Abstract
Efficient hydrolysis of cellulose in agricultural waste (e.g., coconut fiber) is critical for biorefining processes such as second-generation bioethanol (2G ethanol) production. However, free cellulases suffer from low thermal stability and challenges in recovery. To address this, we developed cross-linked enzyme aggregates (CLEAs) combined with magnetic nanoparticles (magnetic CLEAs, m-CLEAs) to enhance enzyme stability and reusability. In this context, solutions of ethanol, acetone, and ammonium sulfate were used to prepare enzymatic aggregates, with subsequent use of glutaraldehyde and magnetic nanoparticles to obtain the biocatalysts. The addition of bovine serum albumin (BSA) protein was also tested to improve immobilization. Biocatalysts with ethanol and acetone performed better. Acetone (AC) and BSA yielded the highest enzymatic activities (287.27 ± 42.59 U/g for carboxymethyl cellulase (CMCase) with Celluclast; 425.37 ± 48.11 U/g for CMCase with Cellic CTec2). Magnetic nanoparticles were incorporated to expand the industrial applicability, producing m-CLEAs with excellent thermal stability and high catalytic activities. The m-CLEA–Celluclast–AC–BSA–GA 5% maintained 58% of its activity after 72 h at 70 °C. The m-CLEA–Celluclast-AC–BSA–GA 2.5% proved effective in hydrolyzing coconut fiber and isolated cellulose, producing up to 0.91 ± 0.01 g/L of glucose and 2.7 ± 0.15 g/L of glucose, respectively, after 72 h. Therefore, this approach supports sustainability by using coconut fiber, which is often discarded into the environment.
1. Introduction
Enzymes are biological catalysts with great potential for industrial application [1,2], due to their high specificity and ability to operate under mild conditions. Commercial cellulolytic cocktails, such as Cellic CTec2 and Celluclast, are widely used to hydrolyze lignocellulosic biomasses [3]. These cocktails are composed of fungal cellulases developed in a recombinant system to obtain increasingly efficient optimized formulations [4]. Cellic CTec2 stands out for its high β-glucosidase activity in its composition, making it a protagonist in reducing sugars in the 2G bioethanol production process [5]. The enzymatic cocktail Celluclast derives from a strain of Trichoderma reesei and contains high endoglucanase and cellobiohydrolase activities, but low β-glucosidase activity [4]. Despite the high efficiency of these commercial cellulolytic cocktails, they are formed by soluble enzymes that are difficult to recover and reuse, increasing the production cost for each batch.
Immobilization is an effective method that allows the reuse of enzymes, increases their stability, facilitates the separation of products, reduces enzyme inhibition, and, in some cases, improves selectivity and specificity [6,7,8]. Enzyme immobilization, when correctly performed, can increase process productivity and provide an economical and environmentally friendly approach [9]. Within this context, the cross-linked enzyme aggregates (CLEAs) approach has been used with various types of enzymes and has proven to be a fast, practical, efficient, easy, and suitable approach for enzyme optimization [10,11]. These characteristics resulted from a method developed as an alternative to enzymatic crystallization, involving the precipitation of the enzyme without using enzymes with a high purity content [11]. The high concentration of enzymatic activity is due to the catalyst mass being mainly made up of enzymes, unlike the method that uses support, in which 90 to 99% of the mass of the final catalyst is attributed to the support [12,13].
To form CLEAs, enzymes are initially precipitated, forming aggregates, and then crosslinked using an agent, such as glutaraldehyde (GA). Thus, the CLEA formation method traditionally does not require solid support. However, incorporating magnetic nanoparticles into CLEAs solves some problems, such as low mechanical stability and difficulties recovering CLEAs [14]. Magnetic nanoparticles have proven to be versatile and support enzyme immobilization, as they allow easy recovery of biocatalysts simply by applying a magnetic field [15]. They also possess a high surface area, enabling the accommodation of a significant amount of enzymes with minimal internal diffusional limitations, making this material more competitive in large-scale industrial processes [16]. Specifically, ferrites (Fe3O4) exhibit superparamagnetic behavior, preventing permanent agglomeration, which is crucial for maintaining reaction efficiency [17]. Moreover, their surface contains hydroxyl groups, which can incorporate functionalizing agents such as glutaraldehyde and silanes, facilitating immobilization through covalent bonding [18].
Among industrial enzymes, cellulases hydrolyze cellulose isolated or present in lignocellulosic materials. The products of this reaction are fermentable sugars that can be used in the production of 2G ethanol and butanol, among other products of commercial value [19,20]. Among the various lignocellulosic materials, coconut fiber can be cited. Brazil is considered the fifth largest producer of coconut and has the sixth largest planted area globally, presenting the highest production yield per planted area. Lignocellulosic biomass is composed mainly of cellulose, hemicellulose, and lignin, with cellulose being the primary target for bioethanol production [21]. Its complete depolymerization into glucose requires different cellulases: endoglucanases break internal bonds, generating oligosaccharides; exoglucanases act at the ends, releasing cellobiose; and, finally, β-glucosidases convert cellobiose into glucose [22].
In this context, this study deals with producing magnetic CLEAs (m-CLEAs) using different commercial cellulolytic cocktails (Celluclast and Cellic CTec2) to be applied to hydrolyze green coconut fiber. It is essential to mention that many papers already approach the production of fermentable sugars from lignocellulosic materials using soluble cellulases [23,24,25]. Still, there is little about the hydrolysis of lignocellulosic materials using immobilized enzymes [26], highlighting the importance of this study to the literature. Thus, this study contributes to producing fermentable sugars from green coconut fiber using immobilized enzymes, aiming at a 2G ethanol scheme. Therefore, in the context of sustainability, the use of coconut waste can reduce environmental impact, aligning with more sustainable practices. Furthermore, the use of m-CLEAs as a biocatalyst makes the enzymatic hydrolysis process more sustainable, since it reduces process costs allowing the reuse of the biocatalyst using the magnetic field.
2. Materials and Methods
2.1. Materials
Glutaraldehyde, carboxymethyl cellulose, and the cellulolytic cocktails, Celluclast and Cellic CTec2, were obtained from Sigma-Aldrich (São Paulo, Brazil). The precipitating agents were absolute ethyl alcohol (Química Moderna, São Paulo, Brazil), acetone (Neon, São Paulo, Brazil), and ammonium sulfate (Kinetics Reagents and Solutions, São Paulo, Brazil). The green coconut was collected at kiosks on Ponta Negra beach in Natal, Rio Grande do Norte (Natal, Brazil). Other reagents were used in the analytical grade.
2.2. Methods
2.2.1. Preparation of Green Coconut Fiber: Alkaline Pre-Treatment with NaOH
The green coconut shell was cut into small pieces to be ground (Wiley mill, TE–680, Tecnal, Niort, France) and sieved (48 mesh). After that, the biomass was washed with water to remove residual compounds from the material and was dried at 50 °C for 72 h, according to Campos et al. [27].
In the alkaline pretreatment, the green coconut fiber was subjected to a mixture of 1:5 (m/v) of biomass and 2% (m/v) NaOH. Subsequently, the biomass was taken to the autoclave at 121 °C for 1 h. Then, the material obtained was washed with water until it reached a neutral pH. The pretreated solid material was dried at 50 °C [27].
2.2.2. Enzyme Cocktails Activity and Protein Concentration
Enzyme Activity Using Filter Paper (FP) as Substrate (FPase)
In the procedure, Whatman No. 1 filter paper strips (1.0 × 6.0 cm) were immersed in sodium citrate buffer (50.0 mM, pH 4.8) and placed in a water bath (50 °C, 5 min), with the addition of the enzyme sample, reacting during 60 min. After incubation, the amount of reducing sugars released was determined using the DNS method [28] at a wavelength of 540 nm. The reducing sugar concentration was determined using glucose as a standard (0 to 2.23 µM). This methodology for quantifying the activity of cellulolytic cocktails was based on the method described by [29], with the activity defined as the amount of enzyme capable of releasing 1.0 μmol of glucose per minute at assay conditions.
