Black Carbon’s Properties and Role in the Environment: A Comprehensive Review
Abstract
:1. Introduction
2. Properties of Black Carbon
2.1. Origin, Types and Composition
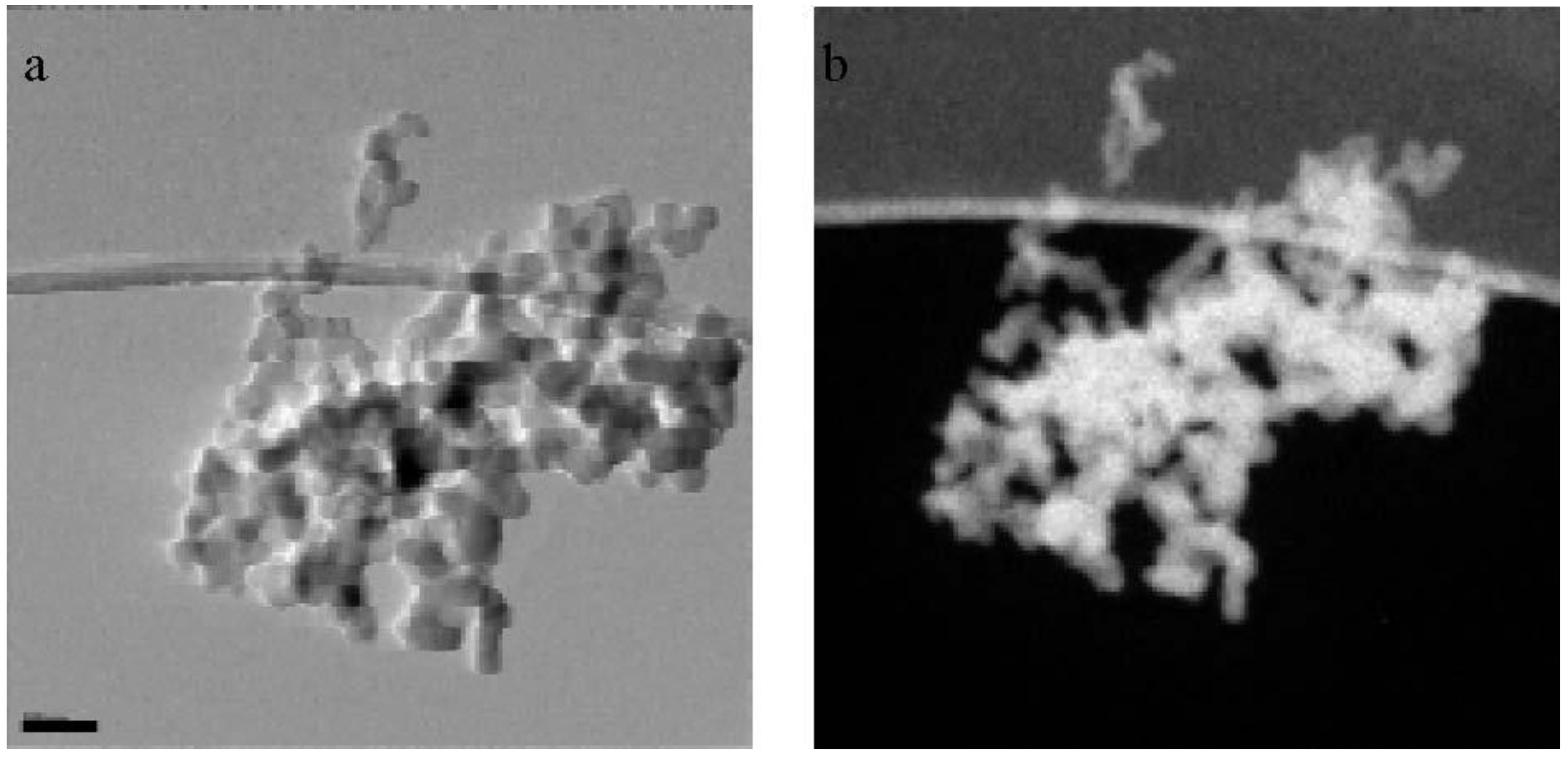
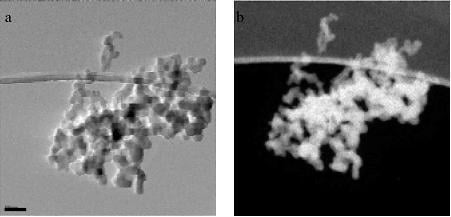
Black carbon versus elemental carbon
2.2. Fossil Fuel and Biomass Origins of BC: Attributing Sources of and with BC
2.2.1. Radiocarbons
2.2.2. PAH isomers
2.2.3. Particulate matter pollution
3. Formation Models for BC
4. Sorption Properties
5. Stability of Black Carbon in the Environment
Black Carbon Oxidation and Degradation: Review of Past Experiments
6. Black Carbon Extraction, Detection, Analysis
7. The Significance of BC in the Environment
7.1. The ‘Missing’ Sink of Carbon
| Carbon Stocks | Gt Carbon |
|---|---|
| Atmosphere | 750 |
| Oceans | 38,000 |
