Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives
Abstract
1. Introduction
2. Diversity and Classification of Lignocellulose-Degrading Microorganisms
2.1. Classification of Fungi in Lignocellulose Degradation
2.2. Classification of Bacteria in Lignocellulose Degradation
3. Diversity and Classification of Lignocellulose-Degrading Enzymes
3.1. Cellulose-Degrading Enzymes
3.2. Hemicellulases
3.3. Lignin-Degrading Enzymes
4. Factors Influencing Lignocellulose Degradation
4.1. Impact of Environmental Factors
4.2. Impact of Substrate Characteristics
4.3. Impact of Microbial Diversity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, G.L.; Tišma, M.; He, B.R.; Zhai, X.H.; Yuan, C.Y.; Su, Z.D.; Shi, J.P.; Zhang, B.G. Valorization of the Caragana waste via two-stage bioaugmentation: Optimizing nutrition composition, palatability, and microbial contaminant control. J. Bioresour. Bioprod. 2024, 9, 518–533. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility—A review. Biofuels Bioprod. Biorefining 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Tessera, G.M.; Habtu, N.G.; Abera, M.K.; Misganaw, F.W. Advances in electromagnetic radiation-assisted pretreatment of lignocellulosic biomass as a green method: A review. Biomass Convers. Biorefinery 2024, 1–25. [Google Scholar] [CrossRef]
- Lu, H.D.; Yadav, V.; Bilal, M.; Iqbal, H.M.N. Bioprospecting microbial hosts to valorize lignocellulose biomass—Environmental perspectives and value-added bioproducts. Chemosphere 2022, 288, 132574. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Lu, H.D.; Yadav, V.; Zhong, M.Y.; Bilal, M.; Taherzadeh, M.J.; Iqbal, H.M.N. Bioengineered microbial platforms for biomass-derived biofuel production—A review. Chemosphere 2022, 288, 132528. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Zhu, J.W.; Gong, D.H.; Mao, J.H.; Qi, W. Research Progress of Lignocellulosic Biomass Pretreatment Technology. Adv. New Renew. Energy 2022, 10, 383–392. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Freitas, S. On the production cost of lignocellulose-degrading enzymes. Biofuels Bioprod. Biorefining 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; NREL/TP-5100-47764; National Renewable Energy Laboratory, US Department of Energy: Golden, CO, USA, 2011. [Google Scholar] [CrossRef]
- Barta, Z.; Kovacs, K.; Reczey, K.; Zacchi, G. Process design and economics of on-site cellulase production on various carbon sources in a softwood-based ethanol plant. Enzym. Res. 2010, 2010, 734182. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Nizami, A.-S.; Pour Bafrani, M.; Saville, B.A.; MacLean, H.L. Impact of cellulase production on environmental and financial metrics for lignocellulosic ethanol. Biofuels Bioprod. Biorefining 2013, 7, 303–313. [Google Scholar] [CrossRef]
- Ferreira, R.d.G.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef]
- De Castro, A.M.; López, J.A.; dos Reis Castilho, L.; Freire, D.M.G. Techno-economic analysis of a bioprocess for the production of multienzyme solutions from the cake of babassu industrial processing: Evaluation of five different inoculum propagation strategies. Biomass Convers. Biorefinery 2014, 4, 237–247. [Google Scholar] [CrossRef]
- Iimura, Y.; Abe, H.; Otsuka, Y.; Sato, Y.; Habe, H. Bacterial Community Coexisting with White-Rot Fungi in Decayed Wood in Nature. Curr. Microbiol. 2021, 78, 3212–3217. [Google Scholar] [CrossRef]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Takada, K.; Elkasaby, T.; Pangestu, R.; Toyoshima, M.; Kahar, P.; Ogino, C.; Kaneko, T.; Kondo, A. Recent advances in lignocellulosic biomass white biotechnology for bioplastics. Bioresour. Technol. 2022, 344, 126165. [Google Scholar] [CrossRef]
- Cedeno, F.R.P.; Siqueira, B.B.d.; Chavez, E.G.S.; Roldán, I.U.M.; Ropelato, L.M.; Galán, J.P.M.; Masarin, F. Recovery of cellulose and lignin from Eucalyptus by-product and assessment of cellulose enzymatic hydrolysis. Renew. Energy 2022, 193, 807–820. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, K.K. Fungal lignocellulolytic enzymes and lignocellulose: A critical review on their contribution to multiproduct biorefinery and global biofuel research. Int. J. Biol. Macromol. 2021, 193, 2304–2319. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.Q.; Cui, L.C.; Li, X.; Kulyar, M.F.; Xiong, H.; Zhou, N.; Yin, H.; Li, J.; Li, X. Isolation, characterization, and interaction of lignin-degrading bacteria from rumen of buffalo (Bubalus bubalis). J. Basic Microbiol. 2021, 61, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Ligninolytic Enzymes Mediated Ligninolysis: An Untapped Biocatalytic Potential to Deconstruct Lignocellulosic Molecules in a Sustainable Manner. Catal. Lett. 2020, 150, 524–543. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, H.L.; Yang, Y.; Wang, R.; Wang, C.J.; Wei, X.; Chen, S.W.; Yu, J.; Ma, X. Enhanced Lignin Degradation in Tobacco Stalk Composting with Inoculation of White-Rot Fungi Trametes hirsuta and Pleurotus ostreatus. Waste Biomass Valorization 2020, 11, 3525–3535. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; Woiciechowski, A.L.; Malanski, R.; Letti, L.A.J.; Soccol, C.R. Pulp improvement of oil palm empty fruit bunches associated to solid-state biopulping and biobleaching with xylanase and lignin peroxidase cocktail produced by Aspergillus sp. LPB-5. Bioresour. Technol. 2019, 285, 121361. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Darwesh, O.M.; Matter, I.A.; El-Saied, H. Isolation and characterization of non-cellulolytic Aspergillus flavus EGYPTA5 exhibiting selective ligninolytic potential. Biocatal. Agric. Biotechnol. 2018, 17, 160–167. [Google Scholar] [CrossRef]
- Zhao, B.; Al Rasheed, H.; Ali, I.; Hu, S. Efficient enzymatic saccharification of alkaline and ionic liquid-pretreated bamboo by highly active extremozymes produced by the co-culture of two halophilic fungi. Bioresour. Technol. 2021, 319, 124115. [Google Scholar] [CrossRef]
- Srivastava, N.; Elgorban, A.M.; Mishra, P.K.; Marraiki, N.; Alharbi, A.M.; Ahmad, I.; Gupta, V.K. Enhance production of fungal cellulase cocktail using cellulosic waste. Environ. Technol. Innov. 2020, 19, 100949. [Google Scholar] [CrossRef]
- Naik, S.N.K.; Anuradha, C.M.; Cheemanapalli, S.; Kumar, C. Comparison of Production of Cellulolytic Enzymes by Fusarium sp. Under Optimized and Non-optimized Conditions. J. Environ. Bio-Sci. 2021, 34, 153–168. Available online: https://connectjournals.com/03843.2020.34.153 (accessed on 9 April 2025).