Enzyme Activity Using Carboxymethyl Cellulose (CMC) as Substrate
This method corresponds to the specific activity of endoglucanase (endo-1,4-β-D-glucanase). Therefore, a 4.0% (m/v) CMC solution was prepared using sodium citrate buffer (50 mM, pH 4.8), to which the enzyme sample was added, reacting for 10 min at 50 °C. The method for determining total reducing sugars was the same as previously mentioned, the DNS method [28] at 540 nm. Like using filter paper (FP) as substrate, the activity unit using CMC is defined as the amount of enzyme capable of releasing 1.0 μmol of glucose per minute at assay conditions [29].
2.2.3. Protein Concentration of Enzyme Cocktails
Protein concentration was determined using the methodology developed by Bradford et al. [30]. Bovine serum albumin protein was used as a reference in the standard curve. The quantification method by direct ultraviolet absorption was also used. This method is based on the absorption of proteins in the 280 nm region due to the amino acids tryptophan, tyrosine, and cysteine, which have significant absorptivity in this range [31,32]. Thus, the reading of the enzyme solutions and supernatants was also performed at a wavelength of 280 nm.
2.2.4. Production of Cross-Linked Enzyme Aggregates (CLEAs)
Selection of Precipitating Agent
The precipitation of cellulolytic enzymes from Celluclast and Cellic CTec2 cocktails was performed by adding different precipitating agents (acetone PA, ethanol PA, or a saturated ammonium sulfate solution, approximately 76% m/v) to an enzyme solution (5 mg protein/mL in 50 mM sodium citrate buffer, pH 4.8) in volume ratios of 1:3 (enzyme/precipitant solution), using an ice bath (4 °C), according to the methodology reported by [33]. After adding the precipitating agent, the mixture was shaken (150 rpm) for 2.5 h at 4 °C. The precipitate was recovered by centrifugation (10,400× g at 4 °C, 10 min) and resuspended in sodium citrate buffer (50 mM, pH 4.8). In some specific cases, the precipitation of cellulolytic enzymes in the presence of bovine serum albumin (BSA) was also evaluated, and the mass ratio of 1:1 (enzyme/BSA) was in the precipitation step.
Crosslinking of Aggregated Enzymes for the Formation of CLEAs Using Glutaraldehyde
After precipitation, solutions of glutaraldehyde (2.5% and 5%, v/v) were made using each precipitating agent. These solutions were added to the enzyme aggregate and incubated for 2.5 h, at 4 °C and 150 rpm. Subsequently, the suspensions were centrifugated (10,400× g for 10 min at 4 °C), and the solids were washed twice, by centrifugation, with sodium citrate buffer (50 mM, pH 4.8) (using the same volume of precipitant added in the precipitation step) and stored at 4 °C.
2.2.5. Production of Magnetic Cross-Linked Enzyme Aggregates (m-CLEAs)
Synthesis of Fe3O4 Magnetic Nanoparticles and Coating with 3-Aminopropyltriethoxysilane (APTES)
Magnetic nanoparticles were synthesized by the co-precipitation method in an alkaline medium, according to Zhou et al. [34]. In this case, a NaOH solution (3.0 M) was added dropwise to the iron salt solutions (9.73 g of FeCl3·6H2O and 6.6 g FeSO4·7H2O dissolved in 120 mL of distilled water) and subjected to vigorous stirring (500 rpm) for 90 min, at room temperature. The magnet separated the black-colored product and it was washed five times with distilled water until the supernatant became transparent.
For functionalization with 3-aminopropyltriethoxysilane (APTES), 16 mL of magnetic nanoparticle suspension (37 g/L of nanoparticles) was suspended in 200 mL of distilled water, 100 mL of NaOH (8%, m/v), 4 mL of ethanol, and 2 mL of APTES. The reaction system was subjected to vigorous stirring (300 rpm) for 2 h at room temperature. Finally, the formed nanocomposite was washed several times with distilled water, recovered with a magnet, and dried in a desiccator.
Formation of m-CLEAs
The procedure followed the steps described in topic Selection of Precipitating Agent: adding the magnetic nanoparticles coated with APTES (0.01 g in 8 mL of the precipitation solution) was performed to produce m-CLEAs. The crosslinking step using glutaraldehyde was conducted as described in topic Crosslinking of Aggregated Enzymes for the Formation of CLEAs Using Glutaraldehyde, but the washes were conducted using a magnet.
2.2.6. Biocatalysts Characterization
Immobilization Parameters
The parameters used to evaluate the preparation efficiency of CLEAs were the precipitation yield (PY) and the recovered activity after resuspension of the precipitate (AtR). These parameters were calculated using Equations (1) and (2):
The initial activity (Ati—U/mL) is the activity of the enzyme solution at the beginning of the process, the final activity (Atf—U/mL) is the enzyme activity of the supernatant after precipitation, and Atp is the activity of the precipitate after resuspension (U/mL).
The activity of CLEAs and m-CLEAs was obtained in U/g, as shown in Equation (3):
Abs is the absorbance value of the sample; F (μmol/mL) is the factor obtained from the standard curve of DNS; V (mL) is the total volume of the reaction mixture; D is the sample dilution; t (min) is the reaction time and m (g) is the mass of immobilized enzyme. The recovered activity from the CLEAs was calculated in the same way as Equation (2), but in place of Atp the activity of the immobilized enzyme was used.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis was used to detect the main organic groups in the magnetic nanoparticles before and after coating with APTES and production of m-CLEAs. The FTIR spectra of the samples were obtained using a Cary 630 FTIR spectrometer (Agilent Technologies, Santa Clara, CA, USA) in the region of 650–4500 cm−1 with a resolution of 4 cm−1 and 32 scans.
Thermal Stability Assay
To analyze the thermal stability of the CLEAs obtained, 10 mg of the immobilized enzyme was suspended in 1 mL of sodium citrate buffer (50 mM, pH 4.8) and incubated at 70 °C. The temperature was determined using the thermal resistance assay of soluble CMCase. During the thermal stability tests, samples were collected at certain intervals, with a maximum time of 2 and 72 h for the soluble and immobilized enzymes. The activity was determined using CMC as a substrate (according to Section Enzyme Activity Using Carboxymethyl Cellulose (CMC) as Substrate). The initial point was measured and used as a reference for 100% enzyme activity in the calculation of relative activity (%), established as a ratio between the activity after a specific incubation time and the initial activity. The curves relating relative activity to time were constructed in Origin 8.5 software using the enzymatic deactivation model proposed by Sadana et al. [35].
SDS-PAGE Electrophoresis
SDS-polyacrylamide gel electrophoresis assays were performed according to [36]. The gels were prepared at a concentration of 12% polyacrylamide for the separation gel and 5% for the concentration gel. The disruption buffer was prepared and added to the samples, which were heated at 100 °C for 10 min. Next, 17.0 μL of the 1 mg/mL sample solution was added to each gel well, with only 5.0 μL being used in the standard marker sample. After the run (110 V), the gel was stained with Coomassie blue reagent [37].