| Soils | 1,600 (455 in wetlands) |
| Land Plants | 560 (8 in wetlands) |
| Carbon Fluxes | Gt C yr–1 |
| Inputs | |
| Fossil Fuels | +5.4 |
| Deforestation and Land Use | +0.9 to +1.6 |
| Plant Respiration | +60 |
| Microbial Respiration | +60 |
| River DOC and DIC Transport | +/–0.8 |
| Outputs | |
| Photosynthesis | –120 |
| Peatland Accumulation | –0.07 |
| Ocean Sediment Burial | –0.1 |
| Oceanic Carbonate Equilibrium Processes | –2 |
| Accumulation in Atmosphere | –3.4 |
| Missing Sink | –1.4 to –1.6 |
| Carbon fluxes | Gt C yr–1 | |||
|---|---|---|---|---|
| Period | 1980s | 1990s | 2000–2005 | |
| Known major sources | ||||
| Fossil fuel combustion | +5.4 | +6.3 | +7.6 | |
| Land use change | +1.7 | +1.6 | +1.5 | |
| Total sources of carbon | +7.1 | +7.9 | +9.1 | |
| Known major sinks | ||||
| Atmospheric increase | –3.1 | –3.2 | –4.1 | |
| Ocean uptake | –1.9 | –2.1 | –2.2 | |
| Terrestrial uptake | –0.2 | –1.4 | –1.4 | |
| Total sinks of carbon | –5.2 | –6.7 | –7.7 | |
| Residual or missing sink of carbon | –1.9 | –1.2 | –1.4 | |
| Terrestrial carbon stock | Gt C | |||
| Period | 1800–1994 | 1850–2000 | ||
| Residual or missing sink of carbon | –135 | –116 | ||
7.2. Sorption of Pollutants
7.3. Health Effects
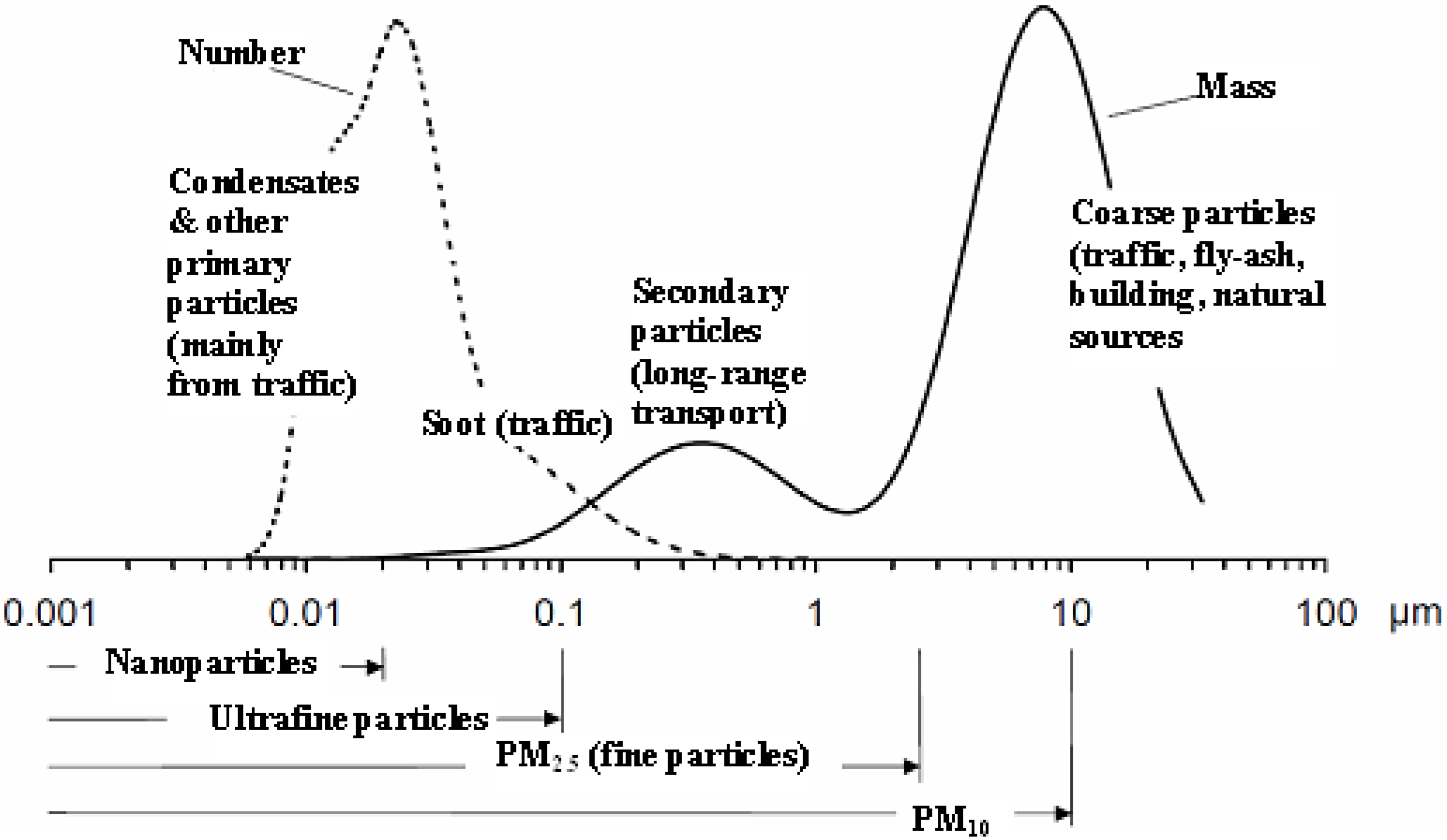
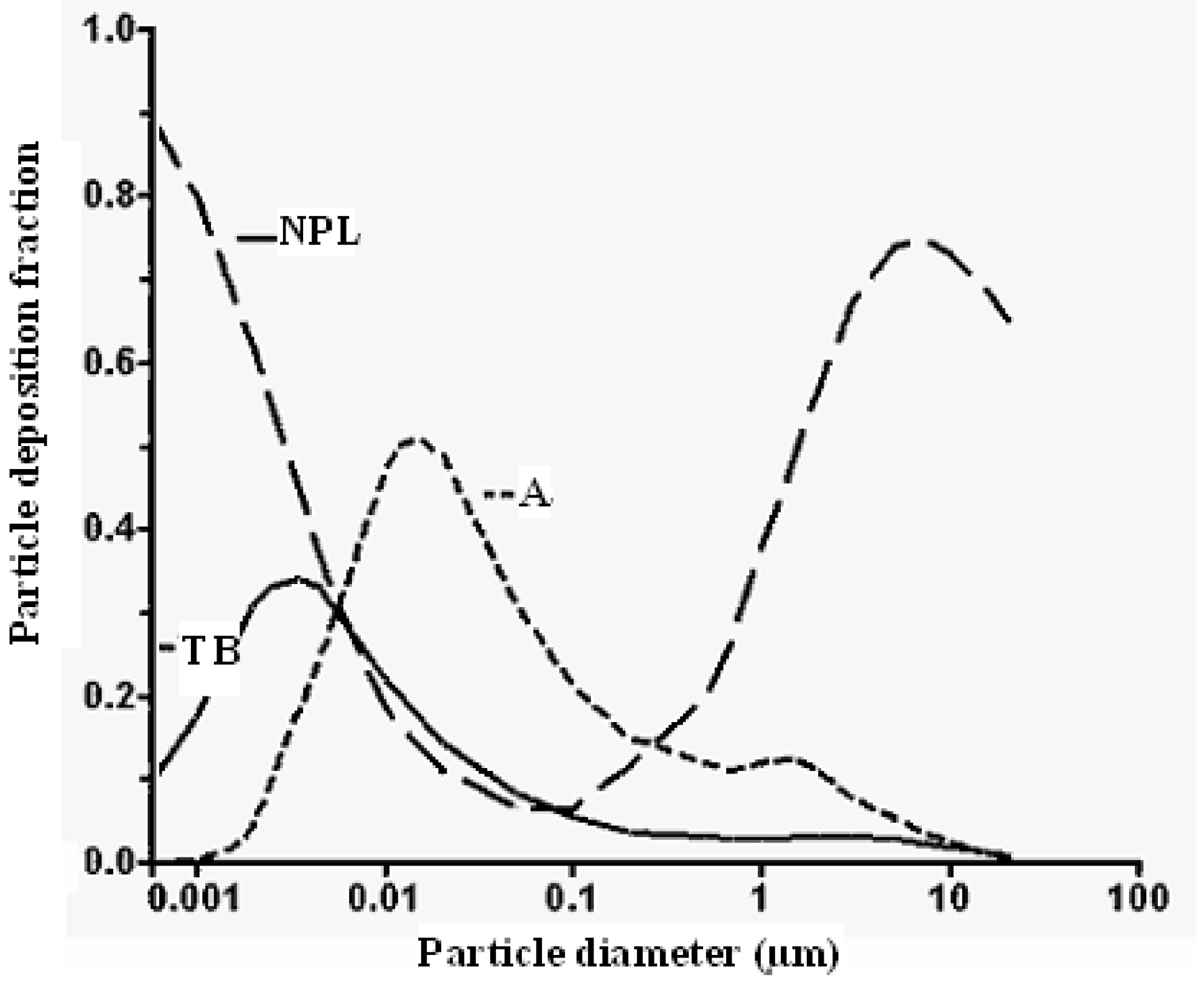
7.4. Climate Impacts
7.5. Sequestration of Carbon and Nutrients in the Pedosphere
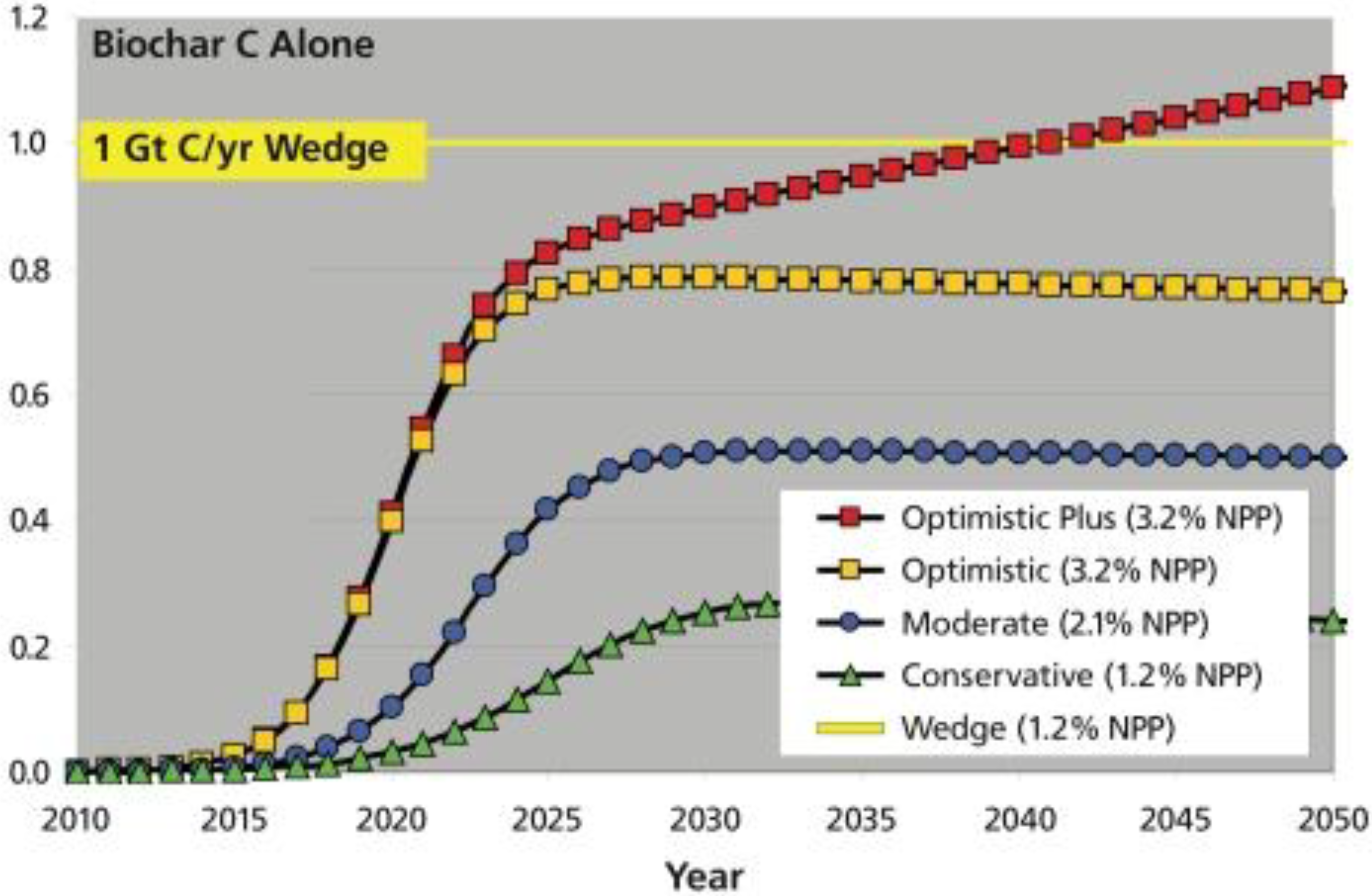
8. Conclusions
Acknowledgments
References
- NOAA/ESRL. Mauna Loa Carbon Dioxide Annual Mean Data. Available online: www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 10 October 2009).