- Santos, G.B.; de Sousa Francisco Filho, Á.; Rêgo da Silva Rodrigues, J.; Rodrigues de Souza, R. Cellulase production by Aspergillus niger using urban lignocellulosic waste as substrate: Evaluation of different cultivation strategies. J. Environ. Manag. 2022, 305, 114431. [Google Scholar] [CrossRef]
- Mahmood, R.T.; Masood, M.; Zia, N.; Safder, A.; Asad, M.; Nasreen, S.; Ahmed, D.; Iqbal, R. Production and Characterization of Endoglucanase from Phaeo-lus spadiceus. Pak. J. Biochem. Biotechnol. 2021, 2, 27–37. [Google Scholar] [CrossRef]
- Alves, T.P.; Triques, C.C.; da Silva, E.A.; Fagundes-Klen, M.R.; Hasan, S.D.M. Multi-enzymatic recovery of fungal cellulases (Aspergillus niger) through solid-state fermentation of sugarcane bagasse. Can. J. Chem. Eng. 2022, 100, 1930–1940. [Google Scholar] [CrossRef]
- Gooruee, R.; Hojjati, M.; Behbahani, B.A.; Shahbazi, S.; Askari, H. Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Convers. Biorefinery 2024, 14, 2777–2786. [Google Scholar] [CrossRef]
- Dhaver, P.; Brett, P.; Bruce, S.; Govinden, R. Isolation, screening, preliminary optimisation and characterisation of thermostable xylanase production under submerged fermentation by fungi in Durban, South Africa. Mycology 2022, 13, 271–292. [Google Scholar] [CrossRef]
- Intasit, R.; Cheirsilp, B.; Suyotha, W.; Boonsawang, P. Purification and characterization of a highly-stable fungal xylanase from Aspergillus tubingensis cultivated on palm wastes through combined solid-state and submerged fermentation. Prep. Biochem. Biotechnol. 2022, 52, 311–317. [Google Scholar] [CrossRef]
- Barbieri, G.S.; Bento, H.B.S.; de Oliveira, F.; Picheli, F.P.; Dias, L.M.; Masarin, F.; Santos-Ebinuma, V.C. Xylanase Production by Talaromyces amestolkiae Valuing Agroindustrial Byproducts. Biotech 2022, 11, 15. [Google Scholar] [CrossRef]
- Ambatkar, N.; Jadhav, D.D.; Nandi, S.; Kumbhar, P.; Kommoju, P.R. Optimized process for the production of fungal peroxidases and efficient saccharification of pre-treated rice straw. Bioresour. Technol. Rep. 2022, 17, 100913. [Google Scholar] [CrossRef]
- Bouacem, K.; Allala, F.; Zaraî Jaouadi, N.; Hamdi, S.; Mechri, S.; Ighilahriz, K.; Rekik, H.; Hacene, H.; Bouanane Darenfed, A.; Jaouadi, B. A novel peroxidase from white-rot Agaricomycetes fungus Phlebia radiata strain KB-DZ15: Its purification, characterisation, and potential application for dye-decolorisation and lignin-biodegradation. Biocatal. Biotransform. 2022, 40, 365–377. [Google Scholar] [CrossRef]
- Ezike, T.C.; Ezugwu, A.L.; Udeh, J.O.; Eze, S.O.O.; Chilaka, F.C. Purification and characterisation of new laccase from Trametes polyzona WRF03. Biotechnol. Rep. 2020, 28, e00566. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.G.; da Silva, T.B.V.; Uber, T.M.; Pasquarelli, D.L.; Bracht, A.; Peralta, R.M. Production of fungal laccase on pineapple waste and application in detoxification of malachite green. J. Environ. Sci. health. Part B Pestic. Food Contam. Agric. Wastes 2022, 57, 90–101. [Google Scholar] [CrossRef]
- Cen, Q.J.; Wu, X.D.; Cao, L.P.; Lu, Y.J.; Lu, X.; Chen, J.W.; Fu, G.M.; Liu, Y.H.; Ruan, R. Green production of a yellow laccase by Coriolopsis gallica for phenolic pollutants removal. AMB Express 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.P.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.X.; Shen, Y.; You, C.P.; Guan, Z.B.; Liao, X.R. Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J. Hazard. Mater. 2020, 382, 121084. [Google Scholar] [CrossRef] [PubMed]
- Lankiewicz Thomas, S.; Choudhary, H.; Gao, Y.; Amer, B.; Lillington Stephen, P.; Leggieri Patrick, A.; Brown Jennifer, L.; Swift Candice, L.; Lipzen, A.; Na, H.; et al. Lignin deconstruction by anaerobic fungi. Nat. Microbiol. 2023, 8, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Li, J.G.; Liu, Q.; Liu, D.F.; Wu, M.; Tian, C.G. Advances in metabolic engineering of filamentous fungi. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2021, 37, 1637–1658. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Y.X. Progress and challenge of the CRISPR-Cas system in gene editing for filamentous fungi. Synth. Biol. J. 2021, 2, 274–286. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, R.; Abomohra, A.E.-F.; Xie, S.X.; Yu, Z.S.; Guo, Q.; Liu, P.; Peng, L.; Li, X.K. A sustainable approach for efficient conversion of lignin into biodiesel accompanied by biological pretreatment of corn straw. Energy Convers. Manag. 2019, 199, 111928. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Han, H.W.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.; Li, X.K. Lignin depolymerization and utilization by bacteria. Bioresour. Technol. 2018, 269, 557–566. [Google Scholar] [CrossRef]
- Khan, M.; Singh, T.; Pal, D.B.; Khan, S.; Ahmad, S.; Jandrajupalli, S.B.; Haque, S.; Singh, R.; Srivastava, N. Enhanced production of bacterial hydrolytic endoglucanase enzyme using waste leaves of water hyacinth and its thermal stability under the influence of TiO2 nanoparticles. Biomass Convers. Biorefinery 2024, 14, 2185–2191. [Google Scholar] [CrossRef]
- Ejaz, U.; Moin, S.F.; Sohail, M.; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, S.M. Characterization of a novel end product tolerant and thermostable cellulase from Neobacillus sedimentimangrovi UE25. Enzym. Microb. Technol. 2023, 162, 110133. [Google Scholar] [CrossRef]