2.2.7. Enzymatic Hydrolysis of Pretreated Biomass
Enzymatic hydrolysis of green coconut fiber pretreated with sodium hydroxide (2.0%, m/v) was performed according to Ribeiro et al. [24]. Using 25.0 mL Erlenmeyer flasks, the biomass was added with 3% (m/v) solids load, along with 10 mg of the best biocatalyst produced in the presence of sodium citrate buffer (50.0 mM, 4.75 mL, pH 4.8) and 0.05% (w/v), 250 μL azide solution that was added to avoid contamination. For comparison, the same solid load was added using commercial cellulose as a substrate. The flasks were incubated (Tecnal, model TE-421) at 50 °C, 150 rpm. At intervals of 4, 24, 48, and 72 h, aliquots were removed and subjected to a boiling bath for 5 min to inactivate the cellulases, followed by centrifugation (Model 5424, Eppendorf, Hamburg, Germany) for 5 min at 10,000 rpm. The supernatant was subjected to quantification of reducing sugars using the DNS method [28]. The supernatant was also filtered using 0.22 μm membranes (Millipore, Burlington, MA, USA), and, to calculate the cellulosic conversion, the sugar concentration was measured by High-Performance Liquid Chromatography (HPLC), according to Bell et al. [38]. The cellulose conversion value was obtained using Equation (4):
Gf (g) is the glucose content after 48 h of enzymatic hydrolysis, Gi (g) is the glucose at 0 h of enzymatic hydrolysis, and C (g) is the cellulose content of the biomass. The value 1.111 was the cellulose-to-glucose conversion factor used.
2.2.8. Statistical Analysis
Tukey’s and mean tests were performed to determine significant differences in the yields regarding activity using CMC and FP as substrates. The software used was Assistat 7.7 (Federal University of Campina Grande, Campina Grande, Brazil).
3. Results and Discussion
3.1. Evaluation of Precipitation Agents for the Preparation of the CLEAs
Following the methodology of Miranda et al. [33], an investigation of ethanol, acetone, and ammonium sulfate as precipitating agents was carried out. Different precipitants directly influence the interaction between enzyme molecules, which generates different enzymatic activities and influences the immiscibility of the enzyme in aqueous solution [38]. Table 1 shows the results of the parameters obtained for the precipitation test.

Table 1.
Precipitation yields (%) of cellulolytic cocktails in aggregate production, measured in terms of CMCase and FPase.
Table 1 shows that precipitation with ammonium sulfate obtained the highest yields in both activity cellulolytic cocktails used. Precipitation by salts occurs by breaking the hydration barriers between protein molecules, causing the water surrounding the protein to move to the solution. The hydrophobic zones on the protein surface are thus exposed, providing sites of attraction between neighboring protein molecules. In this case, proteins with many hydrophobic regions tend to precipitate in concentrated salt solutions (the salting-out effect).
After the ammonium sulfate, the best results were obtained using ethanol as a precipitating agent, which led to practically total precipitation. In the article by Ifko et al. [39], CLEAs and m-CLEAs of T. reesei cellulase were produced. The immobilization yields were between 99% and 100% for all precipitation reagents (acetone, ethanol, and propanol), in which ethanol provided the highest immobilization yield, which was 100% for CLEAs and 99% for m-CLEAs.
Acetone also showed good precipitation (above 85%) but was lower than the other agents. Precipitation by organic solvents occurs due to the addition of a weakly polar escurito the formation of a precipitate. One of these effects is the reduction in water activity, in which solvent molecules replace the water molecules on the protein’s surface. This reduces the hydration layer and favors the electrostatic attraction forces between the protein molecules [39].
In this sense, it can also be inferred that the precipitation with ethanol, acetone, and ammonium sulfate favored more significant precipitation of enzymes from the Celluclast cocktail than the Cellic CTec2 cocktail.
It is known that inadequate solvent selection can result in protein denaturation through interaction with hydrophobic residues within the protein. In this context, the recovered activity is also an important parameter since it measures how active the enzyme remains after precipitation. These results are presented in Figure 1.
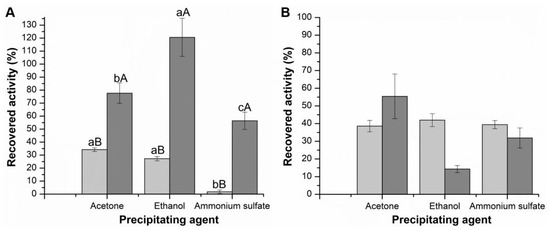
Figure 1.
Recovered activity (%) of the produced CLEAs with three precipitating agents, calculated as described in topic Immobilization Parameters. (A) Celluclast (CMCase—light grey; FPase—dark grey); (B) Cellic CTec2 (CMCase—light grey; FPase—dark grey). Values followed by the same capital letter between experiments for each activity recovered in terms of CMCase and FPase activities do not present statistical differences. Values followed by the same lowercase letter within the same experiment do not present statistical differences.
Regarding recovered activity, ammonium sulfate was not the best precipitating agent for either cellulolytic cocktail. For the precipitates of the Celluclast cocktail (Figure 1A), using the CMC substrate, only 1.79 ± 1.33% of the enzyme remained active. Using FP as substrate, the result was more significant (56.39 ± 6.63%) but still lower than the results obtained for the other agents.
This negative result with ammonium sulfate contradicts some studies’ reports. The cellulase produced by the cellulolytic bacterial strain Stenotrophomonas maltophilia was precipitated using ammonium sulfate, showing a higher recovered activity obtained when ammonium sulfate was used (80% w/v) [40]. According to the study by Li et al. [41], after testing five precipitating agents, the formation of CLEAs of cellulases using ammonium sulfate, 95% (w/v), showed a maximum recovery activity of 71.73%. According to the authors, the addition of these specific agents promoted a significant decrease in the solubility of the enzyme; enzymatic aggregates formed without impacting the protein structure, and therefore, the recovered activity of the CLEAs remained high. In the present study, a lower concentration of 70% (w/v) was applied, which may explain the lower recovered activity of CLEAs.
For Celluclast, it is possible to observe a greater recovered activity after precipitation with ethanol, presenting recovered activities of 27.25 ± 1.67% and 120.59 ± 14.63%, expressed in CMCase and FPase activity, respectively. These results show that the promising ethanol precipitating agent presents good results regarding recovered activity and precipitation yields.
Hojnik Podrepšek et al. [42] compared precipitating agents in their study using the Cellusoft enzyme cocktail and obtained the highest recovered activity (94%) using ethanol compared to the 55% obtained using ammonium sulfate. Perwez et al. [43] reported cellulase precipitation using acetone, ethanol, propanol, and ammonium sulfate, and the aggregate obtained by ethanol showed the highest recovered activity.
Regarding the Tukey test, for the Celluclast there was a significant difference (p < 0.05) in terms of CMCase. Acetone and ethanol results were statistically different from the ammonium sulfate results. Regarding FPase, there was a significant difference between the three agents. For the Cellic CTec2 (Figure 1B), there was no significant difference (p < 0.05) in terms of CMCase and FPase for the three precipitating agents. However, although the precipitation results are all close to 100%, the recovery activity is reduced when the CLEAs are generated, particularly with Cellic CTec2. The way the enzymes are organized in the CLEAs may hinder substrate diffusion or the flexibility required for catalysis. In the case of Cellic CTec2, it is also found to have a higher protein concentration. Overall, the impact on the activity of CLEAs may depend on factors such as aggregation, protein–protein interactions, and possible structural modifications during precipitation.
In summary, it can be stated that the precipitating agent ammonium sulfate presented lower recovered activity and was therefore discarded for subsequent experiments.
3.2. Evaluation of the Addition of Bovine Serum Albumin (BSA)
In the synthesis of CLEAs, BSA can be used as a protein additive, stabilizing protein by increasing the number of lysine residues, and providing sufficient amine groups for crosslinking with glutaraldehyde [39]. Glutaraldehyde is widely used as a crosslinking agent due to its ability to form covalent bonds with amino acid residues on the surface of enzymes [44]. In this sense, adding a protein rich in amino groups, such as BSA, is believed to favor the crosslinking of enzyme molecules with covalent bonds.
Thus, the addition of BSA in the preparation of CLEAs was evaluated. Figure 2 presents the results of the precipitation yield for CMCase and FPase using BSA and the precipitating agents ethanol and acetone.