- Bond, T.C.; Sun, H. Can reducing black carbon emissions counteract global warming? Environ. Sci. Technol. 2005, 39, 5921–5926. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Skjemstad, J.O.; Czimczik, C.I.; Glaser, B.; Prentice, K.M.; Gelinas, Y.; Kuhlbusch, T.A.J. Comparative analysis of black carbon in soils. Global Biogeochem. Cycle. 2001, 15, 163–167. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.F.; Pagels, J.; Zhang, D.; Xue, H.; McMurry, P.H. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. PNAS 2008, 105, 10291–10296. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, Y.; Marlow, W.H. Calculations of the equilibrium vapour-pressure of water over adhering 50–200 nm spheres. Aerosol Sci. Technol. 1995, 22, 43–59. [Google Scholar] [CrossRef]
- Suman, D.O.; Kuhlbusch, T.A.J.; Lim, B. Marine sediments: a reservoir for black carbon and their use as spatial and temporal records of combustion. In Sediment Records of Biomass Burning and Global Change; Clark, J.S., Cachier, H., Goldammer, J.G., Stocks, B.J., Eds.; Springer Verlag: Berlin, Germany, 1997. [Google Scholar]
- Clark, J.S.; Patterson, W.A. Background and local charcoal in sediments: scales of fire evidence in the paleorecord. In Sediment Records of Biomass Burning and Global Change; Clark, J.S., Cachier, H., Goldammer, J.G., Stocks, B.J., Eds.; Springer Verlag: Berlin, Germany, 1997; pp. 23–48. [Google Scholar]
- Masiello, C.A. New directions in black carbon organic geochemistry. Mar. Chem. 2004, 92, 201–213. [Google Scholar] [CrossRef]
- Hedges, J.I.; Eglinton, G.; Hatcher, P.G.; Kirchman, D.L.; Arnosti, C.; Derenne, S.; Evershed, R.P.; Kögel-Knabner, I.; de Leeuw, J.W.; Littke, R.; Michaelis, W.; Rullkötter, J. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 2000, 31, 945–958. [Google Scholar] [CrossRef]
- Dai, X.; Boutton, T.W.; Glaser, B.; Ansley, R.J.; Zech, W. Black carbon in a temperate mixed-grass savanna. Soil Biol. Biochem. 2005, 37, 1879–1881. [Google Scholar] [CrossRef]
- Goldberg, E.D. Black Carbon in the Environment: Properties and Distribution; Wiley: New York, NY, USA, 1985; p. 198. [Google Scholar]
- Elmquist, M.; Cornelissen, G.; Kukulska, Z.; Gustafsson, Ö. Distinct oxidative stabilities of char versus soot black carbon: Implications for quantification and environmental recalcitrance. Global Biogeochem. Cycle. 2006, 20. [Google Scholar] [CrossRef]
- Cope, M.J. Physical and chemical properties of coalified and charcoalified phytoclasts from some British Mesozoic sediments: an organic geochemical approach to paleobotany. In Proceedings of the International Meeting on Organic Geochemistry, Newcastle-Upon-Tyne, UK, 17–20 September 1979.
- Schwarzenbach, R.; Gschwend, P.; Imboden, D. Sorption processes involving organic matter. In Environmental Organic Chemistry, 2nd ed.; Wiley-Interscience: New York, NY, USA, 2002; pp. 275–330. [Google Scholar]
- Andreae, M.O.; Gelencsér, A. Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols. Atmos. Chem. Phys. 2006, 6, 3131–3148. [Google Scholar] [CrossRef]
- Graber, E.R.; Rudich, Y. Atmospheric HULIS: how humic-like are they? A comprehensive and critical review. Atmos. Chem. Phys. 2006, 6, 729–753. [Google Scholar] [CrossRef]
- Lukács, H.; Gelencsr, A.; Hammer, S.; Puxbaum, H.; Pio, C.; Legrand, M.; Kasper-Giebl, A.; Handler, M.; Limbeck, A.; Simpson, D.; Preunkert, S. Seasonal trends and possible sources of brown carbon based on 2-year aerosol measurements at six sites in Europe. J. Geophys. Res. 2007, 112, D23S18. [Google Scholar] [CrossRef]
- Novakov, T.; Andreae, M.O.; Gabriel, R.; Kirchstetter, T.W.; Mayol-Bracero, O.L.; Ramanathan, V. Origin of carbonaceous aerosols over the tropical Indian Ocean: biomass burning or fossil fuels? Geophys. Res. Lett. 2000, 27, 4061–4064. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, C.; Zech, W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Fitzpatrick, E.M.; Jones, J.M.; Pourkashanian, M.; Ross, A.B.; Williams, A.; Bartle, K.D. Mechanistic aspects of soot formation from the combustion of pine wood. Energ. Fuel. 2008, 22, 3771–3778. [Google Scholar] [CrossRef]
- Masiello, C.A.; Druffel, E.R.M. Black carbon in deep-sea sediments. Science 1998, 280, 1911–1913. [Google Scholar] [CrossRef] [PubMed]
- Masiello, C.A.; Druffel, E.R.M.; Currie, L.A. Radiocarbon measurements of black carbon in aerosols and ocean sediments. Geochim. Cosmochim. Acta 2002, 66, 1025–1036. [Google Scholar] [CrossRef]