- Das, T.; Ali, F.; Rahman, M.S. Cellulase activity of a novel bacterial strain Arthrobacter woluwensis TDS9: Its application on bioconversion of paper mill sludge. J. Genet. Eng. Biotechnol. 2022, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Haq, I.U.; Shah, F.I.; Aqeel, A.; Ahmed, Z.; Mir, A.S.; Qureshi, S.S.; Raja, S.I. Genus Thermotoga: A valuable home of multifunctional glycoside hydrolases (GHs) for industrial sustainability. Bioorganic Chem. 2022, 127, 105942. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Liu, S.Y.; Dong, S.; Li, R.M.; Feng, Y.; Cui, Q. Determination of the native features of the exoglucanase Cel48S from Clostridium thermocellum. Biotechnol. Biofuels 2018, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Ma, T.F.; Fang, S.-G.; Han, Z.G. Improving the catalytic activity of thermostable xylanase from Thermotoga maritima via mutagenesis of non-catalytic residues at glycone subsites. Enzym. Microb. Technol. 2020, 139, 109579. [Google Scholar] [CrossRef]
- Xue, D.S.; Zeng, X.H.; Qiang, L.D.; Yao, S.J. Thermostable ethanol tolerant xylanase from a cold-adapted marine species Acinetobacter johnsonii. Chin. J. Chem. Eng. 2019, 27, 1166–1170. [Google Scholar] [CrossRef]
- Bakry, M.M.; Salem, S.S.; Atta, H.M.; El-Gamal, M.S.; Fouda, A. Xylanase from thermotolerant Bacillus haynesii strain, synthesis, characterization, optimization using Box-Behnken Design, and biobleaching activity. Biomass Convers. Biorefinery 2024, 14, 9779–9792. [Google Scholar] [CrossRef]
- Mhiri, S.; Bouanane-Darenfed, A.; Jemli, S.; Neifar, S.; Ameri, R.; Mezghani, M.; Bouacem, K.; Jaouadi, B.; Bejar, S. A thermophilic and thermostable xylanase from Caldicoprobacter algeriensis: Recombinant expression, characterization and application in paper biobleaching. Int. J. Biol. Macromol. 2020, 164, 808–817. [Google Scholar] [CrossRef]
- Mehandia, S.; Sharma, S.C.; Arya, S.K. Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnol. Rep. 2020, 25, e00413. [Google Scholar] [CrossRef]
- Wang, J.J.; Chang, F.; Tang, X.Q.; Li, W.; Yin, Q.; Yang, Y.; Hu, Y. Bacterial laccase of Anoxybacillus ayderensis SK3-4 from hot springs showing potential for industrial dye decolorization. Ann. Microbiol. 2020, 70, 51. [Google Scholar] [CrossRef]
- Abdelgalil, S.A.; Soliman, N.A.; Abo-Zaid, G.A.; Abdel-Fattah, Y.R. Bioprocessing strategies for cost-effective large-scale production of bacterial laccase from Lysinibacillus macroides LSO using bio-waste. Int. J. Environ. Sci. Technol. 2022, 19, 1633–1652. [Google Scholar] [CrossRef]
- Khaled, J.M.; Alyahya, S.A.; Govindan, R.; Chelliah, C.K.; Maruthupandy, M.; Alharbi, N.S.; Kadaikunnan, S.; Issac, R.; Murugan, S.; Li, W.J. Laccase producing bacteria influenced the high decolorization of textile azo dyes with advanced study. Environ. Res. 2022, 207, 112211. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nie, Y.; Tang, Y.Q.; Song, X.M.; Cao, K.; Sun, L.Z.; Wang, Z.J.; Wu, X.-L. Diverse bacteria with lignin degrading potentials isolated from two ranks of coal. Front. Microbiol. 2016, 7, 1428. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Williamson, J.J.; Alberti, F. Microbial hosts for metabolic engineering of lignin bioconversion to renewable chemicals. Renew. Sustain. Energy Rev. 2021, 152, 111674. [Google Scholar] [CrossRef]
- Mycroft, Z.; Gomis, M.; Mines, P.; Law, P.; Bugg, T.D.H. Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways. Green Chem. 2015, 17, 4974–4979. [Google Scholar] [CrossRef]
- Jørgensen, H.; Pinelo, M. Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2017, 11, 150–167. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef]
- Singh, N.; Mathur Anshu, S.; Gupta Ravi, P.; Barrow Colin, J.; Tuli Deepak, K.; Puri, M. Enzyme systems of thermophilic anaerobic bacteria for lignocellulosic biomass conversion. Int. J. Biol. Macromol. 2021, 168, 572–590. [Google Scholar] [CrossRef]
- You, M.C.; Zhao, Q.Y.; Liu, Y.S.; Zhang, W.H.; Shen, Z.W.; Ren, Z.X.; Xu, C.G. Insights into lignocellulose degradation: Comparative genomics of anaerobic and cellulolytic Ruminiclostridium-type species. Front. Microbiol. 2023, 14, 1288286. [Google Scholar] [CrossRef]
- Manesh, M.J.H.; Bing, R.G.; Willard, D.J.; Adams, M.W.W.; Kelly, R.M. Complete genome sequence for the extremely thermophilic bacterium Anaerocellum danielii (DSM:8977). Microbiol. Resour. Announc. 2024, 13, e0122923. [Google Scholar] [CrossRef]
- Peng, X.W.; Qiao, W.B.; Mi, S.F.; Jia, X.J.; Su, H.; Han, Y.J. Characterization of hemicellulase and cellulase from the extremely thermophilic bacterium Caldicellulosiruptor owensensis and their potential application for bioconversion of lignocellulosic biomass without pretreatment. Biotechnol. Biofuels 2015, 8, 131. [Google Scholar] [CrossRef]
- Penttilä, M.E.; André, L.; Lehtovaara, P.; Bailey, M.; Teeri, T.T.; Knowles, J.K.C. Efficient secretion of two fungal cellobiohydrolases by Saccharomyces cerevisiae. Gene 1988, 63, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Whittle, D.J.; Kilburn, D.G.; Warren, R.A.; Miller, R.C., Jr. Molecular cloning of a Cellulomonas fimi cellulose gene in Escherichia coli. Gene 1982, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Li, H.; Zhang, M.Q.; Jiang, X.P.; Chen, C.; Zhang, X.H.; Zhang, R.; Huang, G.Q.; Liu, G.; Gui, Z.Z. Whole Genome Sequencing Analysis of Cellulose-degrading Bacterium DC11 Isolated from Silkworm Excrement and Characterization of Its Key Cellulase Gene ytoP: Running Head: Characterization of strain DC11 and its key gene ytoP. J. Asia-Pac. Entomol. 2024, 27, 102285. [Google Scholar] [CrossRef]