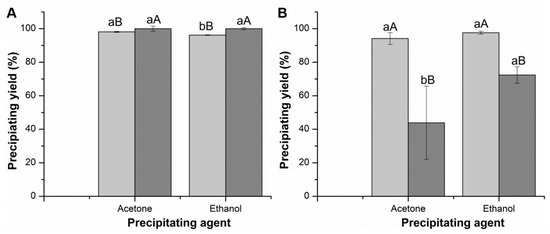
Figure 2.
Precipitation yield of CMCase and FPase of commercial cellulolytic cocktails in the presence of BSA. (A) Celluclast (CMCase—light grey; FPase—dark grey); (B) Cellic CTec2 (CMCase—light grey; FPase—dark grey). Values followed by the same capital letter between experiments, for each activity recovered in terms of CMCase and FPase activities, do not present statistical differences. Values followed by the same lowercase letter within the same experiment do not present statistical differences.
Based on the precipitation yield data of the Celluclast cocktail, the precipitant with the best yield was acetone, obtaining yields expressed in CMCase activity of 98.10 ± 0.27% and 100 ± 1.57% in FPase (Figure 2). Comparing with the data in Table 1, it can be stated that the addition of BSA maintained the yield expressed in CMCase activity (yield without BSA: 97.83 ± 0.64%) and increased the yield expressed in FPase activity (yield without BSA: 85.43 ± 1.43%). Celluclast CLEAs showed a significant difference (p < 0.05) in terms of CMCase when varying the two precipitating agents, while in terms of FPase there was no significant difference.
The precipitation yield results of Cellic CTec2 indicate that using ethanol results in better precipitation efficiency (Table 1). Comparing the data in Figure 2 with the data in Table 1, it can be stated that the addition of BSA promoted an increase in the yield expressed in CMCase activity (yield without BSA: 93.62 ± 0.07%) and decreased the yield expressed in FPase activity (yield without BSA: 88.28 ± 2.92%). There was no significant difference (p < 0.05) in CMCase for this cocktail. In contrast, in terms of FPase and protein concentration, there was a significant difference when varying the two precipitating agents.
After the formation of aggregates using acetone and ethanol as precipitating agents, a 5% (v/v) glutaraldehyde solution was added for the crosslinking step to occur, resulting in the formation of CLEAs. The ones obtained after precipitation with acetone are shown in Figure S1.
It was observed that the CLEAs formed with BSA and acetone turned out more robust, presenting a firmer and more resistant structure, which made a greater quantity of biocatalyst viable and allowed for better handling. Adding BSA to CLEAs improves structural stability by reducing fragility and increasing crosslinking via glutaraldehyde. Its size (~66 kDa) prevents excessive aggregation of enzymes, fills voids, and improves force distribution, making CLEAs more robust [45]. Also, when acting as a spacer it improves activity recovery and thermal stability of CLEAs [45].
When ethanol was used (Figure S2), the precipitate resolubilized after adding glutaraldehyde for the CLEA samples without BSA (Figure S2a). It was possible to obtain CLEAs–Celluclast with BSA using ethanol as a precipitating agent (Figure S2b). However, it was less robust than those obtained with BSA and acetone (Figure S1b). CLEAs–Cellic CTec2 with BSA using ethanol as a precipitating agent (Figure S2c) showed a fragile appearance and did not form a pellet.
Table 2 and Table 3 show the activities of the CLEAs obtained when using acetone and ethanol as precipitating agents in the absence and presence of BSA.

Table 2.
CMCase and FPase activity (U/g) of CLEAs obtained from the Celluclast cocktail and using acetone and ethanol as precipitating agents in the absence and presence of BSA.

Table 3.
CMCase and FPase activity (U/g) of CLEAs obtained from the Cellic CTec2 cocktail using acetone and ethanol as precipitating agents in the absence and presence of BSA.
All precipitates prepared using ethanol as the precipitating agent, and without the addition of BSA, did not form pellets. As a result, it was not possible to measure the catalytic activities of these biocatalysts. The best CLEA activities for the Celluclast cocktail were obtained after precipitation with acetone, with the addition of BSA not being significant (Table 2). However, the CLEAs of both cocktails showed low FPase activity, showing difficulty in interacting this type of aggregate with the solid substrate (filter paper). The differences in the expressed activities can be attributed to the different particle sizes and the different sizes of the internal pores, which can modify the diffusion of the substrates, especially in the case of insoluble substrates [39].
The activity values of CLEAs–Cellic CTec2 (Table 3) were greater than those obtained with CLEAs Celluclast with and without BSA and using both substrates (Table 4). These results can be explained by the fact that the Cellic CTec2 enzyme complex has high β-glucosidase activity in its composition [5]. Because CLEAs with acetone perform better for both cocktails, the following steps will be performed using acetone as the precipitating agent and with BSA.

Table 4.
Immobilization parameters of m-CLEAs obtained from Celluclast and Cellic CTec2 cocktails using acetone as a precipitating agent in the presence of BSA, using glutaraldehyde 5% (v/v) in the crosslinking step.
To better understand the crosslinking mechanism that occurs with these enzyme cocktails, composed mainly of β-glucosidases, endoglucanases, and exoglucanases produced by Trichoderma reesei, we analyzed their structures (obtained from the PDB database) about the lysine residues, which interact directly with glutaraldehyde (Figure 3). It was revealed that β-glucosidases (Cel3A, Figure 3A) [46] have the largest amount, with 25 residues, followed by exoglucanase (Cel7A, Figure 3B) [47] with 13 residues and endoglucanase (Cel5A, Figure 3C) with six residues. From these data, it is possible to suggest that an enzyme cocktail enriched with β-glucosidase, such as Cellic CTec2, may present more significant changes in activity since the interactions promoted by crosslinking affect the rigidity of the enzyme structure and, consequently, its performance. However, the proximity of the lysines to the active site is also relevant.
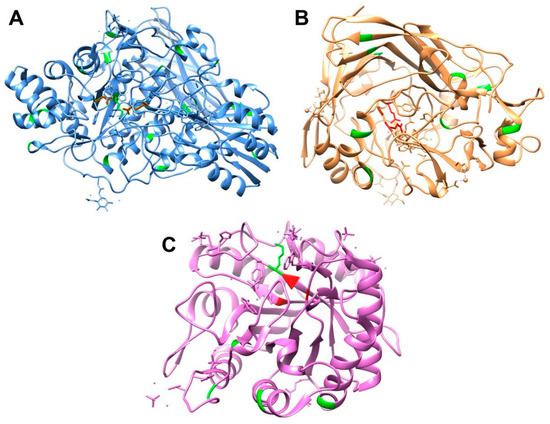
Figure 3.
Structure of β-glucosidases (Cel3A, (A)), exoglucanase (Cel7A, (B)) and endoglucanase (Cel5A, (C)). Lysine residues are in green and active site residues in red. PDB codification: (A)—3ZYZ; (B)—1DY4; and (C)—3QR3.
β-glucosidases have three Lys residues (positions 158, 206, and 370) close to their active site (Asp 236 and Glu 441). Exoglucanase has four Lys residues (positions 286, 346, 354, 415) close to its active site (Glu 212, Asp 214, and Glu 217). Despite having the smallest amount of six Lys residues, endoglucanases have one next to the active site (position 119), and its active site is composed of Thr 258, His 218, and Glu 148. Since cellulose degradation occurs synergistically, the absence or inactivity of endoglucanase compromises the efficiency of the process. Without its action, fewer attack sites are available for exoglucanases, reducing the release of cellobiose. Consequently, the action of β-glucosidases is also affected since they depend on cellobiose to produce glucose. Such insights serve to elucidate the observed decrease in enzyme activity following the crosslinking procedure.