- Reddy, C.M.; Pearson, A.; Xu, L.; McNichol, A.P.; Benner, B.A.; Wise, S.A.; Klouda, G.A.; Currie, L.A.; Eglinton, T.I. Radiocarbon as a tool to apportion the sources of polycyclic aromatic hydrocarbons and black carbon in environmental samples. Environ. Sci. Technol. 2002, 36, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Dickhut, R.M.; Canuel, E.A.; Gustafson, K.E.; Liu, K.; Arzayus, K.Y.; Walker, S.E.; Edgecombe, G.; Gaylor, M.O.; MacDonald, E.H. Automotive Sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay Region. Environ. Sci. Technol. 2000, 34, 4635. [Google Scholar] [CrossRef]
- Mitra, S.; Bianchi, T.S.; McKee, B.A.; Sutula, M. Black carbon from the Mississippi River: quantities, sources, and potential implications for the global carbon cycle. Environ. Sci. Technol. 2002, 36, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.L.; Larson, T.V.; Koenig, J.Q.; Mar, T.F.; Fields, C.; Stewart, J.; Lippmann, M. Associations between health effects, particulate matter, and BC in subjects with respiratory disease. Environ. Health Perspect. 2005, 113, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Ogren, J.A.; Charlson, R.J. Elemental carbon in the atmosphere: cycle and lifetime. Tellus 1983, 35, 241–254. [Google Scholar] [CrossRef]
- Cooke, W.F.; Wilson, J.J.N. A global black carbon aerosol model. J. Geophys. Res. 1996, 101, 19395–19409. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Bruhl, C.; Galbally, I.E. Atmospheric effects from post-nuclear fires. Climatic Change 1984, 6, 323–364. [Google Scholar] [CrossRef]
- Putaud, J.P.; Raes, F.; Dingenen, R.V.; Baltensperger, J.P.U.; Brüggemann, E.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Gehrig, R.; Hansson, H.C.; Hüglin, C.; Laj, P.; Lorbeer, G.; Maenhaut, W.; Mihalopoulos, N.; Müller, K.; Querol, X.; Rodriguez, S.; Schneider, R.; Spindler, J.; Brink, H.; Wehner, T.B.; Wiedensohler, A. A European aerosol phenomenology—2: chemical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos. Environ. 2004, 38, 2579–2595. [Google Scholar] [CrossRef]
- Phalen, R.F.; Kleinman, M.T. Inhalation Toxicology of Combined Acid and Soot Particles; Research Contract No. A5-129-33, Final Report; California Air Resources Board: Sacramento, CA, USA, 1989. Available online: http://www.arb.ca.gov/research/apr/past/a5-129-33.pdf. (accessed on 1 January 2006).
- EPA. National Ambient Air Quality Standards (NAAQS). Available online: http://www.epa.gov/ttn/naaqs/ (accessed on 5 February 2007).
- Esser, G.; Hoffstadt, J.; Mack, F.; Wittenberg, U. High Resolution Biosphere Model, Documentation, Model Version 3.00.00; Institut für Planzenökologie, Justus-Liebig-Universität: Gießen, Germany, 1994. [Google Scholar]
- Vigouroux, R.Z. Pyrolysis of Biomass. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2001. [Google Scholar]
- Demirbas, A. Mechanism of liquefaction and pyrolysis reactions of biomass. Energ. Conv. Manage. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Bridgewater, A.V.; Peacoke, G.V.C. Fast pyrolysis for biomass. Renew. Sust. Energ. Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Eckstrom, C.; Rensfelt, E. Flash pyrolysis of biomass in Sweden. In Proceedings of Specialist’s Workshop on Fast Pyrolysis of Biomass, Copper Mountain, CO, USA, 20 October 1980; pp. 622–1096.
- Kuhlbusch, T.A.J. Black carbon as a product of savanna fires in southern Africa: a sink of biospheric carbon. In Proceedings of the 1993 AGU Fall Meeting, San Francisco, CA, USA, December 1993.
- Kuhlbusch, T.A.J.; Crutzen, P.J. Black carbon, the global carbon cycle, and atmospheric carbon dioxide. In Biomass Burning and Global Change; Levine, J.S., Ed.; MIT Press: Cambridge, MA, USA, 1996; pp. 161–169. [Google Scholar]
- Demirbas, A. Effect of temperature on pyrolysis products from biomass. Energ. Source, Part A 2007, 29, 329–336. [Google Scholar] [CrossRef]
- Penner, J.E.; Chuang, C.C.; Grant, K. Climate forcing by carbonaceous and sulfate aerosols. Clim. Dynam. 1998, 14, 839–851. [Google Scholar] [CrossRef]
- Grant, K.E.; Chuang, C.C.; Grossman, A.S.; Penner, J. E. Modeling the spectral optical properties of ammonium sulfate and biomass burning aerosols: parameterization of relative humidity effects and model results. Atmos. Environ. 1999, 33, 2603–2620. [Google Scholar] [CrossRef]
- Schaap, M.; Denier van der Gon, H.A.C. On the variability of black smoke and carbonaceous aerosols in the Netherlands. Atmos. Environ. 2007, 41, 5908–5920. [Google Scholar] [CrossRef]
- Smith, D.M.; Chughtai, A.R. The surface structure and reactivity of black carbon. Colloid Surface A 1995, 105, 47–77. [Google Scholar] [CrossRef]
- Frenklach, M. Soot Formation in Combustion: Mechanisms and Models; Bockhorn, H., Ed.; Springer-Verlag: Berlin, Germany, 1994; pp. 162–192. [Google Scholar]
- Frenklach, M. Soot modeling. Available online: www.me.berkeley.edu/soot/ (accessed on 10 August 2009).