- El-Khamisi, E.F.; Soliman, E.A.M.; El-Sayed, G.M.; Nour, S.A.; Abdel-Monem, M.O.; Hassan, M.G. Optimization, gene cloning, expression, and molecular docking insights for enhanced cellulase enzyme production by Bacillus amyloliquefaciens strain elh1. Microb. Cell Factories 2024, 23, 191. [Google Scholar] [CrossRef]
- Yang, J.; Yue, H.-R.; Pan, L.-Y.; Feng, J.-X.; Zhao, S.; Suwannarangsee, S.; Champreda, V.; Liu, C.-G.; Zhao, X.-Q. Fungal strain improvement for efficient cellulase production and lignocellulosic biorefinery: Current status and future prospects. Bioresour. Technol. 2023, 385, 129449. [Google Scholar] [CrossRef]
- Yang, Q.; Li, W.Z.; Ju, M.T.; Qi, X.H. Advances in microbial degradation of lignocelluloses biomass solid waste—A review. Microbiology 2015, 42, 1569–1583. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef]
- Hou, L.P.; Ji, D.D.; Dong, W.F.; Yuan, L.; Zhang, F.S.; Li, Y.; Zang, L.H. The Synergistic Action of Electro-Fenton and White-Rot Fungi in the Degradation of Lignin. Front. Bioeng. Biotechnol. 2020, 8, 99. [Google Scholar] [CrossRef]
- Liang, J.S.; Zhang, R.; Chang, J.N.; Chen, L.; Nabi, M.; Zhang, H.B.; Zhang, G.M.; Zhang, P.Y. Rumen microbes, enzymes, metabolisms, and application in lignocellulosic waste conversion—A comprehensive review. Biotechnol. Adv. 2024, 71, 108308. [Google Scholar] [CrossRef]
- Cai, J.L.; Feng, F.; Li, J.X.; Ma, H.Y.; Li, D.M. Research progress of biological decomposition lignin. Appl. Chem. Ind. 2024, 53, 1681–1686. [Google Scholar] [CrossRef]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. A Review on Bacterial Contribution to Lignocellulose Breakdown into Useful Bio-Products. Int. J. Environ. Res. Public health 2021, 18, 6001. [Google Scholar] [CrossRef]
- Liu, W.X.; Xu, L.T.; Sun, J.X.; Cheng, H.N.; Chen, Z.; Zhou, H.B.; Yan, B.H.; Wang, Y.G. The patterns of marine microbial communities in composts with high lignocellulose content. Chem. Eng. J. 2023, 468, 143649. [Google Scholar] [CrossRef]
- Yang, J.; Jang, J.C.; Zhang, N.; Xu, H.; Xie, J.C.; Zhao, J. Research Progress on Lignin Degradation by Microorganism. Biomass Chem. Eng. 2021, 55, 62–70. [Google Scholar] [CrossRef]
- Adsul, M.; Sandhu, S.K.; Singhania, R.R.; Gupta, R.; Puri, S.K.; Mathur, A. Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzym. Microb. Technol. 2020, 133, 109442. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Zhang, B.Y.; Luo, L.L.; Zhang, F.; Yi, Y.L.; Shan, Y.Y.; Liu, B.F.; Zhou, Y.; Wang, X.; Lü, X. A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Moya, E.B.; Syhler, B.; Dragone, G.; Mussatto, S.I. Tailoring a cellulolytic enzyme cocktail for efficient hydrolysis of mildly pretreated lignocellulosic biomass. Enzym. Microb. Technol. 2024, 175, 110403. [Google Scholar] [CrossRef]
- Li, Y.Y.; Song, W.Y.; Han, X.Y.; Wang, Y.C.; Rao, S.Q.; Zhang, Q.; Zhou, J.W.; Li, J.H.; Liu, S.; Du, G.C. Recent progress in key lignocellulosic enzymes: Enzyme discovery, molecular modifications, production, and enzymatic biomass saccharification. Bioresour. Technol. 2022, 363, 127986. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.M.; Mohamed, T.A.; Zheng, G.G.; Qu, F.T.; Wang, F.; Zhao, Y.; Song, C.H. Lignocellulose biomass bioconversion during composting: Mechanism of action of lignocellulase, pretreatment methods and future perspectives. Chemosphere 2022, 286, 131635. [Google Scholar] [CrossRef]
- De Souza, T.S.P.; Kawaguti, H.Y. Cellulases, Hemicellulases, and Pectinases: Applications in the Food and Beverage Industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Cellulose Depolymerization over Heterogeneous Catalysts. Acc. Chem. Res. 2018, 51, 761–768. [Google Scholar] [CrossRef]
- Li, F.; Xie, Y.J.; Gao, X.; Shan, M.X.; Sun, C.C.; Niu, Y.D.; Shan, A.S. Screening of cellulose degradation bacteria from Min pigs and optimization of its cellulase production. Electron. J. Biotechnol. 2020, 48, 29–35. [Google Scholar] [CrossRef]
- Galadima, A.; Masudi, A.; Muraza, O. Conversion of cellulose to glucose and further transformation into fuels over solid acid catalysts: A mini review. Microporous Mesoporous Mater. 2022, 336, 111846. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.Y.; Liu, S.J. Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl. Catal. B Environ. 2015, 174–175, 225–243. [Google Scholar] [CrossRef]
- Yu, C.L.; Li, Z.J.; Diao, W.T.; Wang, B.T.; Liu, D.H. Research progress on lytic polysaccharide monooxygenases. Food Ferment. Ind. 2024, 50, 393–400. [Google Scholar] [CrossRef]
- Santos, C.A.; Morais, M.A.B.; Mandelli, F.; Lima, E.A.; Miyamoto, R.Y.; Higasi, P.M.R.; Araujo, E.A.; Paixão, D.A.A.; Junior, J.M.; Motta, M.L.; et al. A metagenomic ‘dark matter’ enzyme catalyses oxidative cellulose conversion. Nature 2025, 639, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Lübeck, M.; Lübeck, P.S. Isolation and Screening of Cellulolytic Filamentous Fungi. Methods Mol. Biol. 2018, 1796, 37–45. [Google Scholar] [CrossRef]
- Bautista-Cruz, A.; Aquino-Bolaños, T.; Hernández-Canseco, J.; Quiñones-Aguilar, E.E. Cellulolytic Aerobic Bacteria Isolated from Agricultural and Forest Soils: An Overview. Biology 2024, 13, 102. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]
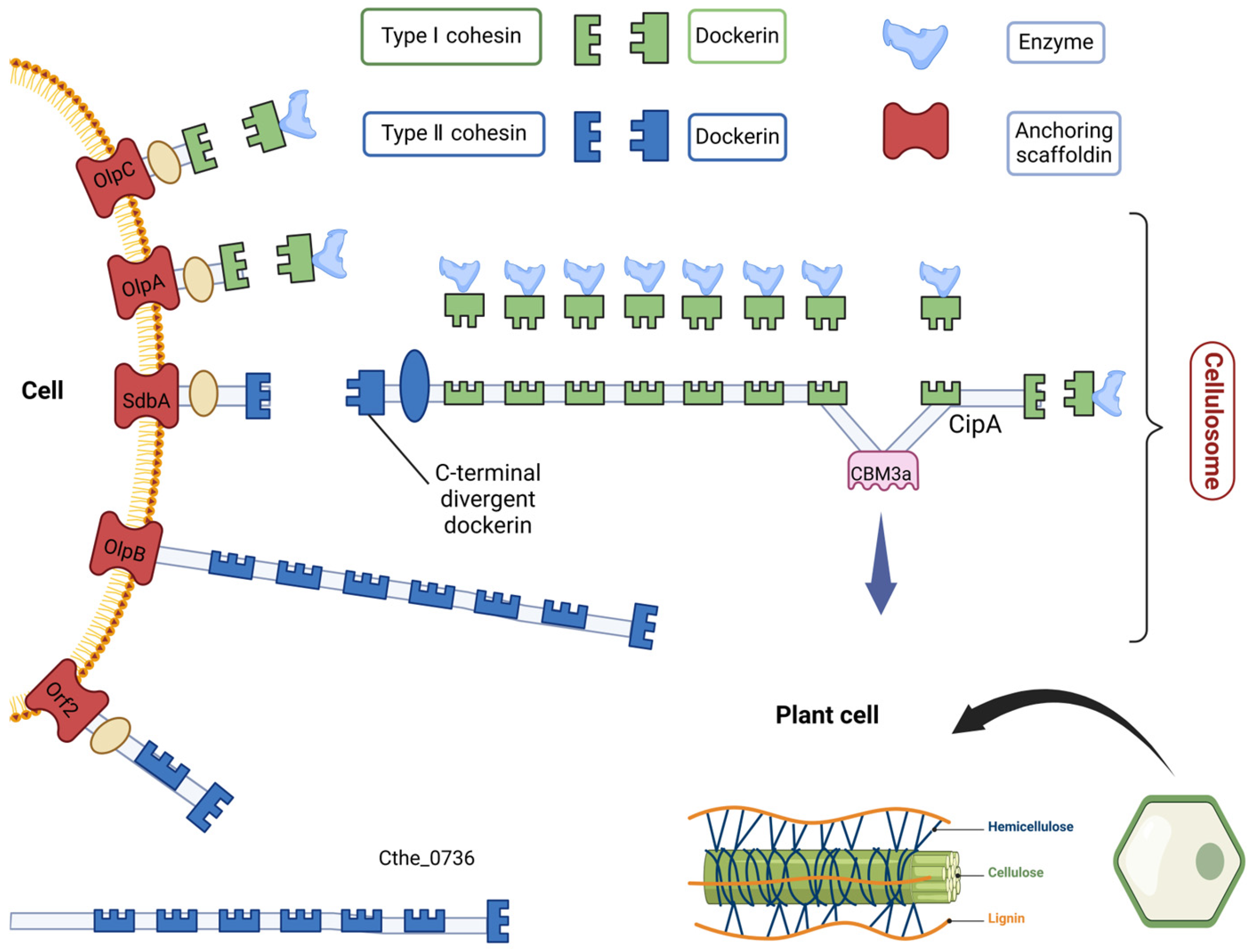
- Fontes, C.M.; Gilbert, H.J. Cellulosomes: Highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 2010, 79, 655–681. [Google Scholar] [CrossRef]
- Gurovic, M.S.V.; Viceconte, F.R.; Bidegain, M.A.; Dietrich, J. Regulation of lignocellulose degradation in microorganisms. J. Appl. Microbiol. 2023, 134, lxac002. [Google Scholar] [CrossRef]