3.3. Addition of Magnetic Nanoparticles (m-CLEAs)
According to Sheldon et al. [11], constant centrifugation can affect the structure of CLEAs and result in a decrease or total loss of catalytic activity. These characteristics may make the reuse of these biocatalysts impracticable. Nanoparticles are inserted into CLEAs, facilitating the separation process from the reaction medium and an increase in mass, facilitating the handling and application of this immobilized material. Because they are non-porous supports, they do not affect direct contact between the substrate and the enzyme, which is essential for cellulases, since they already act on insoluble substrates. In this case, magnetic CLEAs were synthesized using acetone as a precipitating agent and BSA as an additive. Figure S3 shows the appearance of the m-CLEAs obtained for the two cellulolytic cocktails, Celluclast and Cellic CTec2.
The immobilization parameters for the m-CLEAs are summarized in Table 4.
Based on the immobilization yield results obtained in Table 4 of m-CLEAs–Celluclast and compared with Table 2, it can be concluded that adding nanoparticles functionalized with APTES did not significantly influence the yield result. The activity of m-CLEAs–Celluclast was also not greater than that of CLEAs–Celluclast with BSA, with a reduction corresponding to 48% of the activity in CMCase. This result is not necessarily bad since, according to reports in the literature [48], the activity of the nanoparticle is expected to remain more stable and active for a longer time. However, this decreasing behavior is contrary to that reported in the literature.
In the article by Ifko et al. [39], m-CLEAs showed higher expressed activities (in carboxymethyl cellulose) than CLEAs. Similarly, a study by Lucena et al. [49], where cellulase m-CLEAs were synthesized and compared with cellulase CLEAs (in carboxymethyl cellulose), resulting in 33% higher activity for m-CLEAs. Despite the drop in CMCase activity, regarding FPase, an increase in activity of 150 times was observed (from 0.035 ± 0.029 U/g to 5.30 ± 1.75 U/g), revealing that the nanoparticles favored a better interaction with the solid substrate.
Comparing the immobilization yield results obtained in Table 4 for m-CLEAs–Cellic CTec2 with Table 3, it can be analyzed that adding nanoparticles functionalized with APTES favored an increase in the immobilization yield value expressed in FPase activity. However, in terms of activity, m-CLEAs–Cellic CTec2 decreased by around 75% activity compared to CLEAs–Cellic CTec2 with BSA.
By comparing the immobilization parameters, m-CLEAs–Celluclast was more successful in yield and activity. Thus, these results and their ease of handling and reuse caused m-CLEAs to be chosen as the focus of the following characterizations.
3.4. Characterization of m-CLEAs
3.4.1. Variation of Glutaraldehyde Concentration in m-CLEAs
The crosslinking agent and its concentration are essential for the activity, stability, and size of CLEAs. Studies show that the activity of CLEAs increases with the concentration of this agent until reaching an optimum point [49]. Based on this, the production of m-CLEAs with glutaraldehyde at concentrations of 2.5 and 5.0% (v/v) was tested to observe the effect on the activity of CMCase and FPase, with the results shown in Table 5.

Table 5.
Activity of m-CLEAs–Celluclast precipitated with acetone (AC) and BSA with 2.5 and 5.0% glutaraldehyde (GA).
Compared to the results in Table 4, it is noticeable that the decrease in the glutaraldehyde concentration to 2.5% directly influenced the activity, both in CMCase (twice as high) and FPase (5.6 times as high). This result suggests that the concentration of 5.0% exceeded the optimum concentration point of this crosslinking agent. At low concentrations, the bonds between the enzymes are insufficient for forming aggregates, resulting in excessive free enzymes in the solution. On the other hand, high concentrations can lead to the formation of strong bonds between the enzymes, making CLEAs a rigid aggregate that hinders the access of the substrate to the active site of the enzymes immobilized inside the aggregate [50].
3.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
The magnetic nanoparticles before and after immobilization were analyzed through FTIR, seeking to verify the possible changes promoted by the immobilization and by the coating of the nanomagnetic support. The spectra observed in Figure 4 were obtained.
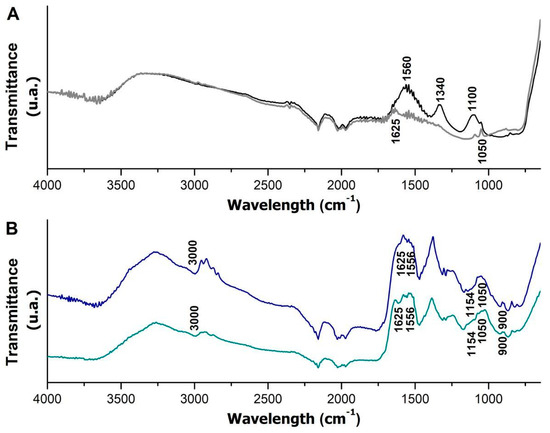
Figure 4.
FTIR spectra of nanoparticles before and after coating with APTES and m-CLEAs obtained from the Celluclast and Cellic CTec2 cocktails. Magnetic nanoparticles (black spectrum) and magnetic nanoparticles coated with APTES (gray spectrum) are represented in graph (A); m-CLEAs–Celluclast (blue spectrum) and m-CLEAs–Cellic CTec2 (green spectrum) are represented in graph (B).
The spectrum of the nanoparticle (black line) and the nanoparticle modified with APTES (gray line) display minor variations. However, after coating, the disappearance of some bands (1560, 1340, and 1100 cm−1) and the appearance of vibrational bands at 1050 and 1625 cm−1, which are related to C-N stretching and NH bending, respectively [51], were observed. These bands also appeared more evidently in the m-CLEA samples (blue and green lines) since these bands can be attributed to both the amino groups present in APTES and the amino groups in cellulases. In addition, the presence of silanes (Si–O–Si groups) in the nanoparticles due to APTES coating can be confirmed by the band at 993 cm−1 [52], which was probably overlapped by nearby bands.
In the m-CLEA spectra, bands were also observed at 1154 cm−1, related to amine vibration (-NH2), and at 1556 cm−1, associated with the deformation of amide groups (N-H) [53]. The presence of these bands is expected in samples with protein in their composition; in this case, it is attributed to the cellulolytic enzyme complex in the cellulase sample immobilized in nanoparticles. These results corroborate previous results, suggesting that the preparation of magnetic biocatalysts was successful [54]. The band at 900 cm−1 is attributed to the bending vibration –CH [55].
3.4.3. SDS-PAGE Electrophoresis
To verify the formation of covalent bonds in the obtained m-CLEAs, an SDS-PAGE electrophoresis assay was performed on the soluble cellulolytic cocktails with and without the addition of BSA and on the produced m-CLEAs. The results are shown in Figure 5.
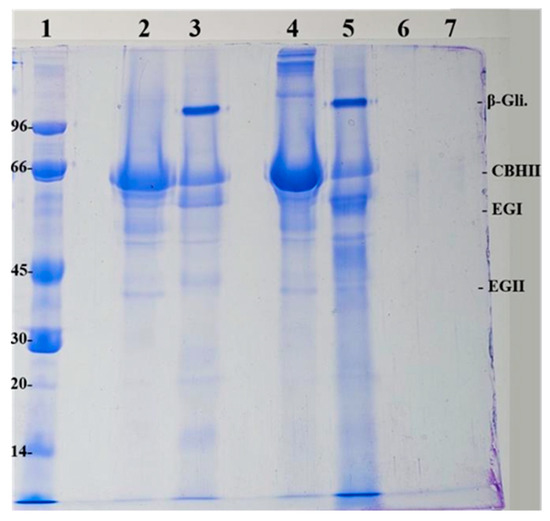
Figure 5.
Electrophoresis of the enzyme cocktails and m-CLEAs obtained. (1) Low molecular weight marker (LMW) in kDa; (2) Celluclast soluble cocktail without BSA; (3) Cellic CTec2 soluble cocktail without BSA; (4) Celluclast soluble cocktail with BSA; (5) Cellic CTec2 soluble cocktail with BSA; (6) m-CLEA–Celluclast with BSA; (7) m-CLEA–Cellic CTec2 with BSA.