- vander Wal, R.L.; Mueller, C.J. Initial investigation of effects of fuel oxygenation on nanostructure of soot from a direct-injection diesel engine. Energ. Fuel. 2006, 20, 2364–2369. [Google Scholar] [CrossRef]
- Violi, A.; Sarofim, A.F.; Voth, G.A. Kinetic monte carlo molecular dynamics approach to model soot inception. Combust. Sci. Technol. 2004, 176, 991–1005. [Google Scholar] [CrossRef]
- Gustafson, O.; Gschwend, P.M. Soot as a strong partition medium for polycyclic aromatic hydrocarbons. In Molecular Markers in Environmental Geochemistry; Eganhouse, R.P., Ed.; American Chemical Society: Washington, DC, USA, 1997; pp. 365–381. [Google Scholar]
- Bärring, H.; Bucheli, T.D.; Broman, D.; Gustafsson, Ö. Soot-water distribution coefficients for polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and polybrominated diphenylethers determined with the soot cosolvency-column method. Chemosphere 2002, 49, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Mattila, T.J.; Verta, M. Modeling the importance of biota and black carbon as vectors of polybrominated diphenyl ethers (PBDEs) in the baltic sea ecosystem. Environ. Sci. Technol. 2008, 42, 4831–4836. [Google Scholar] [CrossRef] [PubMed]
- Dachs, J.; Eisenreich, S.J. Adsorption onto aerosol soot carbon dominates gas-particle partitioning of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2000, 34, 3690. [Google Scholar] [CrossRef]
- Jones, J.M.; Ross, A.B.; Williams, A. Atmospheric chemistry implications of the emission of biomass smoke. J. Energy Inst. 2005, 78, 199–200. [Google Scholar] [CrossRef]
- Oen, A.M.P.; Cornelissen, G.; Breedveld, G.D. Relation between PAH and black carbon contents in size fractions of Norwegian harbor sediments. Environ. Pollut. 2006, 141, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.; Zimmerman, J.R.; Luthy, R.G. PCB and PAH speciation among particle types in contaminated harbor sediments and effects on PAH bioavailability. Environ. Sci. Technol. 2003, 37, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, O.; Haghseta, F.; Chan, C.; MacFarlane, J.; Gschwend, P.M. Quantification of the dilute sedimentary soot phase: Implications for PAH speciation and bioavailability. Environ. Sci. Technol. 1996, 31, 203–209. [Google Scholar] [CrossRef]
- Accardi-Dey, A.; Gschwend, P.M. Assessing the combined roles of natural organic matter and black carbon as sorbents in sediments. Environ. Sci. Technol. 2001, 36, 21–29. [Google Scholar] [CrossRef]
- Forbes, M.S.; Raison, R.J.; Skjemstad, J.O. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ. 2006, 370, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal: a review. Biol. Fert. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Dickens, A.F.; Gelinas, Y.; Hedges, J.I. Physical separation of combustion and rock sources of graphitic black carbon in sediments. Mar. Chem. 2004, 92, 215–223. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Andreae, M.O. Biomass burning in the tropics: impact on atmospheric chemistry and biogeochemical cycles. Science 1990, 250, 1669. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbusch, T.A.J. Method for determining black carbon in residues of vegetation fires. Environ. Sci. Technol. 1995, 29, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Global Biogeochem. Cycle. 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Rumpel, C.; Alexis, A.; Chabbi, A.; Chaplot, V.; Rasse, D.P.; Valentin, C. Black carbon contribution to soil organic matter composition in tropical sloping land under slash and burn agriculture. Geoderma 2006, 130, 35–46. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Reicosky, D.C.; Wilts, A.R.; McGowan, J.A. Charcoal carbon in U.S. agricultural soils. Soil Sci. Soc. Am. J. 2002, 66, 1249–1255. [Google Scholar] [CrossRef]
- Kim, S.; Kaplan, L.A.; Benner, R.; Hatcher, P.G. Hydrogen-deficient molecules in natural riverine water samples—evidence for the existence of black carbon in DOM. Mar. Chem. 2004, 92, 225–234. [Google Scholar] [CrossRef]
- Hockaday, W.C.; Grannas, A.M.; Kim, S.; Hatcher, P.G. The transformation and mobility of charcoal in a fire-impacted watershed. Geochim. Cosmochim. Acta 2007, 71, 3432–3445. [Google Scholar] [CrossRef]
- Lehmann, J.; Liang, B.; Solomon, D.; Lerotic, M.; Luizão, F.; Kinyangi, J.; Schäfer, T.; Wirick, S.; Jacobsen, C. Near-edge X-ray absorption fine structure (NEXAFS) spectroscopy for mapping nano-scale distribution of organic carbon forms in soil: application to black carbon particles. Global Biogeochem. Cycle. 2005, 19, 12. [Google Scholar]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralisation in soil. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Donnet, J.B.; Ehrburger, P.; Voet, A. Etude du mecanisme d’oxydation des noirs de carbone par l’ozone en milieu aqueux. Carbon 1972, 10, 737–746. [Google Scholar] [CrossRef]
- Chughtai, A.R.; Jassim, J.A.; Peterson, J.H.; Stedman, D.H.; Smith, D.M. Spectroscopic and solubility characteristics of oxidized soots. Aerosol Sci. Technol. 1991, 15, 112–126. [Google Scholar] [CrossRef]
- Shneour, E.A. Oxidation of graphitic carbon in certain soils. Science 1966, 151, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Hilscher, A.; Hagedorn, F.; Knicker, H. Incorporation of black carbon into soil organic matter of forested high-elevation soils in Switzerland. Geophys. Res. Absrt. 2006, 8, 06544, SRef-ID: 1607-7962/gra/EGU06-A-06544. [Google Scholar]
- Lim, B.; Cachier, H. Determination of black carbon by chemical oxidation and thermal treatment in recent marine and lake sediments and Cretaceous-Tertiary clays. Chem. Geol. 1996, 131, 143–154. [Google Scholar] [CrossRef]