- Ding, S.Y.; Bayer, E.A. Understanding Cellulosome Interaction with Cellulose by High-Resolution Imaging. ACS Cent. Sci. 2020, 6, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Mikkelsen, D.; Flanagan, B.M.; Dhital, S.; Gaunitz, S.; Henriksson, G.; Lindström, M.E.; Yakubov, G.E.; Gidley, M.J.; Vilaplana, F. Wood hemicelluloses exert distinct biomechanical contributions to cellulose fibrillar networks. Nat. Commun. 2020, 11, 4692. [Google Scholar] [CrossRef] [PubMed]
- Cologna, N.d.M.d.; Gómez-Mendoza, D.P.; Zanoelo, F.F.; Giannesi, G.C.; Guimarães, N.C.d.A.; Moreira, L.R.d.S.; Filho, E.X.F.; Ricart, C.A.O. Exploring Trichoderma and Aspergillus secretomes: Proteomics approaches for the identification of enzymes of biotechnological interest. Enzym. Microb. Technol. 2018, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.X.; Zhao, X.; Zhang, P.Y.; Long, L.K.; Ding, S.J. A novel AA14 LPMO from Talaromyces rugulosus with bifunctional cellulolytic/hemicellulolytic activity boosted cellulose hydrolysis. Biotechnol. Biofuels bioproducts 2024, 17, 30. [Google Scholar] [CrossRef]
- Dhaver, P.; Pletschke, B.; Sithole, B.; Govinden, R. Optimization, purification, and characterization of xylanase production by a newly isolated Trichoderma harzianum strain by a two-step statistical experimental design strategy. Sci. Rep. 2022, 12, 17791. [Google Scholar] [CrossRef]
- Yan, S.; Xu, Y.; Yu, X.W. Rational engineering of xylanase hyper-producing system in Trichoderma reesei for efficient biomass degradation. Biotechnol. Biofuels 2021, 14, 90. [Google Scholar] [CrossRef]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Wenger, J.; Haas, V.; Stern, T. Why Can We Make Anything from Lignin Except Money? Towards a Broader Economic Perspective in Lignin Research. Curr. For. Rep. 2020, 6, 294–308. [Google Scholar] [CrossRef]
- Hossain, M.M.; Rawal, A.; Aldous, L. Aprotic vs Protic Ionic Liquids for Lignocellulosic Biomass Pretreatment: Anion Effects, Enzymatic Hydrolysis, Solid-State NMR, Distillation, and Recycle. ACS Sustain. Chem. Eng. 2019, 7, 11928–11936. [Google Scholar] [CrossRef]
- Rais, D.; Zibek, S. Biotechnological and Biochemical Utilization of Lignin. Adv. Biochem. Eng. Biotechnol. 2019, 166, 469–518. [Google Scholar] [CrossRef]
- Rodríguez Couto, S. Industrial and environmental applications of white-rot fungi. Mycosphere 2017, 8, 456–466. [Google Scholar] [CrossRef]
- Chandra, R.; Chowdhary, P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environmental science. Process. Impacts 2015, 17, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ruiz, M.I.; Ayuso-Fernández, I.; Rencoret, J.; González-Ramírez, A.M.; Linde, D.; Davó-Siguero, I.; Romero, A.; Gutiérrez, A.; Martínez, A.T.; Ruiz-Dueñas, F.J. Agaricales Mushroom Lignin Peroxidase: From Structure-Function to Degradative Capabilities. Antioxidants 2021, 10, 1446. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F.F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Heese, K. Characterization of Lignocellulolytic Enzymes from White-Rot Fungi. Curr. Microbiol. 2015, 70, 485–498. [Google Scholar] [CrossRef]
- Tang, L.; Liao, Q.; Xia, A.; Huang, Y.; Zhu, X.Q.; Zhu, X. Mechanism and characteristics of nature inspired enzyme-fungi synergistic system for lignin pretreatment. Chem. Ind. Eng. Prog. 2021, 40, 5378–5387. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Algora, C.; Baldrian, P. Lignocellulolytic systems of soil bacteria: A vast and diverse toolbox for biotechnological conversion processes. Biotechnol. Adv. 2019, 37, 107374. [Google Scholar] [CrossRef]
- Benatti, A.L.T.; Polizeli, M. Lignocellulolytic Biocatalysts: The Main Players Involved in Multiple Biotechnological Processes for Biomass Valorization. Microorganisms 2023, 11, 162. [Google Scholar] [CrossRef]
- Obeng, E.M.; Adam, S.N.N.; Budiman, C.; Ongkudon, C.M.; Maas, R.; Jose, J. Lignocellulases: A review of emerging and developing enzymes, systems, and practices. Bioresour. Bioprocessing 2017, 4, 16. [Google Scholar] [CrossRef]
- Spasic, J.; Mandic, M.; Djokic, L.; Nikodinovic-Runic, J. Streptomyces spp. in the biocatalysis toolbox. Appl. Microbiol. Biotechnol. 2018, 102, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Hernández, D.A.; García-Pérez, J.S.; López-Pacheco, I.Y.; Iqbal, H.M.N.; Parra-Saldívar, R. Resource recovery of lignocellulosic biomass waste into lactic acid—Trends to sustain cleaner production. J. Environ. Manag. 2022, 301, 113925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.M.; Xie, Z.L.; Tang, S.Y.; Xie, Q.Q.; He, X.Y.; Li, D.J. Synthetic microbial community enhances lignocellulose degradation during composting by assembling fungal communities. Bioresour. Technol. 2025, 419, 132068. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Naik, S.K.; Thombare, N.; Singh, A.K. Potential of lignocellulose degrading microorganisms for agricultural residue decomposition in soil: A review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar] [CrossRef]
- Tang, S.R.; Cheng, W.G.; Hu, R.G.; Guigue, J.; Kimani, S.M.; Tawaraya, K.; Xu, X.K. Simulating the effects of soil temperature and moisture in the off-rice season on rice straw decomposition and subsequent CH4 production during the growth season in a paddy soil. Biol. Fertil. Soils 2016, 52, 739–748. [Google Scholar] [CrossRef]
- Li, Y.K.; Li, Z.X.; Zhang, Y.T.; Yi, Q.R.; Ma, E.N. Water-induced effects of matrix in wood cell wall on cellulose crystalline structure. J. Beijing For. Univ. 2022, 44, 121–131. [Google Scholar] [CrossRef]