In Figure 5, it is possible to identify the bands corresponding to the enzymes that are part of the Celluclast and Cellic CTec2 enzyme cocktails. It can be seen that the 66 kDa band appears in all bands of the soluble cocktails (samples 2 and 3) and has a greater intensity in the bands of the samples that have added BSA (samples 4 and 5). The BSA protein has exactly this molecular mass (66 kDa). Interestingly, the electrophoresis displays high molecular weight proteins in lane 4 that are absent in lane 2 without BSA, indicating the presence of BSA impurities. However, such impurities are not present in lane 5. Thus, this interesting behavior could be exploited in further studies since it deserves attention.
According to the study by Jung et al. [56], the interval between the 50 to 75 kDa bands represents endoglucanases and exoglucanases, present in all bands (lanes 2, 3, 4, and 5) and the interval between the 75 to 120 kDa bands represents β-glucosidase, present with greater intensity in the Cellic CTec2 samples, as this cocktail has a higher amount of this enzyme added to it.
In samples where glutaraldehyde is used in the preparation, strong interaction bonds, such as covalent bonds, are expected, as described by Lee et al. [57]. The denaturation conditions do not break these bonds of the assay (breaking buffer with 4% SDS and 100 °C). Thus, the expected result is that no bands will appear in the samples of the m-CLEAs produced. These behaviors were observed in the gels of both biocatalysts. This suggests that glutaraldehyde effectively created the covalent bonds since even after treatment with the breaking buffer at 100 °C, the enzyme remained retained in the m-CLEAs produced.
3.4.4. Thermal Inactivation
The produced m-CLEAs (m-CLEAs–Celluclast precipitated with acetone and BSA and crosslinked with 5% (v/v) glutaraldehyde) were evaluated for thermal stability at 70 °C compared with the free enzyme. The decay plot of enzyme activity is shown in Figure 6.
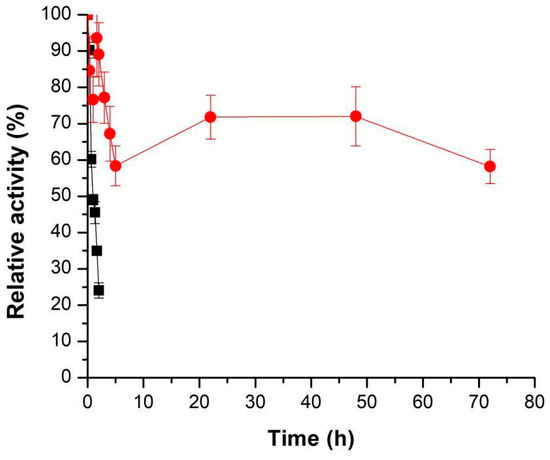
Figure 6.
Thermal stability at 70 °C in 50 mM sodium acetate buffer, pH 4.8, of the soluble cellulolytic cocktail Celluclast (■) and m-CLEAs–Celluclast 1.5 L (with BSA and acetone) (●).
When comparing the soluble enzyme and the immobilized enzyme, it is possible to observe a gain in the thermal stability of the immobilized sample. The half-life time for soluble Celluclast was 1 h 5 min, while the immobilized enzyme did not reach the half-life even after 72 h of incubation (relative activity at 72 h: 58.19 ± 4.68%). This significant increase in cellulase stability can be justified by immobilization, which increases the temperature range in which the enzyme can remain stable, in addition to the establishment of covalent bonds (due to the action of glutaraldehyde), which makes the enzyme structure more rigid. In addition, nanoparticles protect enzymes [48], delaying the impact of temperature on enzymatic activity. This results in a longer preservation of the active form of the enzyme.
The functionalization of nanoparticles with the APTES agent also plays a crucial role in stabilization, forming a protective layer that prevents oxidation of the nanoparticles [57]. According to Doraiswamy et al. [58], the strong covalent bonds between the enzyme and the nanoparticles promote greater rigidity of the enzyme molecules, which favors the enzymatic arrangement and increases heat tolerance. According to the study by Huang et al. [59], after 8 h of incubation at 55 °C, the free cellulase maintained approximately 30% of its initial activity, while the cellulase immobilized in magnetic nanocomposites (Fe3O4@SiO2) maintained 60% of the initial activity.
3.5. Application of m-CLEAs in the Hydrolysis of Pretreated Green Coconut Fiber and Isolated Cellulose
Considering that the biocatalyst m-CLEA–Celluclast-AC-BSA-GA 2.5% showed greater hydrolysis activity of the insoluble substrate FP (Table 5) this biocatalyst was subjected to hydrolysis of the fiber pretreated by the alkaline method and isolated cellulose for comparison. Figure 7 shows the results obtained from the hydrolysis.
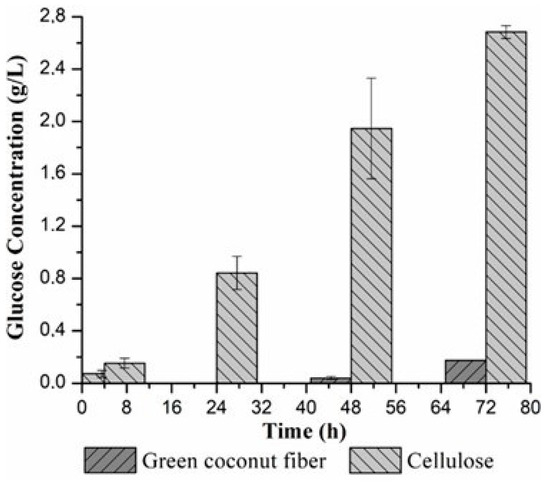
Figure 7.
Enzymatic hydrolysis activity of coconut fiber and cellulose occurred at 50 °C and 125 rpm.
The physicochemical characterization of the pretreated green coconut fiber Cocos nucifera L. was performed and obtained 44.65 ± 1.23% of cellulose, 21.33 ± 0.43% of hemicellulose, and 25.35 ± 0.49% of lignin. Comparing the results obtained by Nogueira et al. [23], it was shown that the pretreatment promoted a decrease in lignin, since the natural coconut fiber has a concentration of approximately 35.70 ± 1.30%. In addition, it eliminates non-productive adsorption sites and makes both hemicellulose and cellulose more accessible for subsequent processes. Alkaline pretreatments tend to preserve hemicellulose polymers, resulting in higher glucose yields during hydrolysis [60].
The hydrolysis results showed that the production of reducing sugars increased gradually when cellulose was used as substrate, indicating that the interaction between the enzyme and the substrate influences this process. In the case of coconut fiber, sugar production was only observed after 48 h and 72 h, probably due to the greater difficulty of the enzyme in accessing this type of substrate.
Regarding the glucose concentration measured by HPLC, 0.79 ± 0.06 g/L and 2.60 ± 0.49 g/L of glucose at 48 h and 0.91 ± 0.01 g/L and 3.13 ± 0.25 g/L of glucose at 72 h were obtained through the hydrolysis catalyzed by m-CLEAs of pretreated green coconut fiber and isolated cellulose, respectively. The final conversion of cellulose from coconut fiber was 6.10% and 8.04% from cellulose, after 72 h.
A comparative evaluation between free cellulase and m-CLEA cellulase was studied by Rai et al. [19] for enzymatic hydrolysis of sugarcane bagasse. Free cellulase presented a bagasse hydrolysis yield of 78%, while in the case of m-CLEAs a yield of 72% was found. In the study developed by [46], the m-CLEAs that were prepared for use in their experiment showed greater efficiency than free cellulase in the hydrolysis of sugarcane bagasse, allowing higher production and sugar yields.