- Jeong, B.S.; Wander, M.M.; Kleineidam, S.; Grathwohl, P.; Ligouis, B.; Werth, C.J. The role of condensed carbonaceous materials on the sorption of hydrophobic organic contaminants in subsurface sediments. Environ. Sci. Technol. 2008, 42, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Werth, C.J. Evaluation of methods to obtain geosorbent fractions enriched in carbonaceous materials that affect hydrophobic organic chemical sorption. Environ. Sci. Technol. 2005, 39, 3279–3288. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H.; Muller, P.; Hilscher, A. How useful is chemical oxidation with dichromate for the determination of ‘black carbon’ in fire-affected soils? Geoderma 2007, 142, 178–196. [Google Scholar] [CrossRef]
- Rumpel, C.; Gonzalez-Perez, J.A.; Bardoux, G.; Largeau, C.; Gonzalez-Vila, F.J.; Valentin, C. Composition and reactivity of morphologically distinct charred materials left after slash-and-burn practices in agricultural tropical soils. Org. Geochem. 2007, 38, 911–920. [Google Scholar] [CrossRef]
- Simpson, M.J.; Hatcher, P.G. Overestimates of black carbon in soils and sediments. Naturwissenschaften 2004, 91, 436–440. [Google Scholar]
- Arnarson, T.S.; Keil, R.G. Organic-mineral interactions in marine sediments studied using density fractionation and X-ray photoelectron spectroscopy. Org. Geochem. 2001, 32, 1401–1415. [Google Scholar] [CrossRef]
- Anderson, R.S.; Smith, S.J. The sedimentary record of fire in montane meadows, Sierra Nevada, California, USA: a preliminary assessment. In Sediment Records of Biomass Burning and Global Change I; Clark, J.S., Cachier, H., Goldammer, J.G., Stocks, B.J., Eds.; Springer Verlag: Berlin, Germany, 1997; Vol. 51, pp. 313–327. [Google Scholar]
- Krull, E.S.; Swanston, C.W.; Skjemstad, J.O.; McGowan, J.A. Importance of charcoal in determining the age and chemistry of organic carbon in surface soils. J. Geophys. Res. 2006, 111, G04001. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The DRI thermal/optical reflectance carbon analysis system: description, evaluation and application in U.S. air quality studies. Atmos. Environ. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Eatough, D.J.; Obeidi, F.; Pang, Y.; Ding, Y.; Eatough, N.L.; Wilson, W.E. Integrated and real-time diffusion denuder sampler for PM2.5. Atmos. Environ. 1999, 33, 2835–2844. [Google Scholar] [CrossRef]
- Schmidt, W.I. Black Carbon in Soils Looking for the Missing Carbon; Universität Zürich: Zurich, Switzerland, 2005. [Google Scholar]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ‘Terra Preta’ phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Druffel, E.R.M.; Williams, P.M.; Livingston, H.D.; Koide, M. Variability of natural and bomb-produced radionuclide distributions in abyssal red clay sediments. Earth Planet. Sci. Lett. 1984, 71, 205–214. [Google Scholar] [CrossRef]
- Haberstroh, P.R.; Brandes, J.A.; Gelinas, Y.; Dickens, A.F.; Wirick, S.; Cody, G. Chemical composition of the graphitic black carbon fraction in riverine and marine sediments at sub-micron scales using carbon X-ray spectromicroscopy. Geochim. Cosmochim. Acta 2006, 70, 1483–1494. [Google Scholar] [CrossRef]
- Penner, J.E.; Eddleman, H.; Novakov, T. Towards the development of a global inventory of black carbon emissions. Atmos. Environ. 1993, 27, 1277–1295. [Google Scholar] [CrossRef]
- Schmidt, M.W.I. Carbon budget in the black. Nature 2004, 427, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.A.; Hackler, J.L.; Lawrence, K.T. The US carbon budget: contributions from land-use change. Science 1999, 285, 574. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Black and buried carbons’ impacts on soil quality and ecosystem services. Soil Till. Res. 2008, 99, 1–3. [Google Scholar] [CrossRef]
- IPCC. Land Use, Land-Use Change and Forestry; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Climate Change 2007: The Physical Science Basis—Summary for Policymakers; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2007.
- Koelmans, A.A.; Jonker, M.T.O.; Cornelissen, G.; Bucheli, T.D.; van Noort, P.C.M.; Gustafsson, O. Black carbon: the reverse of its dark side. Chemosphere 2006, 63, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Albert, R.E. The effect of particle size on the regional deposition of inhaled aerosols in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 1969, 30, 257–275. [Google Scholar] [CrossRef] [PubMed]
- ICRP. ICRP Publication 66: Human Respiratory Tract Model for Radiological Protection I; Elsevier: Amsterdam, The Netherlands, 1994; Vol. 24. [Google Scholar]
- Forsberg, C. Will an increased greenhouse impact in Fennoscandia give rise to more humic and coloured lakes? Hydrobiologia 1992, 229, 51–58. [Google Scholar] [CrossRef]
- Kocbach, A.; Li, Y.; Yttri, K.; Cassee, F.; Schwarze, P.; Namork, E. Physicochemical characterisation of combustion particles from vehicle exhaust and residential wood smoke. Part. Fibre Toxicol. 2006, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 3. C-1-C-29 organic compounds from fireplace combustion of wood. Environ. Sci. Technol. 2001, 35, 1716. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Yu, C.P. The carcinogenic potential of inhaled diesel exhaust: a particle effect? J. Aerosol Sci. 1990, 21, S397–S401. [Google Scholar] [CrossRef]
- Schlesinger, R.B. Disposition of inhaled particles and gases. In Pulmonary Immunotoxicology; Cohen, M., Zelikoff, J., Schlesinger, B., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2000. [Google Scholar]
- Health Effects Assessment of Exposure to Particles from Wood Smoke; Environmental Project No. 1235; Danish Environmental Protection Agency: Copenhagen, Denmark, 2008.