- Towey, R.; Webster, K.; Darr, M. Influence of Storage Moisture and Temperature on Lignocellulosic Degradation. AgriEngineering 2019, 1, 332–342. [Google Scholar] [CrossRef]
- Yang, L.; Tan, L.P.; Liu, T.J. Progress in detoxification of inhibitors generated during lignocellulose pretreatment. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2021, 37, 15–29. [Google Scholar] [CrossRef]
- Feng, H.L.; Li, Y.M.; Du, C.; Yuan, W.J. Effect of microaeration on cell growth and glucose/xylose fermentation of Kluyveromyces marxianus from the imitate lignocellulosic-derived hydrolysate. Process Biochem. 2021, 101, 247–255. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Nitrogen Release from Crop Residues and Organic Amendments as Affected by Biochemical Composition. Commun. Soil Sci. Plant Anal. 2003, 34, 2441–2460. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.Q.; Xie, X.Y.; Zhang, R.J.; Wei, Z.M. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Yao, T.; Yang, Q.L.; Qi, G.T.; Liu, X.Y.; Gun, S.B. Characteristics and selection of efficient lignocellulose degradation microbial community. Chin. J. Eco-Agric. 2013, 21, 621–627. [Google Scholar] [CrossRef]
- Albrecht, R.; Le Petit, J.; Calvert, V.; Terrom, G.; Périssol, C. Changes in the level of alkaline and acid phosphatase activities during green wastes and sewage sludge co-composting. Bioresour. Technol. 2010, 101, 228–233. [Google Scholar] [CrossRef]
- Long, C.; Wang, S.Y.; Lu, H.L.; Ye, J.; Xu, J.M.; Wang, K.; Jiang, J.C. Selective activation of C—C bonds in lignin model compounds and lignin for production of value-added chemicals. J. Bioresour. Bioprod. 2024, 9, 433–464. [Google Scholar] [CrossRef]
- Yao, X.Z.; Chen, C.; Wang, Y.F.; Dong, S.; Liu, Y.J.; Li, Y.F.; Cui, Z.L.; Gong, W.B.; Perrett, S.; Yao, L.S.; et al. Discovery and mechanism of a pH-dependent dual-binding-site switch in the interaction of a pair of protein modules. Sci. Adv. 2020, 6, eabd7182. [Google Scholar] [CrossRef]
- Miao, Y.Z.; Chen, X.; Li, T.; Zhu, H.; Tang, S.Y.; Liu, D.Y.; Shen, Q.R. Proteomic analysis reflects an environmental alkalinization-coupled pH-dependent mechanism of regulating lignocellulases in Trichoderma guizhouense NJAU4742. Biotechnol. Biofuels 2020, 13, 6. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J. Temperature and the catalytic activity of enzymes: A fresh understanding. FEBS Lett. 2013, 587, 2738–2743. [Google Scholar] [CrossRef]
- Kausar, H.; Ismail, M.R.; Saud, H.M.; Othman, R.; Habib, S. Use of Lignocellulolytic Microbial Consortium and pH Amendment on Composting Efficacy of Rice Straw. Compost. Sci. Util. 2013, 21, 121–133. [Google Scholar] [CrossRef]
- Zavala, M.A.L.; Funamizu, N.; Takakuwa, T. Temperature effect on aerobic biodegradation of feces using sawdust as a matrix. Water Res. 2004, 38, 2415. [Google Scholar] [CrossRef]
- Fan, Y.V.; Lee, C.T.; Klemeš, J.J.; Chua, L.S.; Sarmidi, M.R.; Leow, C.W. Evaluation of Effective Microorganisms on home scale organic waste composting. J. Environ. Manag. 2018, 216, 41–48. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, M.; Su, J.M.; Hu, H.Y.; Yang, M.; Huang, Z.Q.; Chen, D.; Wu, J.; Feng, Z.F. Overcoming biomass recalcitrance by synergistic pretreatment of mechanical activation and metal salt for enhancing enzymatic conversion of lignocellulose. Biotechnol. Biofuels 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Huang, R.L.; Su, R.X.; Qi, W.; Zhang, Y.M.; He, Z.M. An overview on lignocellulose pretreatment and recalcitrant characteristics. Huagong Xuebao/CIESC J. 2012, 63, 677–687. [Google Scholar] [CrossRef]
- Wang, Y.; Leng, L.; Islam, M.K.; Liu, F.H.; Lin, C.S.K.; Leu, S.-Y. Substrate-Related Factors Affecting Cellulosome-Induced Hydrolysis for Lignocellulose Valorization. Int. J. Mol. Sci. 2019, 20, 3354. [Google Scholar] [CrossRef] [PubMed]
- Vikman, M.; Karjomaa, S.; Kapanen, A.; Wallenius, K.; Itävaara, M. The influence of lignin content and temperature on the biodegradation of lignocellulose in composting conditions. Appl. Microbiol. Biotechnol. 2002, 59, 591–598. [Google Scholar] [CrossRef]
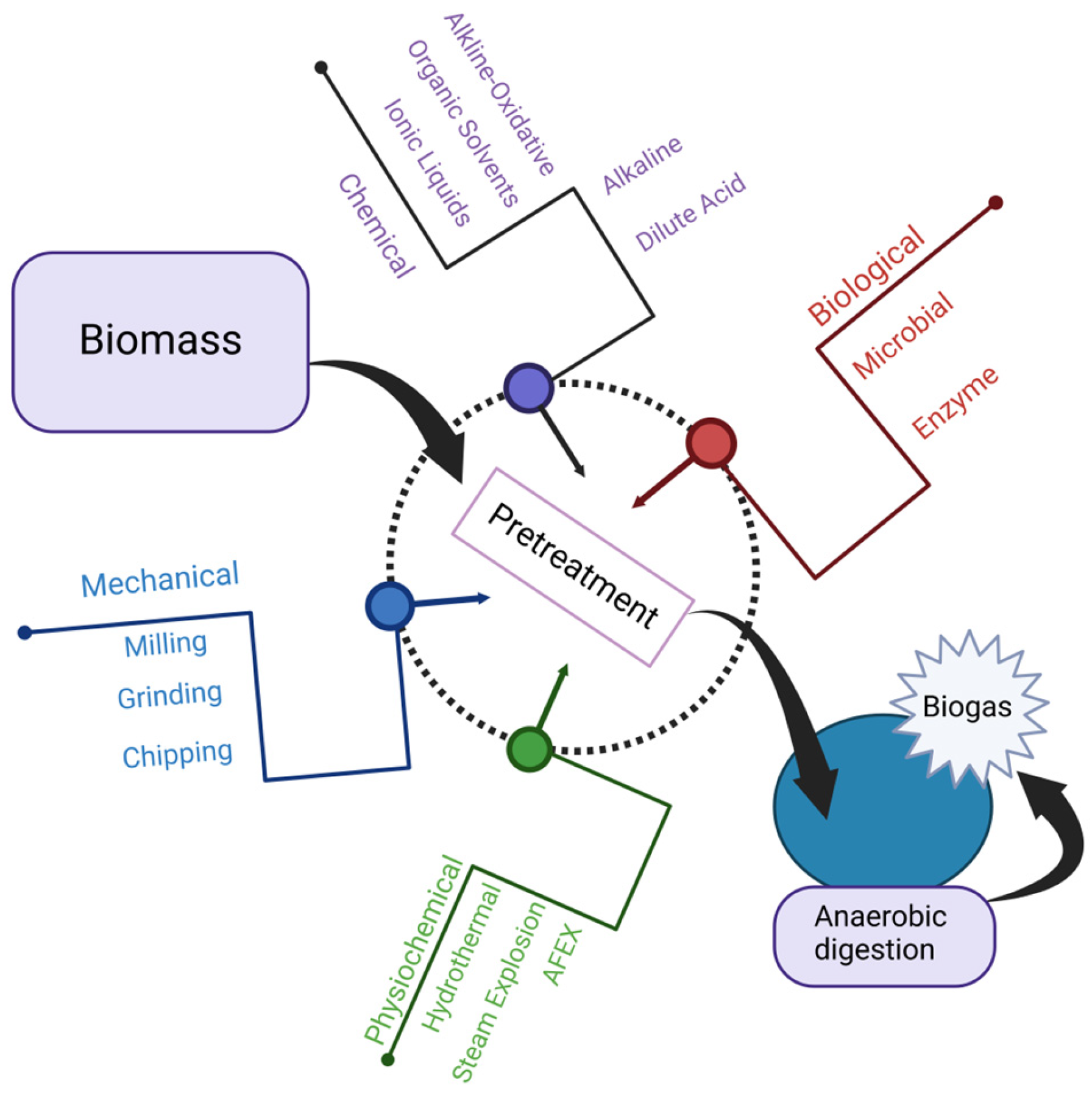
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Ingle, A.P.; Chandel, A.K.; Antunes, F.A.F.; Rai, M.; Silva, S.S.d. New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioprod. Biorefining 2019, 13, 776–788. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.d.S.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Guo, X.Y.; Jiang, B.; Wu, W.J.; Zhang, T.W.; Sweeney, M.; Ahmad, M.; Jin, Y.C. Effect of various aromatic compounds with different functional groups on enzymatic hydrolysis of microcrystalline cellulose and alkaline pretreated wheat straw. J. Bioresour. Bioprod. 2024, 9, 211–221. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Biobutanol production from apple pomace: The importance of pretreatment methods on the fermentability of lignocellulosic agro-food wastes. Appl. Microbiol. Biotechnol. 2017, 101, 8041–8052. [Google Scholar] [CrossRef]
- Owolabi, A.F.; Haafiz, M.K.M.; Hossain, M.S.; Hussin, M.H.; Fazita, M.R.N. Influence of alkaline hydrogen peroxide pre-hydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int. J. Biol. Macromol. 2017, 95, 1228–1234. [Google Scholar] [CrossRef]
- Xie, D.; Liu, Z.L.; Cao, Y.F.; Yang, S.I.; Su, C.; Li, M. Improving antioxidant activities of water-soluble lignin-carbohydrate complex isolated from wheat stalk through prolonging ball-milling pretreatment and homogeneous extraction. J. Bioresour. Bioprod. 2024, 9, 113–125. [Google Scholar] [CrossRef]
