cereal grain. J. Sci. Food Agric. 1995, 67, 309–315. [Google Scholar]
- Parker, M.A. Nonadaptive evolution of disease resistance in an annual legume. Evolution 1991, 45, 1209–1217. [Google Scholar]
- Hedrick, P.W. Genetic hitchhiking—A new factor in evolution? BioScience 1982, 32, 845–848. [Google Scholar]
- Saghai Maroof, M.A.; Webster, R.K.; Allard, R.W. Evolution of resistance to scald, powdery mildew, and net blotch in barley composite cross II populations. Theor. Appl. Genet. 1983, 66, 279–283. [Google Scholar]
- Liu, B. Epigenetic variation and crop genetic improvement. J. Jilin Agric. Univ. 2008, 30, 386–393. [Google Scholar]
- Rapp, R.A.; Wendel, J.F. Epigenetics and plant evolution. New Phytol. 2005, 168, 81–91. [Google Scholar]
- Finnegan, J.E. Epialleles—A source of random variation in times of stress. Curr. Opin. Plant Biol. 2002, 5, 101–106. [Google Scholar]
- Steele, K.; Edwards, G.; Zhu, J.; Witcombe, J. Marker-evaluated selection in rice: Shifts in allele frequency among bulks selected in contrasting agricultural environments identify genomic regions of importance to rice adaptation and breeding. Theor. Appl. Genet. 2004, 109, 1247–1260. [Google Scholar]
- Kovács, G. Development of Composite Cross Populations from Cereals. Research Report on ALKOBEER Project; Agricultural Research Institute (in Hungarian): Martonvásár, Hungary, 2008. [Google Scholar]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. U. S. A. 1984, 81, 8014–8018. [Google Scholar]
- Döring, T.F.; Wolfe, M.; Jones, H.; Pearce, H.; Zhan, J. Breeding for resilience: A strategy for organic and low-input farming systems? Proceedings of EUCARPIA 2nd Conference of the Organic and Low-Input Agriculture Section, Paris, France, 1–3 December 2010.
- Lammerts van Bueren, E.T.; Struik, P.C.; Tiemens-Hulscher, M.; Jacobsen, E. Concepts of intrinsic value and integrity of plants in organic plant breeding and propagation. Crop Sci. 2003, 43, 1922–1929. [Google Scholar]
- International Federation of Organic Agriculture Movements (IFOAM). D1 Plant Breeding Draft Standards; IFOAM: Bonn, Germany, 2005. Available online: http://www.ifoam.org/about_ifoam/standards/norms/draft_standards/DraftPlantBreedingStandardsD1050729.pdf (accessed on 30 September 2011).
- Goldringer, I.; Prounin, C.; Rousset, M.; Galic, N.; Bonnin, I. Rapid differentiation of experimental populations of wheat for heading time in response to local climatic conditions. Ann. Bot. 2006, 98, 805–817. [Google Scholar]
- Frey, K.J. Mass selection for seed width in oat populations. Euphytica 1967, 16, 341–349. [Google Scholar]
- Döring, T.; Crowley, O.; Wolfe, M. Against the grain. Org. Farming 2011, 107, 42–43. [Google Scholar]
- Hubbard, J.D.; Pomeranz, Y.; Lai, F.S. Note on protein contents and amino acid composition of dark hard and yellow hard kernels separated from red winter wheat. Cereal Chem. 1977, 57, 778–783. [Google Scholar]
- Danquah, E.Y.; Barrett, J.A. Grain yield in composite cross five of barley: Effects of natural selection. J. Agric. Sci. 2002, 138, 171–176. [Google Scholar]
- Matanguihan, J.B.; Murphy, K.M.; Jones, S.S. Control of common bunt in organic wheat. Plant Dis. 2011, 95, 92–103. [Google Scholar]
- Finckh, M.R. Evolutionsverbot per Gesetz, oder: Die Konsequenzen der Verhinderung der Ko-Evolution in der Landwirtschaft. In Kant, das Prinzip “Vorsorge” und die Wiederentdeckung der “Allmende”; Lange, B., Lange, B., Eds.; Ergon Verlag: Würzburg, Germany, 2007; pp. 109–120. [Google Scholar]
- Dreisigacker, S.; Kishii, M.; Lage, J.; Warburton, M. Use of synthetic hexaploid wheat to increase diversity for CIMMYT bread wheat improvement. Aust. J. Agric. Res. 2008, 59, 413–420. [Google Scholar]
- Mujeeb-Kazi, A.; Rosas, V.; Roldan, S. Conservation of the genetic variation of Triticum tauschii (Coss.) Schmalh. (Aegilops squarrosa auct. non L.) in synthetic hexaploid wheats (T. turgidum L. s.lat. × T. tauschii; 2n = 6x = 42, AABBDD) and its potential utilization for wheat improvement. Genet. Resour. Crop Evol. 1996, 43, 129–134. [Google Scholar]
- Dawson, J.C.; Rivière, P.; Berthellot, J.F.; Mercier, F.; de Kochko, P.; Galic, N.; Pin, S.; Serpolay, E.; Thomas, M.; Giuliano, S.; et al. Collaborative plant breeding for organic agricultural systems in developed countries. Sustainability 2011, 3, 1206–1223. [Google Scholar]
- Conflict of Interest: The authors declare no conflict of interest.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Döring, T.F.; Knapp, S.; Kovacs, G.; Murphy, K.; Wolfe, M.S. Evolutionary Plant Breeding in Cereals—Into a New Era. Sustainability 2011, 3, 1944-1971. https://doi.org/10.3390/su3101944
Döring TF, Knapp S, Kovacs G, Murphy K, Wolfe MS. Evolutionary Plant Breeding in Cereals—Into a New Era. Sustainability. 2011; 3(10):1944-1971. https://doi.org/10.3390/su3101944
Chicago/Turabian StyleDöring, Thomas F., Samuel Knapp, Geza Kovacs, Kevin Murphy, and Martin S. Wolfe. 2011. "Evolutionary Plant Breeding in Cereals—Into a New Era" Sustainability 3, no. 10: 1944-1971. https://doi.org/10.3390/su3101944
APA StyleDöring, T. F., Knapp, S., Kovacs, G., Murphy, K., & Wolfe, M. S. (2011). Evolutionary Plant Breeding in Cereals—Into a New Era. Sustainability, 3(10), 1944-1971. https://doi.org/10.3390/su3101944