4. Conclusions
This study demonstrated that ammonium sulfate stood as the best precipitating agent in terms of yield; however, in terms of recovered activity, ethanol and acetone proved to be efficient and ideal. The addition of BSA resulted in improvements in enzyme activity. FTIR characterization confirmed the presence of the enzymes in immobilized form, and electrophoresis tests indicated strong covalent interactions in the presence of glutaraldehyde, reinforcing the structural integrity of CLEAs. Notably, the thermal stability of the biocatalysts was improved, which suggests significant potential for industrial applications, such as in the production of second-generation ethanol. The ability of CLEAs to efficiently hydrolyze green coconut fiber highlights their practical applicability and suggests a promising path for the valorization of agricultural residues. For future research, it would be beneficial to explore the hydrolysis of different types of biomass under various process conditions, in addition to testing different crosslinking agents and their concentrations, so that CLEAs can achieve a more versatile hydrolysis capacity, suggesting a promising route for valorization of multiple agro-residues. The applicability of CLEAs in other biotechnological contexts, particularly in bioethanol production, also deserves detailed investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17094221/s1, Figure S1: CLEAs produced from cellulolytic cocktails using acetone as a precipitating agent; Figure S2: CLEAs produced from cellulolytic cocktails using ethanol as a precipitating agent; Figure S3: Magnetic CLEAs produced with Celluclast (a) and Cellic CTec2 (b); Table S1: Enzymatic hydrolysis activity of coconut fiber and cellulose by DNS assay occurred at 50 °C and 125 rpm.
Author Contributions
Conceptualization, N.S.R. and E.S.d.S.; methodology, N.S.R. and E.S.d.S.; software, J.R.F.M. and I.O.C.; validation, C.E.A.P., N.S.R. and E.S.d.S.; formal analysis, N.S.R.; investigation, J.R.F.M. and I.O.C.; resources, N.S.R. and E.S.d.S.; data curation, N.S.R. and E.S.d.S.; writing—original draft preparation, J.R.F.M. and N.S.R.; writing—review and editing, N.S.R. and E.S.d.S.; visualization, N.S.R.; supervision, E.S.d.S.; project administration, E.S.d.S.; funding acquisition, N.S.R. and E.S.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (Brazil), Finance Code 001, and CNPq (Brazil), grant number 304571/2023-7.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
The authors thank the Group on Applied Thermofluid Dynamics (GPTA) at the Federal University of Ceará (UFC) for FTIR spectroscopy. Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APTES | 3-aminopropyltriethoxysilane |
| CLEAs | Cross-linked enzyme aggregates |
| CMC | Carboxymethyl cellulose |
| CMCase | Enzyme activity using carboxymethyl cellulose as substrate |
| FP | Filter paper |
| FPase | Enzyme activity using filter paper as substrate |
| m-CLEAs | Magnetic cross-linked enzyme aggregates |
| SDS-PAGE | Sodium dodecyl–sulfate polyacrylamide electrophoresis |
References
- Schmid, A.; Dorndick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.P.; Souza, M.R.L.; Ferreira, F.E.X. An overview of holocellulose-degrading enzyme immobilization for bioethanol production. J. Mol. Catal. B Enzym. 2016, 133, 127–135. [Google Scholar] [CrossRef]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Boyer, A.; Subramanian, S. Bioconversion of lignocellulosic biomass to fermentable sugars by immobilized magnetic cellulolytic enzyme cocktails. Langmuir 2018, 34, 6546–6555. [Google Scholar] [CrossRef]
- Santos, F.D.D. Desenvolvimento de Coquetéis Enzimáticos Customizados Para a Hidrólise de Substratos Com Elevados Teores de Hemicelulose e Lignina. Tese de Doutorado, Universidade de São Paulo, São Paulo, Brazil, 2021. [Google Scholar]
- Araújo, B.M.C.; Costa, I.O.; Brito, H.G.; Rios, N.S.; Santos, E.S. Enzyme technology in bioethanol production from lignocellulosic biomass: Recent trends with a focus on immobilized enzymes. BioResources 2023, 18, 8653–8667. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lohka, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of enzyme loading and immobilization conditions on the catalytic features of lipase from Pseudomonas fluorescens immobilized on octyl-agarose beads. Front. Bioeng. Biotech. 2020, 8, 36. [Google Scholar] [CrossRef]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Ganero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green. Sust. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Mo, H.; Qiu, J. Preparation of chitosan/magnetic porous biochar as support for cellulase immobilization by using glutaraldehyde. Polymers 2020, 12, 2672. [Google Scholar] [CrossRef]
- Asgher, M.; Shahid, M.; Kamal, S.; Iqbal, H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Catal. B Enzym. 2014, 101, 56–66. [Google Scholar] [CrossRef]
- Lozanor, V.S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2016, 1, 166–177. [Google Scholar]
- Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’magnetic CLEAs: Biocatalysis in a bio- based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; Van Rantwijk, F.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotech. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]
- Cao, L.; Van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of Penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Basso, A.E.; Brady, D. New fronteirs in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, J.; Alcántara, A.A.; Fernandez-Lafuente, R.; Fernandez-Lucas, J. Magnetic micro-macro biocatalysts applied to industrial bioprocesses. Bioresour. Technol. 2021, 322, 12457. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, M.; Mohammadlou, A.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef]
- Ashtari, K.; Khajeh, K.; Fasihi, J.; Ashtari, P.; Ramazani, A.; Vali, H. Silica-encapsulated magnetic nanoparticles: Enzyme immobilization and cytotoxic study. Int. J. Biol. Macromol. 2012, 50, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Galvão, W.S.; Neto, D.M.A.; Freire, R.M.; Fechine, P.B.A. Super-paramagnetic nanoparticles with spinel structure: A review of synthesis and biomedical applications. Solid. State Phenom. 2016, 241, 139–176. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Biswas, J.K.; Silva, S.S. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal. Rev.-Sci. 2019, 61, 1–26. [Google Scholar] [CrossRef]
- Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and bioprocess design issues in enzymatic hydrolysis of lignocellulosic biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Bronzato, G.R.F.; Reis, V.A.; Borro, J.A.; Leão, A.L.; Cesarino, I. Second generation ethanol made from coir husk under the biomass Cascade approach. Mol. Cryst. Liq. 2019, 693, 107–114. [Google Scholar] [CrossRef]
- Costa, B.E.B.; Rangel, F.C.; Meneghetti, S.M.P. Comparação entre a hidrólise química e enzimática da biomassa lignocelulósica para a produção de bioetanol: Uma revisão. Rev. Virtual Quim. 2021, 13, 242–259. [Google Scholar]
- Nogueira, C.C.; Padilha, C.E.A.; Santos, E.S. Enzymatic hydrolysis and simultaneous saccharification and fermentation of green coconut fiber under high concentrations of ethylene oxide-based polymers. Renew. Energy 2021, 163, 1536–1547. [Google Scholar] [CrossRef]
- Ribeiro, V.T.; Campolina, A.C.; Costa, W.A.; Padilha, C.E.A.; Costa Filho, J.D.B.; Leitão, A.L.O.S.; Rocha, J.C.; Santos, E.S. Ethanol production from green coconut fiber using a sequential steam explosion and alkaline pretreatment. Biomass Convrs. Biorefin. 2024, 14, 17955–17970. [Google Scholar] [CrossRef]