- Yu, G.W.; Guo, T.T.; Huang, Q.D. Preparation of rapeseed oil with superhigh canolol content and superior quality characteristics by steam explosion pretreatment technology. Food Sci. Nutr. 2020, 8, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yao, B.; Su, X.Y. Linking Enzymatic Oxidative Degradation of Lignin to Organics Detoxification. Int. J. Mol. Sci. 2018, 19, 3373. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. International microbiology. Off. J. Span. Soc. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K.; Sarkar, L.; Verma, A.K. Role of extremophiles and their extremozymes in biorefinery process of lignocellulose degradation. Extremophiles. Life Extrem. Cond. 2021, 25, 203–219. [Google Scholar] [CrossRef]
- Zambare, V.P.; Bhalla, A.; Muthukumarappan, K.; Sani, R.K.; Christopher, L.P. Bioprocessing of agricultural residues to ethanol utilizing a cellulolytic extremophile. Extremophiles. Life Extrem. Cond. 2011, 15, 611–618. [Google Scholar] [CrossRef]
- Nguyen, T.A.D.; Kim, K.-R.; Kim, M.S.; Sim, S.J. Thermophilic hydrogen fermentation from Korean rice straw by Thermotoga neapolitana. Int. J. Hydrogen Energy 2010, 35, 13392–13398. [Google Scholar] [CrossRef]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef]
- Boucias, D.G.; Cai, Y.P.; Sun, Y.J.; Lietze, V.U.; Sen, R.; Raychoudhury, R.; Scharf, M.E. The hindgut lumen prokaryotic microbiota of the termite Reticulitermes flavipes and its responses to dietary lignocellulose composition. Mol. Ecol. 2013, 22, 1836–1853. [Google Scholar] [CrossRef]
- Scharf, M.E. Omic research in termites: An overview and a roadmap. Front. Genet. 2015, 6, 76. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nature reviews. Microbiology 2014, 12, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Yelle, D.J.; Li, C.; Yang, M.Y.; Ke, J.; Zhang, R.J.; Liu, Y.; Zhu, N.; Liang, S.Y.; Mo, X.C.; et al. Lignocellulose pretreatment in a fungus-cultivating termite. Proc. Natl. Acad. Sci. USA 2017, 114, 4709–4714. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.H.; Lu, M.L.; Zhang, Y.; Lin, B.F.; Chen, L.; Huang, Z.; Li, K.; Li, J.B. Functional enzyme analysis and metabolic regulation mechanism of the combined microflora LXB in the degradation of lignocellulose. Biochem. Eng. J. 2024, 206, 109285. [Google Scholar] [CrossRef]
- Du, G.L.; Zhou, Y.; Zhang, J.X.; Han, S.W.; Liu, X.C.; Yuan, C.Y.; Ndayisenga, F.; Shi, J.P.; Zhang, B.G. Optimized strategy valorizing unautoclaved cottonseed hull as ruminant alternative feeds via solid-state fermentation: Detoxifying polyphenols, restraining hazardous microflora and antibiotic-resistance gene hosts. Environ. Technol. Innov. 2022, 28, 102937. [Google Scholar] [CrossRef]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Piñeiro, J.; Mack, M.C.; Marks, J.C.; Bell, S.L.; Miller, S.N.; Schwartz, E.; et al. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. ISME J. 2021, 15, 2738–2747. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, Y.J.; Lee, J.K.; Nurika, I.; Suhartini, S.; Choe, D.; Kim, D.H.; Choi, H.; Murphy, N.P.; Kim, H.Y.; et al. Production of chitosan-based composite film reinforced with lignin-rich lignocellulose nanofibers from rice husk. J. Bioresour. Bioprod. 2024, 9, 174–184. [Google Scholar] [CrossRef]
- Ma, H.W.; Cheng, Z.Y.; Li, X.B.; Li, B.; Fu, Y.J.; Jiang, J.C. Advances and challenges of cellulose functional materials in sensors. J. Bioresour. Bioprod. 2023, 8, 15–32. [Google Scholar] [CrossRef]
- Seidi, F.; Jiang, W.S.; Yu, Z.C.; Deng, C. Cellulose-MXene composites: New platforms with outstanding multifunctional characteristics. J. Bioresour. Bioprod. 2024, 9, 243–245. [Google Scholar] [CrossRef]
- Fatani, S.; Saito, Y.; Alarawi, M.; Gojobori, T.; Mineta, K. Genome sequencing and identification of cellulase genes in Bacillus paralicheniformis strains from the Red Sea. BMC Microbiol. 2021, 21, 254. [Google Scholar] [CrossRef]
- Abd Elhameed, E.; Sayed, A.R.M.; Radwan, T.E.E.; Hassan, G. Biochemical and Molecular Characterization of Five Bacillus Isolates Displaying Remarkable Carboxymethyl Cellulase Activities. Curr. Microbiol. 2020, 77, 3076–3084. [Google Scholar] [CrossRef]
- Shikata, A.; Sermsathanaswadi, J.; Thianheng, P.; Baramee, S.; Tachaapaikoon, C.; Waeonukul, R.; Pason, P.; Ratanakhanokchai, K.; Kosugi, A. Characterization of an Anaerobic, Thermophilic, Alkaliphilic, High Lignocellulosic Biomass-Degrading Bacterial Community, ISHI-3, Isolated from Biocompost. Enzym. Microb. Technol. 2018, 118, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Tian, L.; Li, Y.X.; Zhong, C.; Tian, C.J. Effects of exogenous cellulose-degrading bacteria on humus formation and bacterial community stability during composting. Bioresour. Technol. 2022, 359, 127458. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.Y.; Geng, A.L. Construction of CRISPR-Cas9 genome editing platform for white-rot fungus Cerrena unicolor BBP6 and its effects on extracellular ligninolytic enzyme biosynthesis. Biochem. Eng. J. 2022, 185, 108527. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q.; Xiao, L.; Wang, Y.; Feng, J.X.; Bu, Q.T.; Xiao, Y.; Hao, K.; Guo, M.L.; Chen, W.S.; et al. Multiplexed CRISPR/Cas9-Mediated Knockout of Laccase Genes in Salvia miltiorrhiza Revealed Their Roles in Growth, Development, and Metabolism. Front. Plant Sci. 2021, 12, 647768. [Google Scholar] [CrossRef]