- Ramanathan, V. Indian Ocean experiment: an integrated analysis of the climate forcing and effects of the great Indo-Asian haze. J. Geophys. Res. 2001, 106, 28371–28398. [Google Scholar] [CrossRef]
- Chung, C.; Ramanathan, V.; Kim, D.; Podgorny, I.A. Global anthropogenic aerosol direct forcing derived from satellite and ground-based observations. J. Geophys. Res. 2005, 110, D24207. [Google Scholar] [CrossRef]
- Ramanathan, V. Warming trends in Asia amplified by brown cloud solar absorption. Nature 2007, 448, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, V. Role of Black Carbon in Global and Regional Climate Changes; Testimonial to House Committee on Oversight and Government Reform: Washington, DC, USA, 2007; Vol. 17. [Google Scholar]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. 2007: Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Boucher, O.; Reddy, M.S. Climate trade-off between black carbon and carbon dioxide emissions. Energ. Policy 2008, 36, 193–200. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Control of fossil-fuel particulate black carbon and organic matter, possibly the most effective method of slowing global warming. J. Geophys. Res. 2002, 107, 4410. [Google Scholar] [CrossRef]
- Seinfeld, J. Atmospheric science: black carbon and brown clouds. Nature Geosci. 2008, 1, 15–16. [Google Scholar] [CrossRef]
- Schuster, G.L.; Dubovik, O.; Holben, B.N.; Clothiaux, E.E. Inferring black carbon content and specific absorption from Aerosol Robotic Network (AERONET) aerosol retrievals. J. Geophys. Res. 2005, 110, D10S17. [Google Scholar] [CrossRef]
- Sato, M. Global atmospheric black carbon inferred from AERONET. Proc. Natl. Acad. Sci. USA 2003, 100, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Stier, P.; Seinfeld, J. H.; Kinne, S.; Boucher, O. Aerosol absorption and radiative forcing. Atmos. Chem. Phys. 2007, 7, 5237–5261. [Google Scholar] [CrossRef]
- Meehl, G.A.; Arblaster, J.M.; Collins, W.D. Effects of black carbon aerosols on the Indian monsoon. J. Climate 2007, 21, 2861–2881. [Google Scholar]
- Lau, K.M.; Ramanathan, V.; Wu, G.X.; Li, Z.; Tsay, S.C.; Hsu, C.; Sikka, R.; Holben, B.; Lu, D.; Tartari, G.; Chin, M.; Koudelova, P.; Chen, H.; Ma, Y.; Huang, J.; Taniguchi, K.; Zhang, R. The joint aerosol-monsoon experiment: a new challenge for monsoon climate research. B. Am. Meteorol. Soc. 2008, 369–383. [Google Scholar] [CrossRef]
- Ramanathan, V. Atmospheric brown clouds: impacts on South Asian climate and hydrologic cycle. Proc. Natl. Acad. Sci. USA 2005, 102, 5326–5333. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Nazarenko, L. Soot climate forcing via snow and ice albedos. Proc. Natl. Acad. Sci. USA 2004, 101, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Noone, K. Soot in the Arctic: a cause for perturbation in radiative transfer. J. Geophys. Res. 1985, 19, 2045–2053. [Google Scholar]
- Flanner, M.G.; Zender, C.S.; Randerson, J.T.; Rasch, P.J. Present-day climate forcing and response from black carbon in snow. J. Geophys. Res. 2007, 112, D11202. [Google Scholar] [CrossRef]
- Ramanathan, V.; Carmichael, G. Global and regional climate changes due to black carbon. Nature Geosci. 2008, 1, 221–227. [Google Scholar] [CrossRef]
- Thompson, L.G. Tropical glacier and ice core evidence of climate changes on annual to millenial time scales. Climatic Change 2003, 59, 137–155. [Google Scholar] [CrossRef]
- Lehmann, J.; Skjemstad, J.; Sohi, S.; Carter, J.; Barson, M.; Falloon, P.; Coleman, K.; Woodbury, P.; Krull, E. Australian climate-carbon cycle feedback reduced by soil black carbon. Nature Geosci. 2008, 1, 832–835. [Google Scholar] [CrossRef]
- Neves, E.G.; Petersen, J.B.; Bartone, R.N.; Heckenberger, M.J. The timing of terra preta formation in the central Amazon: archaeological data from three sites. In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W., Eds.; Springer: Berlin, Germany, 2004; pp. 125–134. [Google Scholar]
- Lehmann, J.; Kern, D.; Glaser, B.; Woods, W. Amazonian Dark Earths: Origin, Properties, Management; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Baldock, J A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—a review. Mitig. Adapt. Strat. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Ball, R. Combustion of biomass as a global carbon sink. Open Thermodyn. J. 2008, 2, 106–108. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; Neves, E.G. Black carbon increases cation exchange capacity in soils. Soil. Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Lenton, T.M.; Vaughan, N.E. The radiative forcing potential of different climate geoengineering options. Atmos. Chem. Phys. Disc. 2009, 9, 2559–2608. [Google Scholar] [CrossRef]
- International Biochar Initiative. How much carbon can biochar systems offset-and when? Available online: http://www.biochar-international.org/images/final_carbon.pdf. (accessed on 20 October 2009).
- Tenenbaum, D.J. Biochar: carbon mitigation from the ground up. Environ. Health Perspect. 2009, 117, A70–A73. [Google Scholar] [CrossRef] [PubMed]
- Climate Change Science Compendium; United Nations Environment Programe (UNEP): Nairobi, Kenya, 2009.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shrestha, G.; Traina, S.J.; Swanston, C.W. Black Carbon’s Properties and Role in the Environment: A Comprehensive Review. Sustainability 2010, 2, 294-320. https://doi.org/10.3390/su2010294
Shrestha G, Traina SJ, Swanston CW. Black Carbon’s Properties and Role in the Environment: A Comprehensive Review. Sustainability. 2010; 2(1):294-320. https://doi.org/10.3390/su2010294
Chicago/Turabian StyleShrestha, Gyami, Samuel J. Traina, and Christopher W. Swanston. 2010. "Black Carbon’s Properties and Role in the Environment: A Comprehensive Review" Sustainability 2, no. 1: 294-320. https://doi.org/10.3390/su2010294
APA StyleShrestha, G., Traina, S. J., & Swanston, C. W. (2010). Black Carbon’s Properties and Role in the Environment: A Comprehensive Review. Sustainability, 2(1), 294-320. https://doi.org/10.3390/su2010294