- Ribeiro, V.T.; Costa Filho, J.D.B.; Padilha, C.E.A.; Santos, E.S. Using Tween 80 in pretreatment, enzymatic hydrolysis, and fermentation processes for enhancing ethanol production from green coconut fiber. Biomass Convrs. Biorefin. 2024, 14, 8579–8589. [Google Scholar] [CrossRef]
- Costa, I.O.; Morais, J.R.F.; Dantas, J.M.M.; Gonçalves, R.L.B.; Santos, E.S.; Rios, N.S. Enzyme immobilization technology as a tool to innovate in the production of biofuels: A special review of the Cross-Linked Enzyme Aggregates (CLEAs) strategy. Enzyme Microbiol. Technol. 2023, 170, 110300. [Google Scholar] [CrossRef]
- Campos, A.O. Produção de Enzimas Celulolíticas por Trichoderma reesei CCT2768 por Fermentação em Estado Sólido Usando Fibra de coco Verde (Cocos nucifera) Pré-Tratada Por Explosão a Vapor Como Substrato. Dissertação de Mestrado, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2022. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Groves, W.E.; Davis, F.C.; Sells, B.H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal. Biochem. 1968, 22, 195–210. [Google Scholar]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- Miranda, L.P.; Guimaraes, J.R.; Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Composites of crosslinked aggregates of eversa® transform and magnetic nanoparticles. Performance in the ethanolysis of soybean oil. Catalysts 2020, 10, 817. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Zang, Y. Preparation of magnetic polyethylenimine lignin and its adsorption of Pb (II). Int. J. Biol. Macromol. 2020, 155, 981–990. [Google Scholar]
- Sadana, A.; Henley, J.P. Analysis of enzyme deactivations by a series-type mechanism: Influence of modification on the activity and stability of enzymes. Ann. N. Y. Acad. Sci. 1987, 501, 73–79. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.S.; Morais, E.G.; Santos, G.W.; Andrade, N.D.N.; Santos, J.C.S.; Bohn, F.; Correa, M.A.; Fechine, P.B.A.; Fernandez-Lafuente, F.; Gonçalvez, L.R.B. Further stabilization of lipase from Pseudomonas fluorescens immobilized onoctyl coated nanoparticles via Chemical modification with bifunctional agents. Int. J. Biol. Macromol. 2019, 141, 313–324. [Google Scholar] [CrossRef]
- Bell, D.J.; Hoare, M.; Dunnill, P. The formation of protein precipitates and their centrifugal recoverys. Adv. Biochem. Engi./Biotechnol. 1983, 76, 1–72. [Google Scholar]
- Ifko, D.; Vasić, K.; Knez, Ž.; Leitgeb, M. Magnetic) Cross-linked enzyme aggregates of cellulase from T. reesei: A stable and efficient biocatalyst. Molecules 2023, 28, 1305. [Google Scholar] [CrossRef]
- Tamilanban, R.; Velayudhan, S.S.; Rajadas, S.E.; Harshavardhan, S. Purification and characterization of an extracellular cellulase produced using alkali pretreated rice straw by Stenotrophomonas maltophilia. Int. J. Biol. Res. 2017, 2, 45–54. [Google Scholar]
- Li, T.; Gong, X.; Yang, G.; Li, Q.; Huang, J.; Zhou, N.; Jia, J. Cross-linked enzyme aggregates (CLEAs) of cellulase with improved catalytic activity, adaptability and reusability. Bioprocesses Biosyst. Eng. 2022, 45, 865–875. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Activation of cellulase cross-linked enzyme aggregates (CLEAs) in scCO2. J. Supercrit. Fluids 2019, 154, 104629. [Google Scholar] [CrossRef]
- Perwez, M.; Ahmed Mazumder, J.; Sardar, M. Preparation and characterization of reusable magnetic combi-CLEA of cellulase and hemicellulase. Enzyme Microbiol. Technol. 2019, 131, 109389. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Mafra, A.C.O. Biocatalyst Engineering Applied to the Improvement of Cross-Linked Enzymes Aggregates Aiming at the Multienzymatic Conversion of Sucrose to Gluconic acid and Fructose Syrup. Doctorate Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2027. [Google Scholar]
- Karkehabadi, S.; Helmich, K.E.; Kaper, T.; Hansson, H.; Mikkelsen, N.-E.; Gudmundsson, M.; Piens, K.; Fujdala, M.; Barnejee, G.; Scott-Craig, J.S.; et al. Biochemical characterization and crystal structures of a fungal family 3 β-glucosidase, Cel3A from Hypocrea jecorina. J. Biol. Chem. 2014, 289, 31624–31627. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, J.; Henriksson, H.; Divne, C.; Isaksson, R.; Pettersson, G.; Johansson, G.; Jones, T.A. Structural basis for enantiomer binding and separation of a common β-blocker: Crystal structure of cellobiohydrolase Cel7A with bound (S)-propanolol at 1.9 Å resolution. J. Mol. Biol. 2001, 5, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, R.T.; Shi, Y.P. Recent advances on support materials for lipase immobilization and applicability as biocatalysts in inhibitors screening methods—A review. Anal. Chim. Acta 2020, 1101, 9–22. [Google Scholar] [CrossRef]
- Lucena, C.G.; Santos, C.C.; Pinto, G.C.; Piazza, R.D.; Guedes, W.N.; Jafelicci Júnior, M.; Paula, A.V.; Marques, R.F.C. Synthesis and characterization of magnetic cross-linked enzyme aggregate and its evaluation of the alternating magnetic field (AMF) effects in the catalytic activity. J. Magn. Magn. Mater. 2020, 516, 167326. [Google Scholar] [CrossRef]
- Dal Magro, L.; Hertz, P.L.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Cai, T.; Ge, X.; Park, S.Y.; Li, Y. Comparison of Synechocystis sp. PCC6803 and Nannochloropsis salina for lipid production using artificial seawater and nutrients from anaerobic digestion effluent. Bioresour. Technol. 2013, 144, 255–260. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Paikra, S.K.; Baliarsingh, A.; Panda, D.; Rath, S.; Mishra, M.; Sahoo, H. Surface functionalization of graphene oxide using amino silane magnetic nanocomposite for Chromium (VI) removal and bacterial treatment. Nano Express 2020, 1, 010062. [Google Scholar] [CrossRef]
- Rocha, C.O. Influência de Campo Magnético Alternado em Celulases Imobilizadas em Nanopartículas Magnéticas: Aplicação na Hidrólise de Bagaço de Cana. Tese de Doutorado, Universidade Estadual Júlio de Mesquita Filho, Araraquara, Brazil, 2021. [Google Scholar]
- Abbas, M.; Parvatheeswara Rao, B.; Naga, S.M.; Takahashi, M.; Kim, C. Synthesis of high magnetization hydrophilic magnetite (Fe3O4) nanoparticles in single reaction—Surfactantless polyol process. Ceram. Int. 2013, 39, 7605–7611. [Google Scholar] [CrossRef]
- Silverstein, R.W.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Jung, D.H.; Jung, J.H.; Seo, D.H.; Ha, S.J.; Kweon, D.K.; Park, C.S. One-pot bioconversion of sucrose to trehalose using enzymatic sequential reactions in combined cross-linked enzyme aggregate. Bioresour. Technol. 2013, 130, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Chung, M.; Kim, M.I.; Ha, S.H. Preparation of glutaraldehyde-treated lipase-inorganic hybrid nanoflowers and their catalytic performance as immobilized enzymes. Enzyme Microbiol. Technol. 2017, 105, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, N.; Sarathi, M.; Pennathur, G. Cross-linked esterase aggregates (CLEAs) using nanoparticles as immobilization matrix. Prep. Biochem. Biotechnol. 2019, 49, 270–278. [Google Scholar] [CrossRef]
- Huang, W.; Pan, S.; Li, Y.; Yu, L.; Liu, R. Immobilization and characterization of cellulase on hydroxy and aldehyde functionalized magnetic Fe2O3/Fe3O4 nanocomposites prepared via a novel rapid combustion process. Int. J. Biol. Macromol. 2020, 162, 845–852. [Google Scholar] [CrossRef]
- Nascimento, R.J.A.; Macedo, G.R.; Santos, E.S.; Oliveira, J.A. Real time and in situ near-infrared spectroscopy (nirs) for quantitative monitoring of biomass, glucose, ethanol and glycerine concentrations in an alcoholic fermentation. Braz. J. Chem. Eng. 2017, 34, 457–468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).