- Fan, F.F. Transcriptional Regulation and Funtional Analysis of Laccase Gene from White Rot Fugi. Ph.D. Thesis, Huazhong University of Science of Technology, Wuhan, China, 2013. [Google Scholar] [CrossRef]
| Reference | [11] | [12] | [13] | [14] | [15] | [16] |
|---|---|---|---|---|---|---|
| Process characteristics | ||||||
| Enzyme product | Cellulase mix | Cellulase mix | Cellulase mix | Cellulase mix | β-glucosidase | Multienzyme |
| Microbial platform | Trichoderma reesei | Trichoderma reesei | Trichoderma reesei | Trichoderma reesei | Escherichia coli (recombinant) | Aspergillus awamori |
| Cultivation mode | SmC | SmC | SmC | SmC | SmC | SSC |
| Production titer (g/L) | 50 | 50 | 11–35 | ? | 5 | ? |
| Productivity (g∙L−1∙h−1) | 0.26 | 0.42 | 0.10–0.32 | ? | 0.21 | ? |
| Product yield (g protein/g carbon source) | 20% | 24% | 26% | 21% | 2.5% | 2.4% |
| Enzyme cost composition | ||||||
| Facility-dependent/capital-related | 48% | 21% | +++ | 20% | 45% | 43% |
| Raw materials/ nutrients | 28% | 62% | ++ | 60% | 25% | 31% |
| Utilities/ electricity | 10% | 13% | + | 15% | 2% | 4% |
| Consumables | 4% | 0% | 0% | 23% | 5% | |
| Labor/fixed cost | 7% | 4% | + | 5% | 4% | 18% |
| Other costs | 3% | 0% | 0% | 1% | 0% | |
| Enzyme cost (US$ kg−1) | 10 | 5 | 4 | 5 | 316 | 59 |
| Enzyme | Microorganism | Optimal pH | Optimal Temperature (°C) | References |
|---|---|---|---|---|
| Endoglucanases | Cladosporium cladosporioides | 4 | 30 | [29] |
| Fusarium sp. | 5.5 | 30 | [30] | |
| Aspergillus niger | 5.5 | 30 | [31] | |
| Exoglucanases | Fusarium sp. | 5.5 | 30 | [30] |
| Aspergillus niger | 5.5 | 30 | [31] | |
| Phaeolus spadiceus | 4.5 | 25–30 | [32] | |
| β-glycosidases | Cladosporium cladosporioides | 4 | 30 | [29] |
| Aspergillus niger | 5–9 | 25–45 | [33] | |
| Fusarium sp. | 5.5 | 30 | [30] | |
| Trichoderma sp. | 5 | 28 | [34] | |
| Trichoderma harzianum | 6 | 70 | [35] | |
| Xylanases | Aspergillus tubingensis | 3–8 | 30–60 | [36] |
| Talaromyces amestolkiae | 7 | 30 | [37] | |
| Peroxidases | Pleurotus ostreatus | 3.3 | 25 | [38] |
| Hypsizygus ulmarius | 7 | 28 | [39] | |
| Pleurostuus florida | 7 | 28 | [39] | |
| Laccases | Trametes polyzona | 4.5 | 55 | [40] |
| Trametes versicolor | 4–5 | 40–50 | [41] | |
| Coriolopsis gallica | 6–8 | 40–60 | [42] | |
| Pycnoporus sp. | 6 | 0 | [43] |
| Enzyme | Microorganism | Optimal pH | Optimal Temperature (°C) | Reference |
|---|---|---|---|---|
| Endoglucanases | Bacillus subtilis | 5 | 60 | [50] |
| Neobacillus sedimentimangrovi | 7 | 60 | [51] | |
| Arthrobacter woluwensis | 8 | 50 | [52] | |
| Thermotoga naphtophila | 6 | 90 | [53] | |
| Exoglucanases | Clostridium thermocellum | 5.7 | 70 | [54] |
| Xylanases | Thermotoga marítima TmxB | 5 | 100 | [55] |
| Acinetobacter johnsonii | 6 | 55 | [56] | |
| Bacillus haynesii | 7 | 40 | [57] | |
| Caldicoprobacter algeriensis | 6.5 | 80 | [58] | |
| Laccases | Pseudomonas spp. | 3–8 | 20–80 | [59] |
| Bacillus ayderensis SK3-4 | 7 | 75 | [60] | |
| Endoglucanases | Lysinibacillus macroides | 7 | 30 | [61] |
| Pseudomonas parafulva | 8 | 50 | [62] |
| Compare Items | Fungi | Bacteria | Reference |
|---|---|---|---|
| Degrading enzyme species | Extracellular enzymes such as cellulase, hemicellulase, and ligninase are secreted to enzymatically hydrolyze lignocellulose | Extracellular enzymes such as cellulase and hemicellulase are secreted, and some bacteria can produce ligninase | [77] |
| Degradation products | Mainly carbon dioxide, water and some small molecule organic compounds | Mainly simple sugars, organic acids and a small amount of carbon dioxide | [78,79,80] |
| Degradation efficiency | It is usually slower, but it can degrade lignocellulose more thoroughly, especially for lignin | It is relatively fast, but the overall degree of degradation of lignocellulose is not as good as that of fungi, and it is difficult to completely degrade lignin | [81] |
| Application scenarios | It has a wide range of applications in the fields of chemicals, pulp, bioenergy production, composting, etc., and can be used to produce high-quality biofuels and bio-based products | It is widely used in wastewater treatment, silage, composting, bioenergy development, etc., and can be used to remove organic pollutants in wastewater and produce a variety of organic acids and clean energy such as methane | [45,82] |
| Environmental adaptability | It has strict requirements for environmental conditions, such as temperature, humidity and pH, etc., and the growth rate is relatively slow | It has strong adaptability to the environment, can grow in a wide range of temperature, humidity and pH value, and has a fast growth rate | [83,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Li, Q.; Liu, C.; Meng, E.; Zhang, B. Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives. Sustainability 2025, 17, 4223. https://doi.org/10.3390/su17094223
Chen M, Li Q, Liu C, Meng E, Zhang B. Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives. Sustainability. 2025; 17(9):4223. https://doi.org/10.3390/su17094223
Chicago/Turabian StyleChen, Mengke, Qinyu Li, Changjun Liu, Er Meng, and Baoguo Zhang. 2025. "Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives" Sustainability 17, no. 9: 4223. https://doi.org/10.3390/su17094223
APA StyleChen, M., Li, Q., Liu, C., Meng, E., & Zhang, B. (2025). Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives. Sustainability, 17(9), 4223. https://doi.org/10.3390/su17094223








